Synthesis and Spectroscopic Analysis of Novel 1H-Benzo[d]imidazoles Phenyl Sulfonylpiperazines
Abstract
:1. Introduction
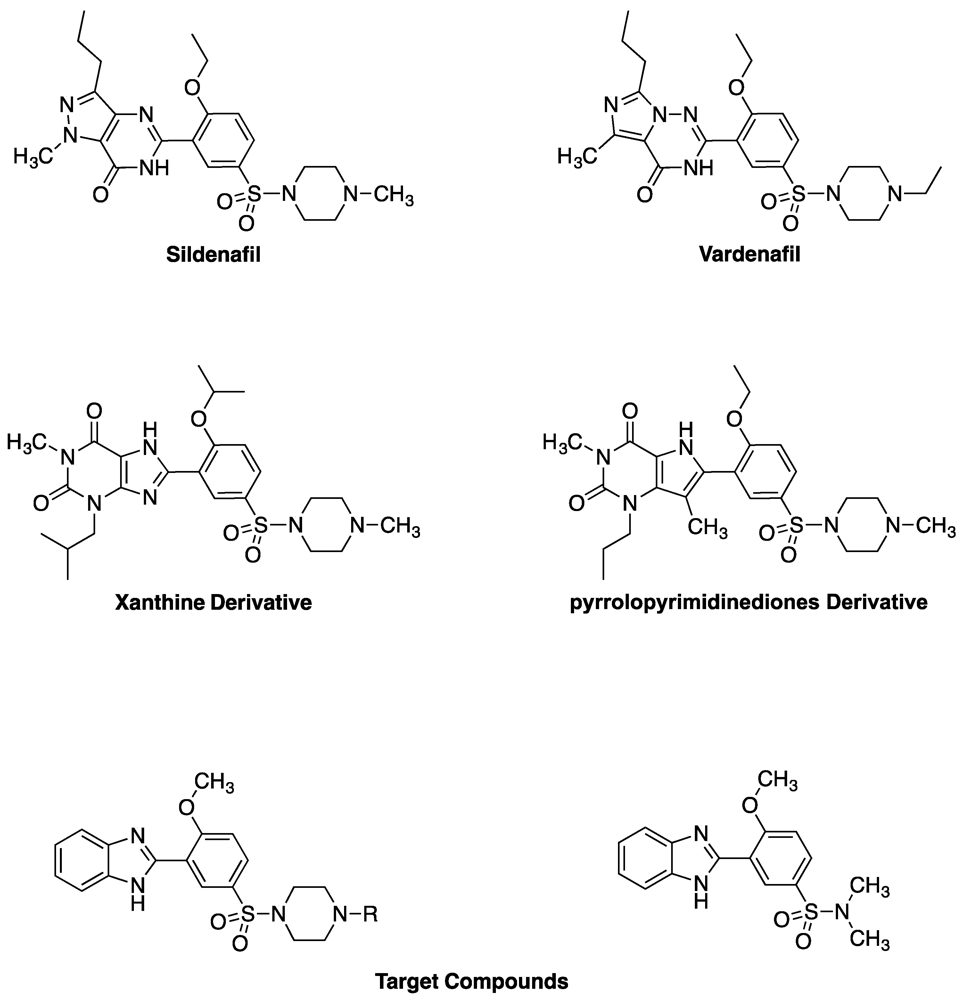
2. Results and Discussion
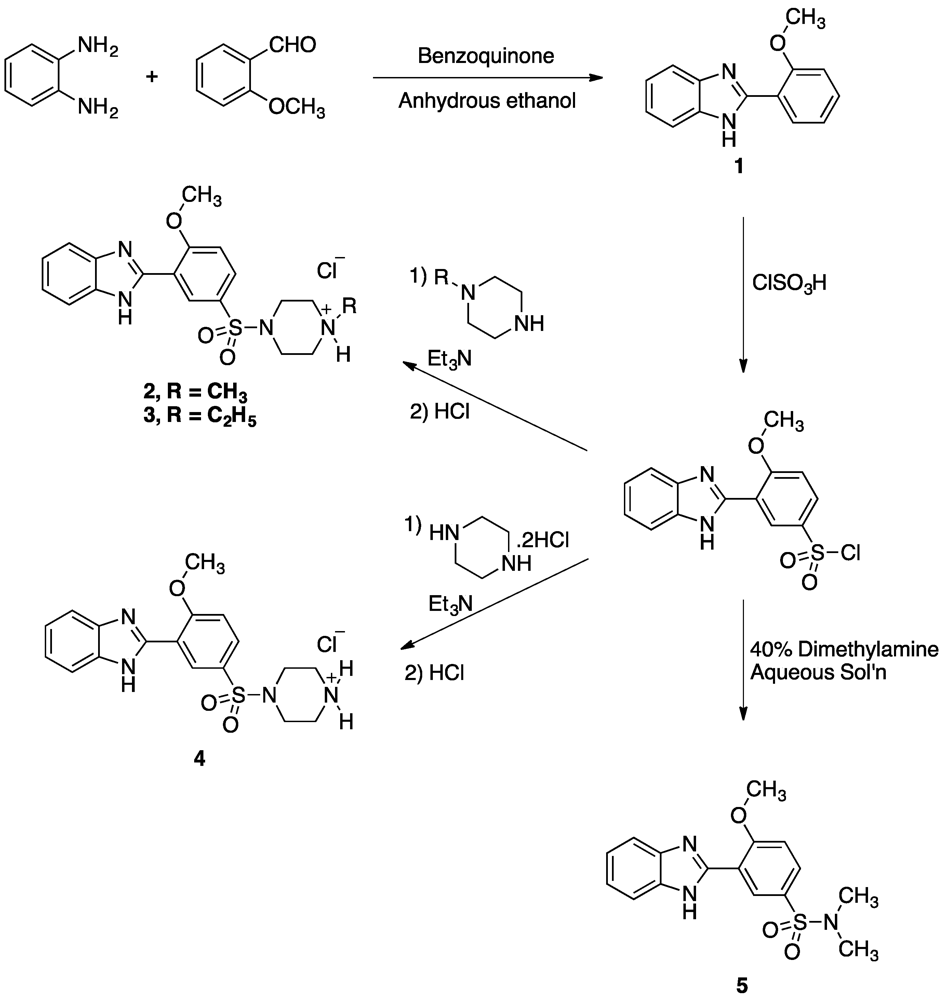

3. Experimental
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of 2-(2-methoxyphenyl)-1H-benzo[d]imidazole (1)
3.2.2. General Procedure for the Synthesis of compounds 2 and 3
3.2.3. 2-(2-methoxy-5-((4-piperazin-1-yl)sulfonyl)phenyl)-1H-benzo[d]imidazole hydrochloride (4)
3.2.4. 3-(1H-Benzo[d]imidazol-2-yl)-4-methoxy-N,N-dimethylbenzenesulfonamide (5)
4. Conclusions
Acknowledgments
References
- Flores Toque, H.A.; Priviero, F.B.M.; Teixeira, C.E.; Perissutti, E.; Fiorino, F.; Severino, B.; Frecentese, F.; Lorenzetti, R.; Baracat, J.S.; Santagada, V.; et al. Synthesis and Pharmacological Evaluations of Sildenafil Analogues for Treatment of Erectile Dysfunction. J. Med. Chem. 2008, 51, 2807–2815. [Google Scholar]
- Boolell, M.; Allen, M.J.; Ballard, S.A.; Gepi-Attee, S.; Muirhead, G.J.; Naylor, A.M.; Osterloh, I.H.; Gingell, C. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996, 8, 47–52. [Google Scholar]
- Badwan, A.A. Novel pyrazolopyrimidones and their use as PDE inhibitors. Eur. Pat. EP1460077, 22 September 2004. [Google Scholar]
- Badwan, A.A. Novel Compounds. US 2005/0080088 A1, 14 April 2005. [Google Scholar]
- Kim, D.-K.; Lee, N.; Lee, J.Y.; Ryu, D.H.; Kim, J.-S.; Lee, S.-H.; Choi, J.-Y.; Chang, K.; Kim, Y.-W.; Im, G.-J.; et al. Synthesis and phosphodiesterase 5 inhibitory activity of novel phenyl ring modified sildenafil analogues. Bioorg. Med. Chem. 2001, 9, 1609–1616. [Google Scholar]
- Khatib, S.Y.; Al-Kinabi, M.; Alawnah, N. Phosphodiesterase-5 inhibitor “Ordonafil” prevents myocardial hypertrophy. J. Mol. Cell. Cardiol. 2008, 44, 816. [Google Scholar]
- Borrmann, T.; Hinz, S.; Bertarelli, D.C.G.; Li, W.; Florin, N.C.; Scheiff, A.B.; Müller, C.E. 1-Alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: Development and Characterization of Adenosine A2B Receptor Antagonists and a New Radioligand with Subnanomolar Affinity and Subtype Specificity. J. Med. Chem. 2009, 52, 3994–4006. [Google Scholar]
- Lima, L.d.M.; Castro, P.; Machado, A.L.; Fraga, C.A.M.; Lugnier, C.; de Moraes, V.L.G.; Barreiro, E.J. Synthesis and anti-inflammatory activity of phthalimide derivatives, designed as new thalidomide analogues. Bioorg. Med. Chem. 2002, 10, 3067–3073. [Google Scholar]
- Bingham, A.H.; Davenport, R.J.; Fosbeary, R.; Gowers, L.; Knight, R.L.; Lowe, C.; Owen, D.A.; Parry, D.M.; Pitt, W.R. Synthesis and structure–activity relationship of aminopyrimidine IKK2 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3622–3627. [Google Scholar]
- Dale, D.J.; Dunn, P.J.; Golightly, C.; Hughes, M.L.; Levett, P.C.; Pearce, A.K.; Searle, P.M.; Ward, G.; Wood, A.S. ChemInform Abstract: The Chemical Development of the Commercial Route to Sildenafil: A Case History. Org. Process Res. Dev. 2000, 4, 17–22. [Google Scholar]
- Haning, H.; Niewöhner, U.; Schenke, T.; Es-Sayed, M.; Schmidt, G.; Lampe, T.; Bischoff, E. Imidazo[5,1-f]triazin-4(3H)-ones, a new class of potent PDE 5 inhibitors. Bioorg. Med. Chem. Lett. 2002, 12, 865–868. [Google Scholar]
- Arnold, R.; Beer, D.; Bhalay, G.; Baettig, U.; Collingwood, S.P.; Craig, S.; Devereux, N.; Dunstan, A.; Glen, A.; Gomez, S.; et al. 8-Aryl xanthines potent inhibitors of phosphodiesterase 5. Bioorg. Med. Chem. Lett. 2002, 12, 2587–2590. [Google Scholar]
- Giovannoni, M.P.; Vergelli, C.; Graziano, A.; Dal Piaz, V. PDE5 inhibitors and their applications. Curr. Med. Chem. 2010, 17, 2564–2587. [Google Scholar]
- Rotella, D.P.; Sun, Z.; Zhu, Y.; Krupinski, J.; Pongrac, R.; Seliger, L.; Normandin, D.; Macor, J.E. N-3-Substituted Imidazoquinazolinones: Potent and Selective PDE5 Inhibitors as Potential Agents for Treatment of Erectile Dysfunction. J. Med. Chem. 2000, 43, 1257–1263. [Google Scholar]
- Xia, G.; Li, J.; Peng, A.; Lai, S.; Zhang, S.; Shen, J.; Liu, Z.; Chen, X.; Ji, R. Synthesis and phosphodiesterase 5 inhibitory activity of novel pyrido[1,2-e]purin-4(3H)-one derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 2790–2794. [Google Scholar]
- Bahrami, K.; Khodaei, M.M.; Kavianinia, I. A Simple and Efficient One-Pot Synthesis of 2-Substituted Benzimidazoles. Synthesis 2007, 547–550. [Google Scholar]
- Bahrami, K.; Khodaei, M.M.; Naali, F. Mild and Highly Efficient Method for the Synthesis of 2-Arylbenzimidazoles and 2-Arylbenzothiazoles. J. Org. Chem. 2008, 73, 6835–6837. [Google Scholar]
- Du, L.-H.; Wang, Y.-G. A Rapid and Efficient Synthesis of Benzimidazoles Using Hypervalent Iodine as Oxidant. Synthesis 2007, 675–678. [Google Scholar]
- Beaulieu, P.L.; Haché, B.; von Moos, E. A Practical Oxone®-Mediated, High-Throughput, Solution-Phase Synthesis of Benzimidazoles from 1,2-Phenylenediamines and Aldehydes and its Application to Preparative Scale Synthesis. Synthesis 2003, 1683–1692. [Google Scholar]
- Maiti, D.K.; Halder, S.; Pandit, P.; Chatterjee, N.; De Joarder, D.; Pramanik, N.; Saima, Y.; Patra, A.; Maiti, P.K. Synthesis of Glycal-Based Chiral Benzimidazoles by VO(acac)2−CeCl3 Combo Catalyst and Their Self-Aggregated Nanostructured Materials. J. Org. Chem. 2009, 74, 8086–8097. [Google Scholar]
- Yang, D.; Fokas, D.; Li, J.; Yu, L.; Baldino, C.M. A Versatile Method for the Synthesis of Benzimidazoles from o-Nitroanilines and Aldehydes in One Step via a Reductive Cyclization. Synthesis 2005, 47, 56. [Google Scholar]
- Kim, Y.; Kumar, M.R.; Park, N.; Heo, Y.; Lee, S. Copper-Catalyzed, One-Pot, Three-Component Synthesis of Benzimidazoles by Condensation and C–N Bond Formation. J. Org. Chem. 2011, 76, 9577–9583. [Google Scholar]
- Hopkins, K.T.; Wilson, W.D.; Bender, B.C.; McCurdy, D.R.; Hall, J.E.; Tidwell, R.R.; Kumar, A.; Bajic, M.; Boykin, D.W. Extended Aromatic Furan Amidino Derivatives as Anti-Pneumocystis carinii Agents. J. Med. Chem. 1998, 41, 3872–3878. [Google Scholar]
- Qandil, A.M.; Hassan, M.A.; Al-Shar'i, N.A. Synthesis and anticandidal activity of azole-containing sulfonamides. Arch. Pharmazie. 2008, 341, 99–112. [Google Scholar]
- Qandil, A.M.; Hassan, M.A.; Al-Jeaidi, B.A. Design and Synthesis of a Series of 3-Aminobenzenesulfonamide Derivatives and Their Screening for Antimicrobial and Cytotoxic Activity. J. J. Pharm. Sci. 2008, 1, 41–53. [Google Scholar]
- Shetty, H.U.; Hawes, E.M.; Midha, K.K. Piperazine Ring Cleavage in the Electron Impact Induced Fragmentation of Piperazine Type Phenothiazine Antipsychotic Agents. Biomed. Mass Spectrom. 1983, 10, 601–607. [Google Scholar]
- John, C.J.C.R. Direct intramolecular gas-phase transfer reactions during fragmentation of sildenafil and thiosildenafil analogs in electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 927–936. [Google Scholar]
- Lin, M.-C.; Liu, Y.-C.; Lin, Y.-L.; Lin, J.-H. Solation and Identification of a Novel Sildenafil Analogue Adulterated in Dietary Supplements. J. Food Drug Anal. 2008, 16, 15–20. [Google Scholar]
- Weinmann, W.; Lehmann, N.; Muller, C.; Wiedemann, A.; Svoboda, M. Identification of lorazepam and sildenafil as examples for the application of LC/ionspray-MS and MS-MS with mass spectra library searching in forensic toxicology. Forensic Sci. Int. 2000, 113, 339–344. [Google Scholar]
Correction
A correction was published on 24 September 2012: https://www.mdpi.com/1424-8247/5/9/1044 (PDF, 110 KB)
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qandil, A.M. Synthesis and Spectroscopic Analysis of Novel 1H-Benzo[d]imidazoles Phenyl Sulfonylpiperazines. Pharmaceuticals 2012, 5, 460-468. https://doi.org/10.3390/ph5050460
Qandil AM. Synthesis and Spectroscopic Analysis of Novel 1H-Benzo[d]imidazoles Phenyl Sulfonylpiperazines. Pharmaceuticals. 2012; 5(5):460-468. https://doi.org/10.3390/ph5050460
Chicago/Turabian StyleQandil, Amjad M. 2012. "Synthesis and Spectroscopic Analysis of Novel 1H-Benzo[d]imidazoles Phenyl Sulfonylpiperazines" Pharmaceuticals 5, no. 5: 460-468. https://doi.org/10.3390/ph5050460



