1. Introduction
Development of novel antimicrobial peptides (AMPs) is urgently needed to combat against rapidly appearing drug resistant strains [
1,
2,
3]. AMPs are generally short in length and composed of relative proportions of cationic and hydrophobic residues [
3,
4,
5,
6]. AMPs may be categorized based on their structures namely (a) α-helix, (b) β-sheet or β-hairpin stabilized by disulfide bridges, (c) extended and (d) loops with or without disulfide bonds. The cytolytic activity of most AMPs stems from membrane- targeted non-receptor mediated mechanisms and hence development of drug resistance towards such peptides would be difficult [
7,
8,
9,
10,
11]. The structurally divergent nature and simple mode of action of AMPs make them suitable candidates for the development of anti-infective agents [
12,
13]. Lipopolysaccharide (LPS) of the outer membrane may restrict activity of AMPs to Gram-negative bacteria [
14,
15,
16,
17,
18]. As a mode of action, broad spectrum AMPs would disrupt the permeability barrier of LPS-outer membrane, which is an important step for killing bacterial cells [
18,
19]. In addition, LPS or endotoxin induces inflammatory pathways leading to the production of cytokines that often results in septic shock [
19,
20,
21]. Sepsis or septicemia occupies the tenth position among the most dreadful diseases and there are no recent significant improvements for the treatment of sepsis-affected patients [
22,
23,
24].
Among the various classes of antimicrobial peptides, β-hairpins stabilized by disulfide bridges, including defensins, protegrins, polyphemusins and tachyplesins, occupy a prominent position [
25,
26,
27]. The disulfide bridges are found to play an important role in the antibacterial activity and cell selectivity [
28,
29,
30]. Moreover, disulfide bridges can stabilize the folded conformations of AMPs in aqueous solutions that upon membrane interactions may form higher order structures [
31]. Addition of fatty acyl chains to AMPs shows increased microbial sterilization probably by increasing incorporation of peptides deeply into the membrane [
32,
33,
34,
35,
36,
37,
38,
39,
40]. We are interested in developing AMPs that disrupt the LPS outer membrane as a mode of action [
41,
42,
43,
44,
45,
46]. In previous reports, we have designed a set of short (~12 amino acid long) cationic/hydrophobic peptides with antibacterial and antiendotoxic activities [
41,
42]. The designed peptides have been termed β-boomerangs as they adopt boomerang-like structures in complex with LPS. From an initial design, the second generation of β-boomerang peptides demonstrated enhanced LPS neutralization and antibacterial activity. Solution NMR studies revealed packing of two aromatic residues (W4 and F9) and amphipathic segregation of four cationic residues is critical for antimicrobial and antiendotoxic activities [
42]. In this work, we have utilized the most active analog of the second generation of β-boomerang peptide, YI12WF (YVLWKRKRFIFI-amide) as a starting template for further design of potent antimicrobial activity and LPS neutralization peptide analogs. Toward this end, we have prepared analogs of YI12WF containing five basic residues (YVLW
KRKRKFCFI-amide) in the loop of the β-boomerang structure and which are acylated at the N-termini, by C4 and C8 compounds. Further, residue I11 has been replaced by residue Cys to prepare disulfide linked variants of β-boomerang lipopeptides. All of the peptides are tested for antimicrobial and LPS neutralization and RBC lysis. Biophysical studies, using optical spectroscopic methods, ITC and dynamic light scattering have provided molecular insights into the mode of action of the designed peptides in Gram-negative bacterial outer membrane and endotoxin neutralization. Atomic-resolution structure, by NMR spectroscopy, has been determined for one of the potent peptides. Collectively, current results describe structure and activity of highly potent β-boomerang peptides of superior activity in comparison to parent peptides. These β-boomerang lipopeptides may be used in developing non-toxic antibacterial therapeutics.
2. Experimental Section
Materials: LPS of E. coli 0111:B4, FITC-LPS of E. coli 055:B5, acrylamide, NPN (1-N-phenylnaphthylamine) were purchased from Sigma (Saint Louis, MO, USA). All bacterial strains were obtained from the American Type Culture Collection (Manassas, VA, USA). Designed peptides were synthesized commercially by GL Biochem (Shanghai, China) and further purified by a reverse phase HPLC (Waters), using a C18 column (300 Å pore size, 5 μm particle size). The dimeric variants of β-boomerang lipopeptides were treated with 20% DMSO for 18 hours and the oxidized peptides were further purified by HPLC. A linear gradient of acetonitrile/water mixture was used for the purification and fractions eluting with higher purity were collected and freeze-dried.
Antibacterial assay: The minimum inhibitory concentration (MIC) of the designed peptides was determined against four Gram-negative strains (E. coli, P. aeruginosa ATCC 27853, K. pneumoniae ATCC 13883, S. enterica ATCC 14028) and four Gram-positive strains (B. subtilis, S. aureus ATCC 25923, S. pyogenes ATCC 19615 and E. faecalis ATCC 29212). In brief, mid logarithmic phase of overnight grown bacterial cultures were obtained in LB broth and adjusted to OD600 0.2 in 10 mM phosphate buffer, pH 6.8. About 50 μL of these cells were added to equal volume of designed peptides at two-fold dilutions in 96-well sterile polypropylene plates followed by incubation for 3hrs at 37 °C. Aliquots of the above titrated wells were streaked onto Mueller-Hinton agar plates and the concentration at which no visible bacterial growth was observed considered as the MIC value of the peptide.
Hemolytic assay: Blood was collected from healthy mice in a tube containing EDTA. Cells with EDTA were centrifuged at 800 g for 10 min, to remove the buffy plasma coat layer, and washed three times in PBS (35 mM phosphate buffer, 150 mM NaCl, pH 7.0). 50 μL of RBC solution was added to equal volume of two-fold dilution of the peptides in 96-well micro titer plates and incubated for one hour. The final erythrocyte concentration estimated to be ~4% (v/v). After one hour, the mixture was centrifuged and the release of hemoglobin in the supernatant was determined spectrophotometrically at OD
540. Buffer and 1% Triton-X in the place of peptides used as negative control and positive control, respectively. The percentage of hemolysis was calculated using the following method:
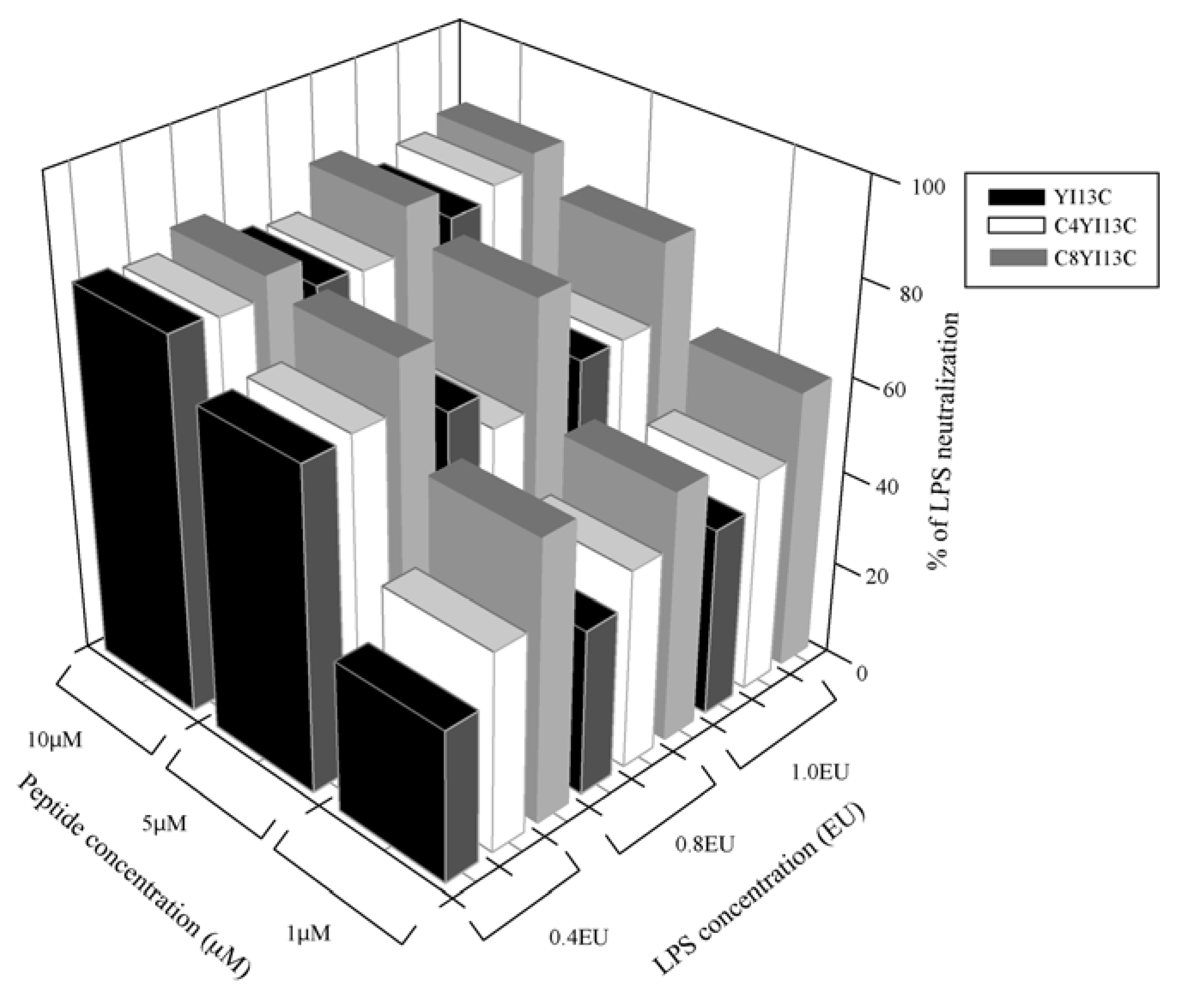
LPS neutralization assay: LAL chromogenic kit (QCL 100 Cambrex, Walkersville, MD, USA) was used to determine endotoxin neutralization of the designed peptides following protocol of the vendor. LPS in Gram negative bacteria activates a proenzyme in limulus amoebocyte lysate (LAL). This activated enzyme in turn catalytically release a colored product pNA from the colorless substrate Ac-Ile-Glu-Ala-Arg-pNA which is detected spectrophotometrically at OD
410. All peptides were dissolved in the pyrogen-free water supplied with the kit and pH was adjusted to 7.0 with 1N HCl or 1N NaOH (which is prepared in pyrogen-free water). The increasing concentrations of the peptides were incubated with three different concentrations of endotoxins,
i.e., at 0.4, 0.8 and 1.0 endotoxin units (EU) in a total volume of 50 μL for 30 minutes at 37 °C. About 50 μL of LAL reagent was added to peptide-LPS complex and further incubated for 10 minutes followed by addition of 100 μL substrate. After incubation of six minutes for the reaction, the release of colored product was recorded at OD
410. Water in the place of peptides served as negative control (blank) that is considered as 0% inhibition and percentage of LPS neutralization was calculated by:
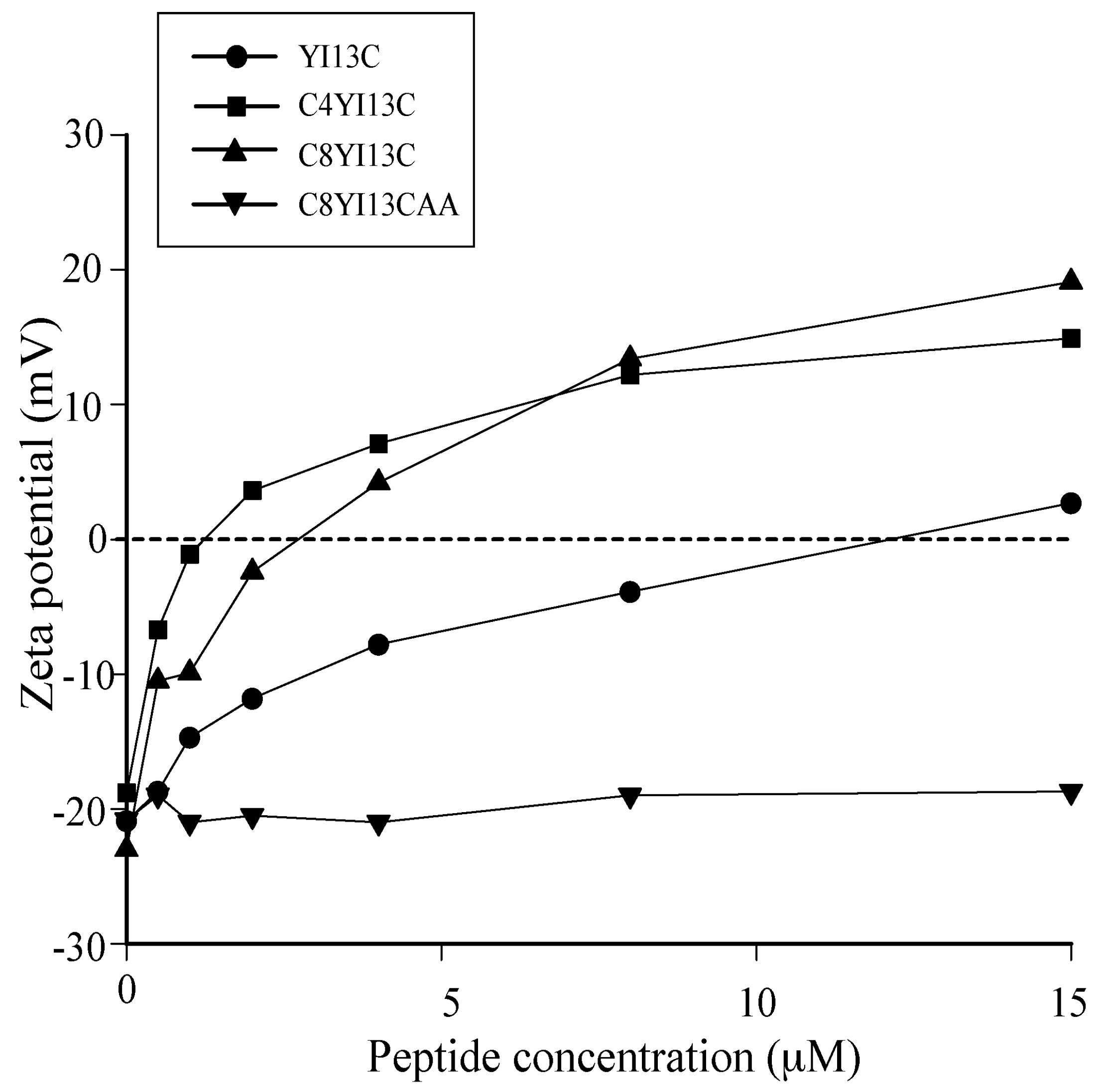
Zeta potential measurements: The zeta potential measurements were carried out on a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) equipped with a 633 nm He lasers. Mid-log phase grown bacterial cells were diluted to an OD600 of 0.2 and a basal measurement in the absence of peptides was acquired in disposable zeta cells with gold electrodes. Increasing concentrations of the designed peptides were then added to the cells and the measurements were made. A total of five measurements of 100 runs each were carried out for all dilutions.
Intrinsic tryptophan fluorescence and acrylamide quenching: The intrinsic Trp fluorescence spectra of the designed peptides were obtained by exciting samples at 280 nm and emission was collected from 300–400 nm in 10 mM sodium phosphate buffer. Fluorescence experiments were carried out with a Cary Eclipse (PaloAlto, CA, USA) fluorescence spectrophotometer using a 0.1 cm path length quartz cuvette. Experiments were initiated by obtaining emission spectra peptide (5 μM) alone followed by additions of increasing concentrations of LPS. Quenching experiments were conducted to examine the solvent accessibility of tryptophan residue in aqueous and hydrophobic environments. Acrylamide, a neutral quencher was added at increasing concentrations to free peptide and peptide/LPS complexes. The extent of decrease in fluorescence intensity was then used to calculate Stern-Volmer constant with the formula F0/F = 1 + Ksv[Q]. F0 and F are the fluorescence intensities before and addition of acrylamide, Ksv is the Stern-Volmer constant and [Q] is the quencher concentration.
Outer membrane permeability assay: Outer membrane of Gram bacteria acts as permeability barrier for hydrophobic antibacterial compounds. Hence the ability of the designed peptides to permeabilize through the outer membrane was examined using 1-N-phenylnaphthylamine (NPN) dye. E. coli cells were grown to mid logarithmic phase and diluted to an OD600nm of 0.5 in 10 mM sodium phosphate buffer, pH 7.0. The excitation was set at 350 nm and emission at 390–450 nm. NPN was added to the diluted cells at the concentration of 10 μM and a basal fluorescence was recorded followed by the addition of increasing concentration of peptides.
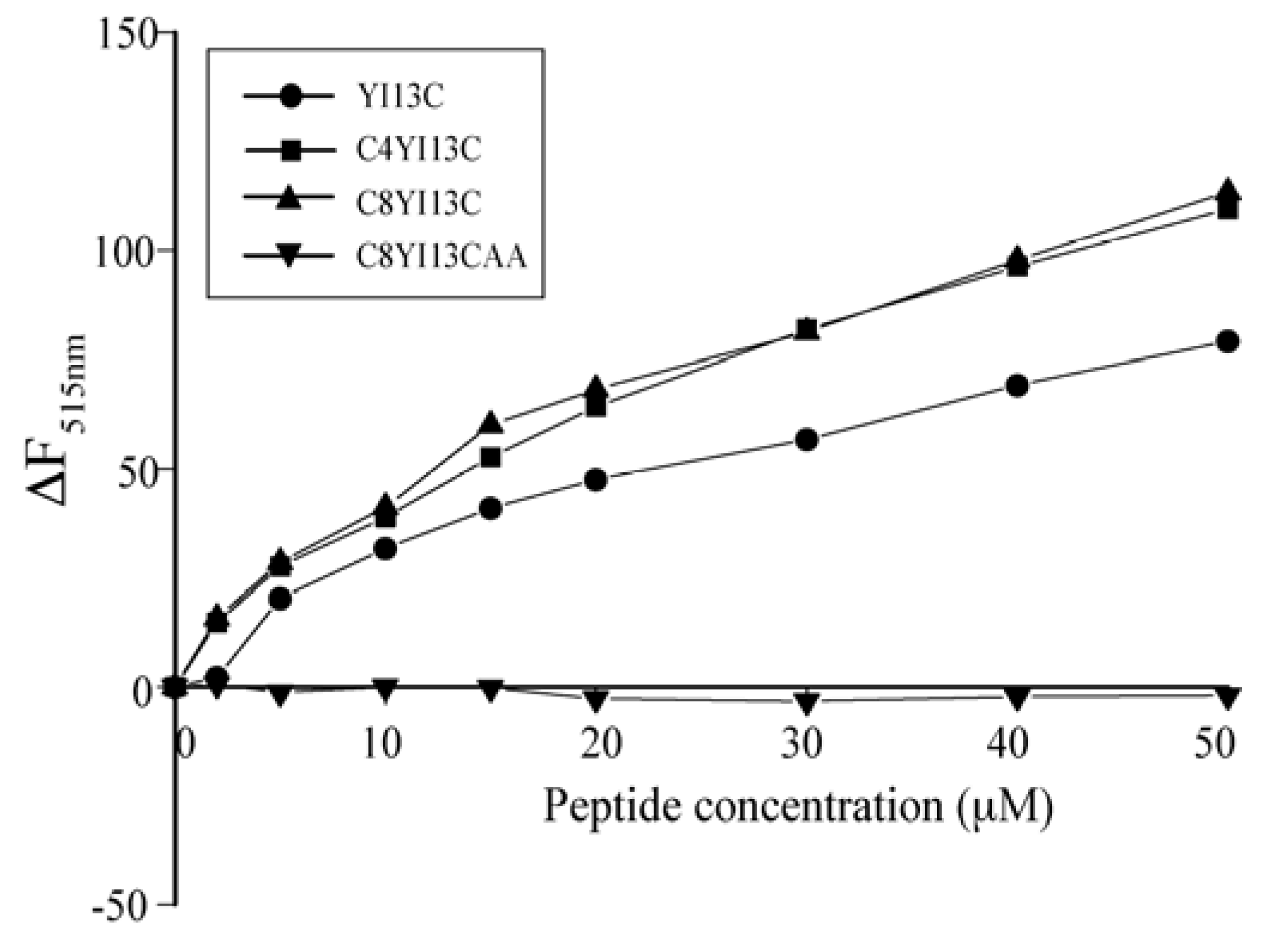
Dissociation of FITC-LPS: LPS molecules form micellar aggregates in water. The competency of the peptides to dissociate LPS micelles were studied using FITC conjugated LPS. In this method, blank fluorescence of 500 nM of FITC-LPS was recorded followed by the addition of increasing concentrations of peptides. The excitation was set at 480 nm and emission at 500–550 nm.
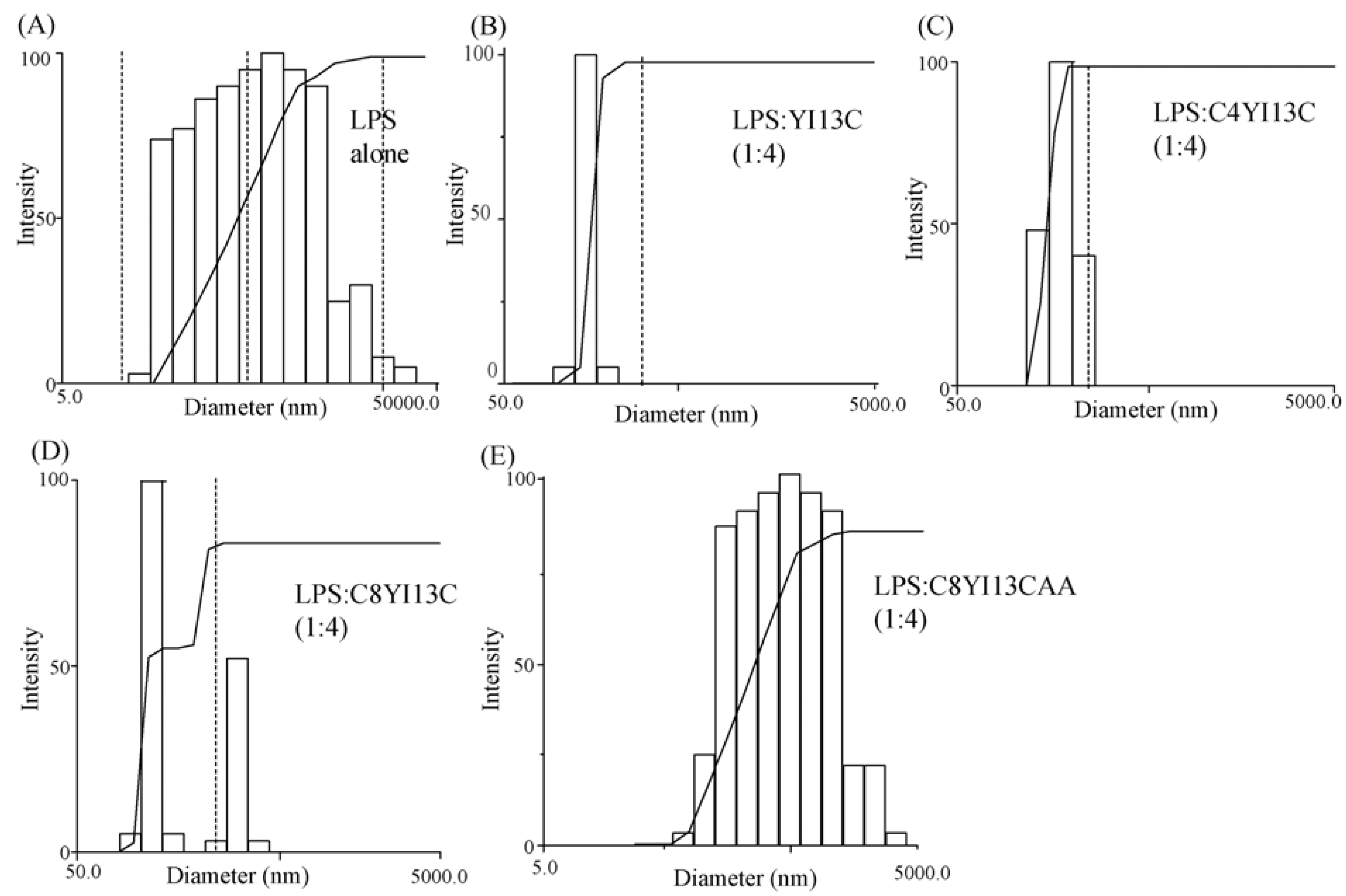
Dynamic light scattering measurements: LPS forms micelles of large sizes, which with the addition of increasing concentration of peptides, dissociates to smaller sizes. DLS measurements were made for 0.5 μM LPS and then with LPS: Peptide ratios of 1:2 and 1:4. The scattering was measured with Dynamic Light Scattering software provided with the instrument (Brookhaven Instruments Corp., Holtsville, NY, USA) and the scattering data was analyzed with CONTIN method.
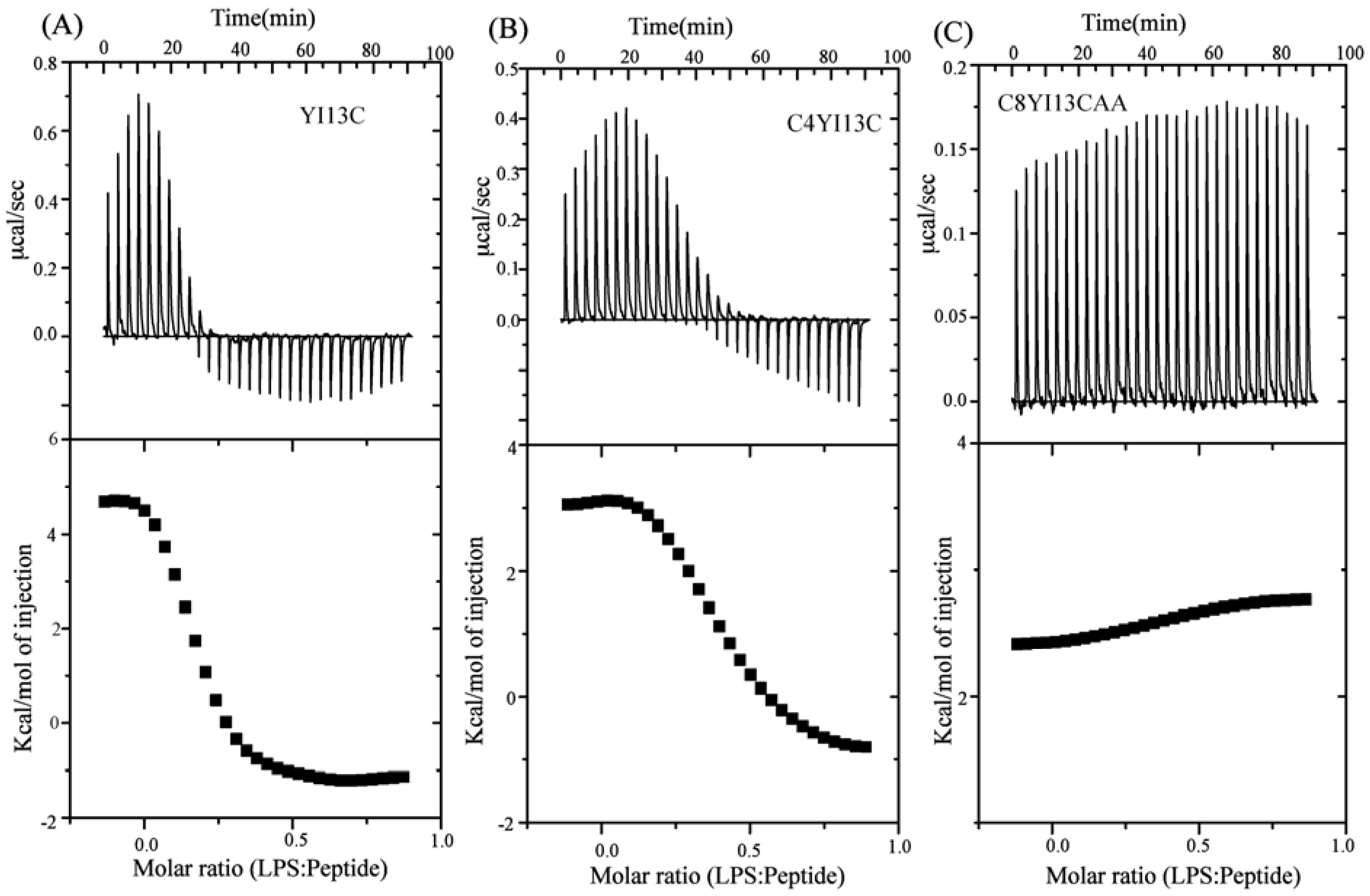
Isothermal titration calorimetry: Binding of designed peptides with LPS micelles was determined using isothermal titration calorimetry in VP-ITC micro calorimeter (Microcal Inc, Northampton, MA, USA). Peptides and LPS samples were dissolved in 10 mM phosphate buffer, pH 7.0 and filtered. LPS at a concentration of 10 μM was loaded into the sample cell and the reference cell was filled with the same buffer. The syringe was filled with 1mM peptide solution. Typically 30 injections of 3.5 μL of the peptides were made into the sample cell at 25 °C. Sample cell was stirred continuously at 300 rpm. Raw data were obtained and analyzed using single site binding model in Microcal Origin 5.0 software. Association constant (Ka) and enthalpy change (ΔH) were directly determined from ITC profiles. Dissociation constant was calculated from Kd = 1/Ka. ΔG and TΔS were estimated from the fundamental equations of thermodynamics, ΔG = −RTlnKa and TΔS = (ΔH − ΔG), respectively.
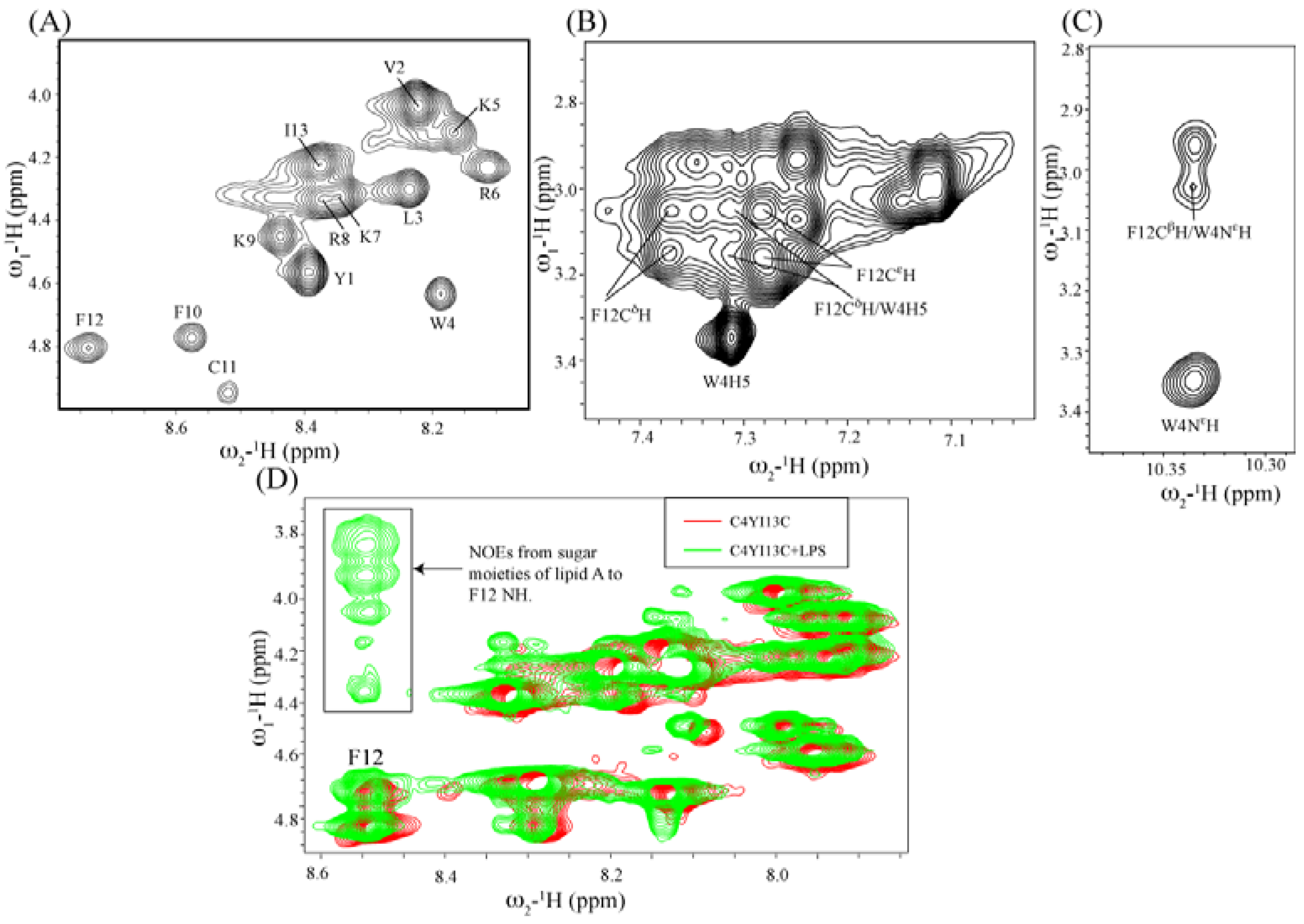
Structural characterization by NMR spectroscopy: NMR spectra were recorded on a Bruker DRX 600 (Fallanden, Switzerland) spectrometer containing a cryo-probe and pulse field gradients. Data acquisition and processing were performed with the Topspin software (Bruker) running on a Linux workstation. Two dimensional TOCSY (total correlation spectroscopy) and NOESY (nuclear Overhauser effect spectroscopy) spectra of peptides in free solution were acquired in aqueous solutions containing 10% D2O at pH 4.5 with 0.5 mM peptide concentration. The mixing times were fixed at 80 ms for TOCSY and 250 ms for NOESY. 2,2-Dimethyl-2-silapentane 5-sulfonate sodium salt (DSS) was used as an internal chemical shift reference. Because of severe spectral overlaps at 298 K, spectra were acquired at low temperature of 278 K. NOESY experiments were performed with 456 increments in t1 and 2 K data points in t2 using the WATERGATE procedure for water signal suppression. NMR data processing and analysis were carried out using Topspin (Bruker) and Sparky (T.D. Goddard and D.G. Kneller, University of California, San Francisco, CA, USA) programs respectively. For tr-NOESY experiments, 0.5 mM peptide solutions were titrated with different concentrations of LPS from 5 μM to 50 μM. The two dimensional tr-NOESY NMR experiments were performed with the same parameters as NOESY experiment except with the mixing time of 150 ms.
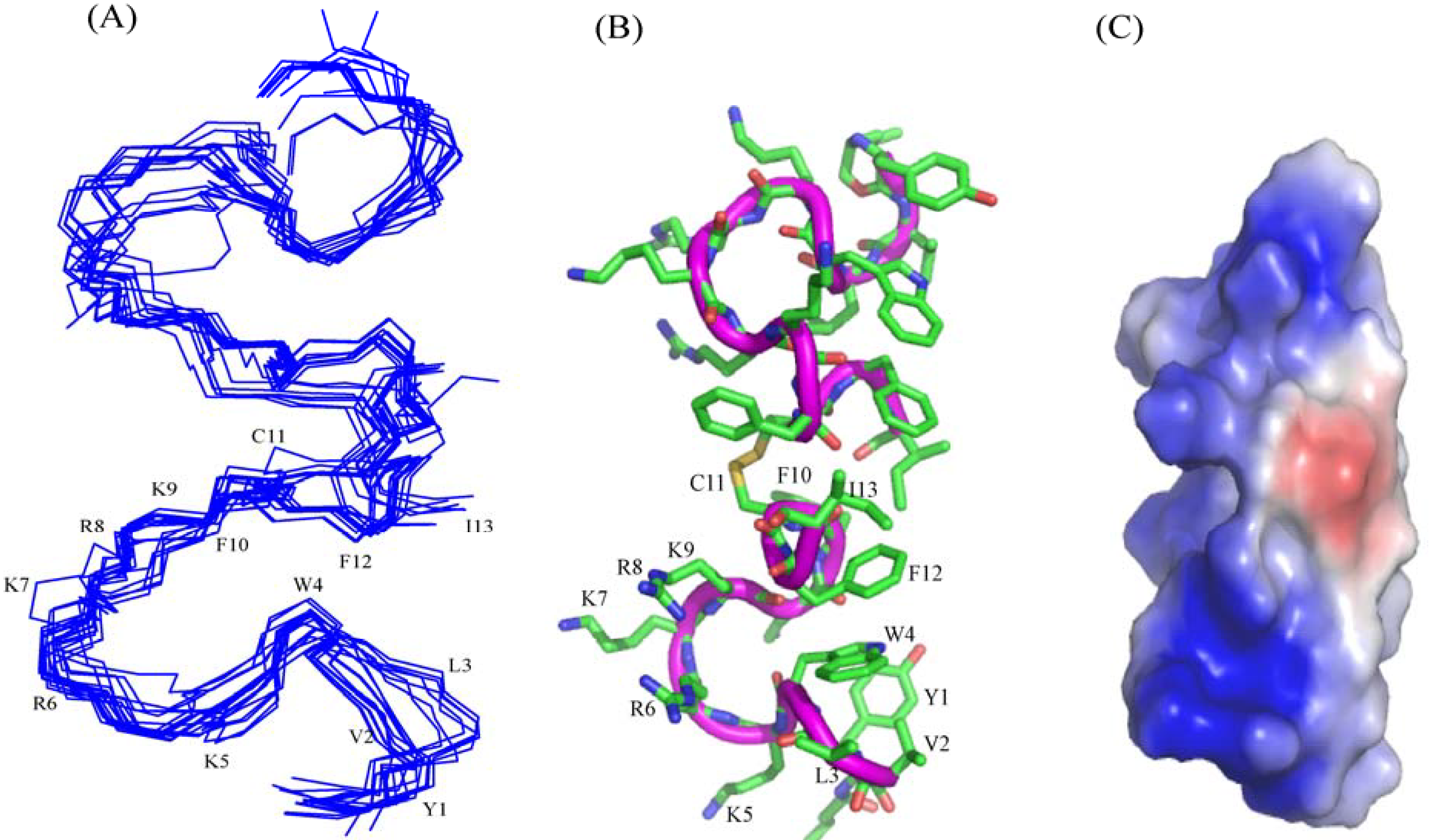
NMR-derived structure calculations: NMR structures were calculated using CYANA program [
47]. Sequential walk of different spin systems were achieved in SPARKY by analyzing two dimensional TOCSY and NOESY spectra of the peptide either in free or in LPS complex. On the basis of cross peak intensities, NOEs were categorized to strong, medium and weak. They were then translated to upper bound distance limits to 2.5, 3.5 and 5.0 Å respectively. PROCHECK was used to analyze Ramachandran plot for the validation of structures calculated [
48].
4. Conclusions
β-Boomerangs are
de novo designed antimicrobial and antiendotoxic peptides. These peptides demonstrate LPS binding as a mode of antibacterial action and endotoxin neutralization. LPS-binding stabilizes the β-boomerang conformation of these peptides. Unique amino acid sequences, novel structures and selective interactions with negatively charged lipids of β-boomerang peptides provide an attractive template for the development of potent antibacterial and antiendotoxic molecules. Several research groups have designed AMPs, based on physicochemical properties and structural propensities including amphipathic α-helical and β-hairpins [
5,
6,
52,
53,
54,
55]. The β-boomerang AMPs appear to be distinct from these designed AMPs, in terms of structures and amphipathicity [
41,
42]. In particular, the mode of action of β-boomerang peptides includes strong perturbation of outer membrane LPS of Gram-negative bacteria. The Gram-positive bacteria contain lipoteichoic acid as a part of the cell wall structure in peptidoglycan layer. At present, interactions of β-boomerang peptides with lipoteichoic acid are yet to be determined.
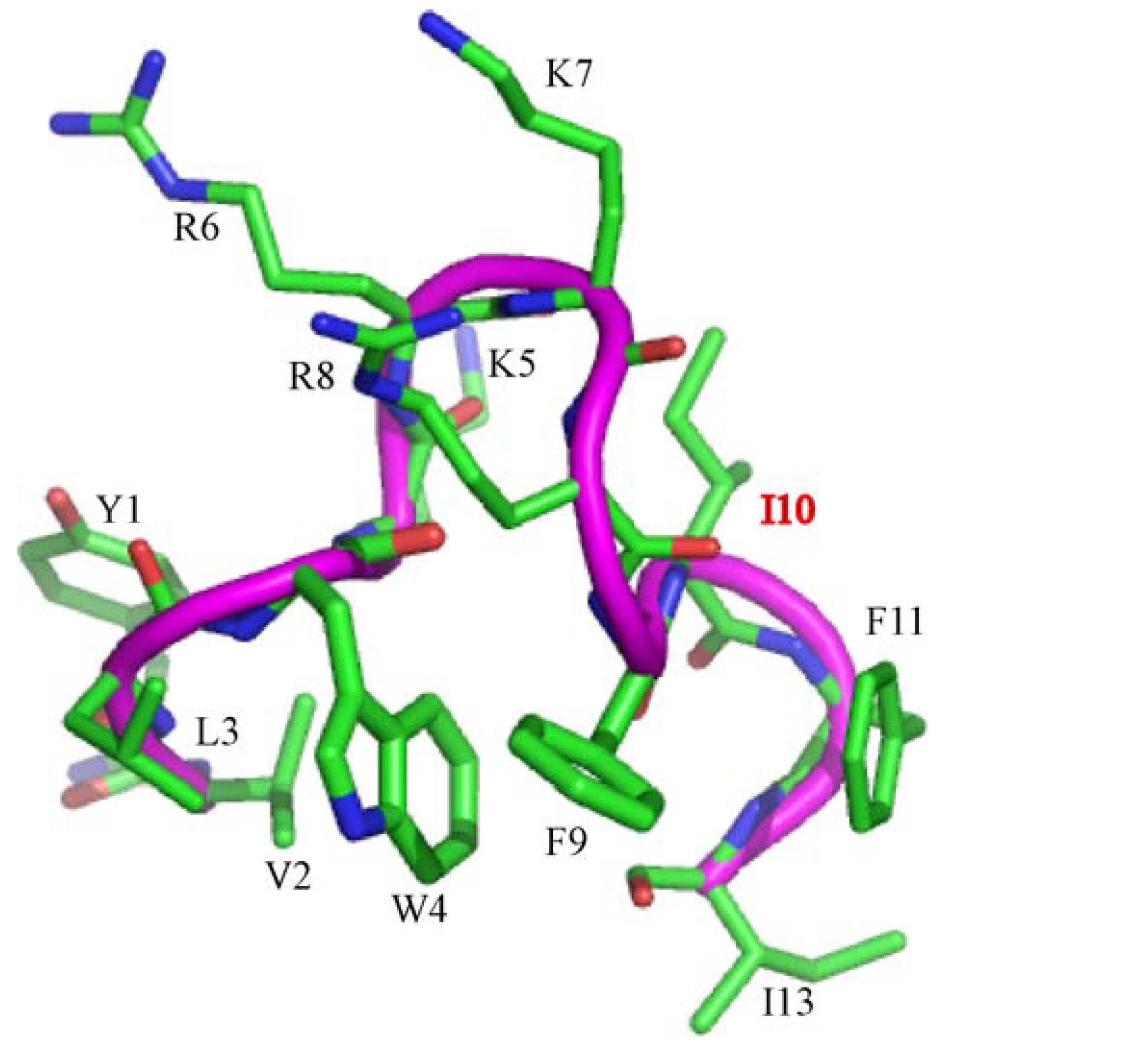
In this work, we have designed and characterized analogs of β-boomerang peptides, termed YI13C, C4YI3C, C8YI13C, for broad spectrum antibacterial activity and LPS neutralization. These peptides contain a short acyl chain, C4 and C8, at their N-termini and dimerize through a disulfide bond at their C-termini. Designed peptides YI13C, C4YI3C, C8YI13C demonstrated potent bactericidal and endotoxin neutralization compared to the parent peptide. The acylated analogs are found to more active in comparison to the non-acylated peptide YI13C. These peptides are low in hemolytic activity and interacted specifically with negatively charged LPS like lipids. The high-affinity interactions of these peptides with LPS are vital for permeabilization of outer membrane, endotoxin neutralization and structural perturbation of LPS micelles. The 3-D structure of the peptide C4YI13C in free solution revealed a β-boomerang-like fold with an amphipathic disposition of the cationic and hydrophobic sidechains.