Stability of Recombinant Tissue Plasminogen Activator at −30 °C over One Year
Abstract
:1. Introduction
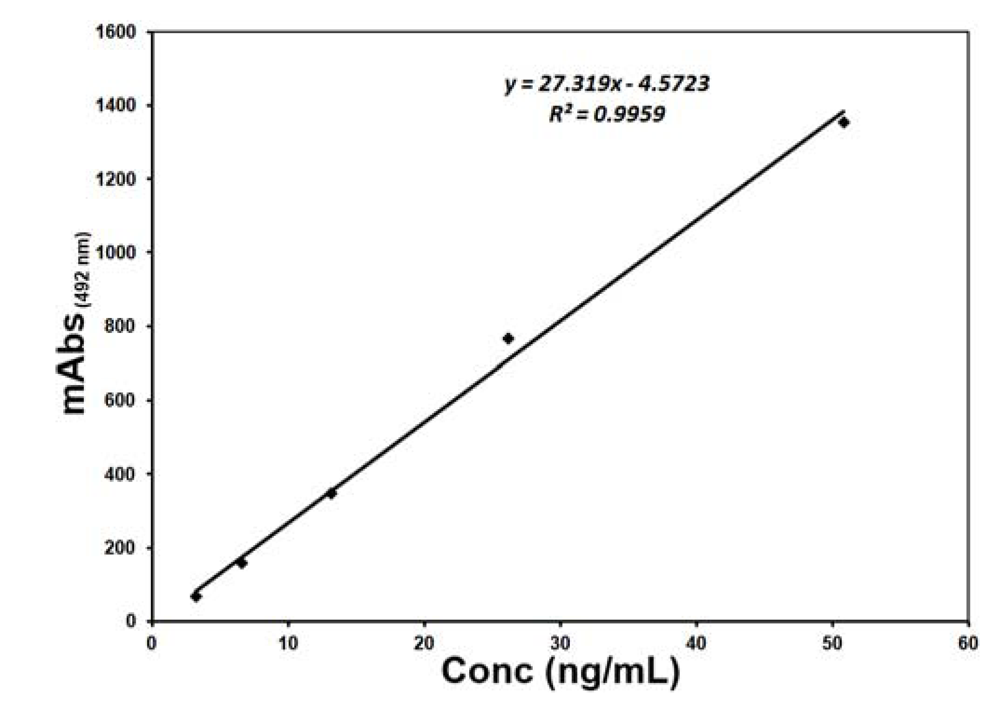
2. Results and Discussion
| Sample | rt-PA Concentration (ng/mL) | Mean ± SEM | % Activity | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Standard | 49.8 | 49.6 | 49.7 | 49.7 ± 0.1 | |
| 1 month | 49.5 | 49.6 | 49.9 | 49.7 ± 0.2 | 99.9 |
| 2 months | 48.1 | 47.2 | 49.0 | 48.1 ± 0.9 | 96.8 |
| 3 months | - | 46.1 | 45.3 | 45.7 ± 0.6 | 92.0 |
| 6 months | 42.6 | 45.3 | 43.4 | 43.8 ± 1.4 | 88.0* |
| 8 months | 41.8 | 43.0 | 42.8 | 42.5 ± 0.6 | 85.5* |
| 12 months | 38.8 | 39.1 | 37.6 | 38.5 ± 0.8 | 77.5* |
3. Experimental
3.1. Materials
3.2. Assessment of Stability
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Todd, J.L.; Tapson, V.F. Thrombolytic Therapy for Acute Pulmonary Embolism A Critical Appraisal. Chest J. 2009, 135, 1321–1329. [Google Scholar] [CrossRef]
- Meers, C.; Toffelmire, E.B. Urokinase efficacy in the restoration of hemodialysis catheter function. J. CANNT 1998, 8, 17–19. [Google Scholar]
- Weisel, J.W.; Litvinov, R.I. The Biochemical and Physical Process of Fibrinolysis and Effects of Clot Structure and Stability on the Lysis Rate. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 161–180. [Google Scholar] [CrossRef]
- Fan, X.; Yu, Z.; Liu, J.; Liu, N.; Hajjar, K.A.; Furie, K.L.; Lo, E.H.; Wang, X. Annexin A2. Stroke 2010, 41, S54–S58. [Google Scholar] [CrossRef]
- Haire, W.D.; Atkinson, J.B.; Stephens, L.C.; Kotulak, G.D. Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: a double-blinded, randomized trial. Thromb. Haemost. 1994, 72, 543–547. [Google Scholar]
- Collen, D.; Lijnen, H.R. The Tissue-Type Plasminogen Activator Story. Arterioscler. Thromb. Vascul. Biol. 2009, 29, 1151–1155. [Google Scholar] [CrossRef]
- Davis, S.; Vermeulen, L.; Banton, J.; Schwartz, B.; Williams, E. Activity and dosage of alteplase dilution for clearing occlusions of venous-access devices. Am. J. Health. Syst. Pharm. 2000, 57, 1039–1045. [Google Scholar]
- Hemmelgarn, B.R.; Moist, L.M.; Lok, C.E.; Tonelli, M.; Manns, B.J.; Holden, R.M.; LeBlanc, M.; Faris, P.; Barre, P.; Zhang, J.; Scott-Douglas, N. Prevention of Dialysis Catheter Malfunction with Recombinant Tissue Plasminogen Activator. New Engl. J. Med. 2011, 364, 303–312. [Google Scholar] [CrossRef]
- Baskin, J.L.; Pui, C.-H.; Reiss, U.; Wilimas, J.A.; Metzger, M.L.; Ribeiro, R.C.; Howard, S.C. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar]
- Daeihagh, P.; Jordan, J.; Chen, G.J.; Rocco, M. Efficacy of Tissue Plasminogen Activator Administration on Patency of Hemodialysis Access Catheters. Am. J. Kidney Dis. 2000, 36, 75–79. [Google Scholar] [CrossRef]
- Wiernikowski, J.T.; Crowther, M.; Clase, C.M.; Ingram, A.; Andrew, M.; Chan, A.K.C. Stability and sterility of recombinant tissue plasminogen activator at -30°C. Lancet 2000, 355, 2221–2222. [Google Scholar]
- Isaac, B. Efficacy of cryopreserved recombinant alteplase for declotting thrombosed central catheters. Ann. Pharmacother. 2000, 34, 533–534. [Google Scholar]
- Kerner, J.A.; Garcia-Careaga, M.G.; Fisher, A.A.; Poole, R.L. Treatment of Catheter Occlusion in Pediatric Patients. J. Parenter. Enteral. Nutr. 2006, 30, S73–S81. [Google Scholar] [CrossRef]
- Atkinson, J.B.; Bagnall, H.A.; Gomperts, E. Investigational use of tissue plasminogen activator (t-PA) for occluded central venous catheters. J. Parenter. Enteral. Nutr. 1990, 14, 310–311. [Google Scholar]
- Iqbal, Y.; Al-Katheri, A.; Al-Sedairy, R.; Al-Omari, A.; Abullah, M.F.; Crankson, S. Cryopreserved recombinant tissue plasminogen activator for the restoration of occluded central venous access devices in pediatric oncology patients. Ann. Saudi. Med. 2002, 22, 300–302. [Google Scholar]
- Genentech Inc. Product Monograph Activase® rt-PA (alteplase); Genentech, Inc.: South San Francisco, CA, USA, 10th April 2001; p. 18.
- Haire, W.D.; Herbst, S.F. Consensus conference on the use of Alteplase (t-PA) for the management of thrombotic catheter dysfunction. J. Vasc. Access. 2000, 1–8. [Google Scholar]
- Calis, K.A.; Cullinane, A.M.; Horne, M.K. 3rd Bioactivity of cryopreserved alteplase solutions. Am. J. Health. Syst. Pharm. 1999, 56, 2056–2057. [Google Scholar]
- Shaw, G.; Sperling, M.; Meunier, J. Long-term stability of recombinant tissue plasminogen activator at -80 C. BMC Res. Notes 2009, 2, 1–3. [Google Scholar] [CrossRef]
- Mataga, N.; Mizuguchi, Y.; Hensch, T.K. Experience-Dependent Pruning of Dendritic Spines in Visual Cortex by Tissue Plasminogen Activator. Neuron 2004, 44, 1031–1041. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alkatheri, A. Stability of Recombinant Tissue Plasminogen Activator at −30 °C over One Year. Pharmaceuticals 2013, 6, 25-31. https://doi.org/10.3390/ph6010025
Alkatheri A. Stability of Recombinant Tissue Plasminogen Activator at −30 °C over One Year. Pharmaceuticals. 2013; 6(1):25-31. https://doi.org/10.3390/ph6010025
Chicago/Turabian StyleAlkatheri, Abdulmalik. 2013. "Stability of Recombinant Tissue Plasminogen Activator at −30 °C over One Year" Pharmaceuticals 6, no. 1: 25-31. https://doi.org/10.3390/ph6010025




