Antimicrobial Effect of Honey Phenolic Compounds against E. coli—An In Vitro Study
Abstract
:1. Introduction
2. Results
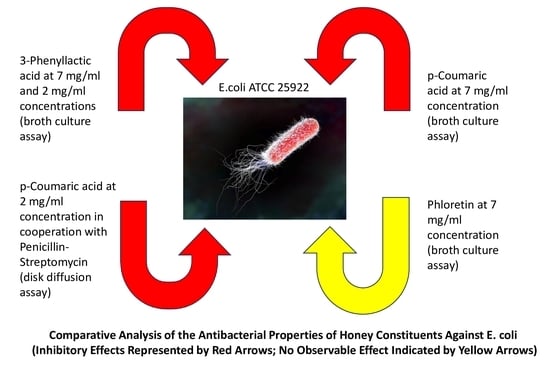
2.1. Comparison of the Antimicrobial Activity of Honey Phytochemicals In Vitro Using a Broth Culture Assay
2.2. Screening for the Antimicrobial Activity of Honey Constituents—Disk Diffusion Assay
2.3. Assessment of the Antimicrobial Activity of Honey Constituents—Well Diffusion Assay
2.4. Determination of the Effects of Sub-Inhibitory Concentrations of p-Coumaric Acid on the Susceptibility of E. coli to Penicillin–Streptomycin
3. Discussion
4. Materials and Methods
4.1. Bacteria
4.2. Honey Constituents
4.3. Comparison of the Antimicrobial Activity of Honey Constituents In Vitro Using Broth Culture Assay
4.4. Measurement of the Inhibitory Properties of Honey Constituents In Vitro Using Diffusion Assays
4.4.1. Disc Diffusion Assay
4.4.2. Well Diffusion Assay
4.5. Investigation of the Effects of Sub-Inhibitory Concentrations of p-Coumaric Acid on the Susceptibility of E. coli ATCC 25922 to Penicillin-Streptomycin
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandal, S.; Deb Mandal, M.; Pal, N.K. Synergistic anti-Staphylococcus aureus activity of amoxicillin in combination with Emblica officinalis and Nymphae odorata extracts. Asian Pac. J. Trop. Med. 2010, 3, 711–714. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Khan, F.R.; Ul Abadin, Z.; Rauf, N. Honey: Nutritional and medicinal value. Int. J. Clin. Pract. 2007, 61, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Deb Mandal, M.; Pal, N.K.; Saha, K. Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella enterica serovar Typhi. Asian Pac. J. Trop. Med. 2010, 3, 961–964. [Google Scholar] [CrossRef]
- Cooper, R.A.; Halas, E.; Molan, P.C. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J. Burn. Care Rehabil. 2002, 23, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. Sci. World J. 2011, 11, 766–787. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Therapeutic and prophylactic effects of crude honey on chronic seborrheic dermatitis and dandruff. Eur. J. Med. Res. 2001, 6, 306–308. [Google Scholar]
- Al-Waili, N.S. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: Partially controlled, single-blinded study. Complement. Ther. Med. 2003, 11, 226–234. [Google Scholar] [CrossRef]
- Al-Waili, N.S. An alternative treatment for pityriasis versicolor, tinea cruris, tinea corporis and tinea faciei with topical application of honey, olive oil and beeswax mixture: An open pilot study. Complement. Ther. Med. 2004, 12, 45–47. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Jenkins, R.E. Honey: A sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef]
- Escuredo, O.; Seijo, M.C. Honey: Chemical Composition, Stability and Authenticity. Foods 2019, 8, 577. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Badawy, O.F.; Shafii, S.S.; Tharwat, E.E.; Kamal, A.M. Antibacterial activity of bee honey and its therapeutic usefulness against Escherichia coli O157:H7 and Salmonella typhimurium infection. Rev. Sci. Tech. 2004, 23, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Cavanagh, H.M. Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J. Med. Food. 2005, 8, 100–103. [Google Scholar] [CrossRef]
- Boukraa, L.; Niar, A. Sahara honey shows higher potency against Pseudomonas aeruginosa compared to north Algerian types of honey. J. Med. Food 2007, 10, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef]
- Ohhira, I.; Kuwaki, S.; Morita, H.; Suzuki, T.; Tomita, S.; Hisamatsu, S.; Sonoki, S.; Shinoda, S. Identification of 3-phenyllactic acid as a possible antibacterial substance produced by Enterococcus faecalis TH10. Biocontrol. Sci. 2004, 9, 77–81. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high-performance liquid chromatography. Phytochem. Anal. 2007, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.T.; Liang, Y.; Duan, L.F.; Zhang, Y.D.; Wang, L.J.; He, G.M.; Xiao, H.T. p-Coumaric Acid Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by MAPK Signaling. Oxidative Med. Cell Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2011, 25, 550–554. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, N.; Zhang, S.; Zhang, L.; Liu, Q. Phloretin inhibited the pathogenicity and virulence factors against Candida albicans. Bioengineered 2021, 12, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Birru, R.L.; Bein, K.; Bondarchuk, N.; Wells, H.; Lin, Q.; Di, Y.P.; Leikauf, G.D. Antimicrobial and Anti-Inflammatory Activity of Apple Polyphenol Phloretin on Respiratory Pathogens Associated With Chronic Obstructive Pulmonary Disease. Front. Cell Infect. Microbiol. 2021, 11, 652944. [Google Scholar] [CrossRef]
- Kim, J.; Durai, P.; Jeon, D.; Jung, I.D.; Lee, S.J.; Park, Y.M.; Kim, Y. Phloretin as a Potent Natural TLR2/1 Inhibitor Suppresses TLR2-Induced Inflammation. Nutrients 2018, 10, 868. [Google Scholar] [CrossRef]
- O’Meara, S.; Cullum, N.; Majid, M.; Sheldon, T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol. Assess. 2000, 4, 1–237. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Musella, V.; Lupia, C.; Palma, E.; Britti, D. Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine. Vet. Sci. 2023, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar] [CrossRef] [PubMed]
- Basualdo, C.; Sgroy, V.; Finola, M.S.; Marioli, J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet. Microbiol. 2007, 124, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Fangio, M.F.; Iurlina, M.O.; Fritz, R. Antimicrobial activity of honey the southeast of Buenos Aires Province against Escherichia coli. Rev. Argent. Microbiol. 2007, 39, 120–123. (In Spanish) [Google Scholar] [PubMed]
- Schneider, M.; Coyle, S.; Warnock, M.; Gow, I.; Fyfe, L. Anti-microbial activity and composition of manuka and portobello honey. Phytother. Res. 2013, 27, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The effect of New Zealand kanuka, manuka and clover honeys on bacterial growth dynamics and cellular morphology varies according to the species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Akujobi, C.O.; Njoku, H.O. Bioassay for Determination of Microbial Sensitivity to Nigerian Honey. Glob. J. Pharmacol. 2010, 4, 36–40. [Google Scholar]
- Dieuleveux, V.; Lemarinier, S.; Guéguen, M. Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int. J. Food Microbiol. 1998, 40, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.N.; Duster, M.; Musuuza, J.S.; Safdar, N. Effect of United States buckwheat honey on antibiotic-resistant hospital acquired pathogens. Pan Afr. Med. J. 2016, 25, 212. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, G.; Nigam, P.; Owusu-Apenten, R.K. Total Phenols, Antioxidant Capacity and Antibacterial Activity of Manuka Honey Chemical Constituents. J. Adv. Biol. Biotechnol. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Elamine, Y.; Imtara, H.; Miguel, M.G.; Anjos, O.; Estevinho, L.M.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Martín, J.; Lyoussi, B. Antibacterial Activity of Moroccan Zantaz Honey and the Influence of Its Physicochemical Parameters Using Chemometric Tools. Appl. Sci. 2021, 11, 4675. [Google Scholar] [CrossRef]
- Kot, B.; Wicha, J.; Piechota, M.; Wolska, K.; Gruzewska, A. Antibiofilm activity of trans-cinnamaldehyde, p-coumaric, and ferulic acids on uropathogenic Escherichia coli. Turk. J. Med. Sci. 2015, 45, 919–924. [Google Scholar] [CrossRef]
- Ellner, P.D.; Stoessel, C.J.; Drakeford, E.; Vasi, F. A new culture medium for medical bacteriology. Am. J. Clin. Pathol. 1966, 45, 502–504. [Google Scholar] [CrossRef]


| Phytochemical/Control | Mean Zone of Inhibition (mm) ± SD |
|---|---|
| 3-Phenyllactic Acid (100 µg per disk) | 0.0 ± 0.0 |
| p-Coumaric Acid (100 µg per disk) | 0.0 ± 0.0 |
| Phloretin (100 µg per disk) | 0.0 ± 0.0 |
| Pen/Strep (100U/100 µg per disc) | 22.0 ± 0.0 |
| Methylglyoxal (400 µg per disc) | 26.0 ± 0.0 |
| Negative Control (Methanol–Water, 20:80) | 0.0 ± 0.0 |
| Phytochemical/Control | Mean Zone of Inhibition (mm) ± SD |
|---|---|
| 3-Phenyllactic Acid (350 µg per disk) | 0.0 ± 0.0 |
| p-Coumaric Acid (350 µg per disk) | 0.0 ± 0.0 |
| Phloretin (350 µg per disk) | 0.0 ± 0.0 |
| Pen/Strep (100U/100 µg per disc) | 28.0 ± 0.0 |
| Methylglyoxal (400 µg per disc) | 22.0 ± 0.0 |
| Negative Control (Methanol–Water, 20:80) | 0.0 ± 0.0 |
| Phytochemical/Control | Mean Zone of Inhibition (mm) ± SD |
|---|---|
| 3-Phenyllactic Acid (700 µg per well) | 0.0 ± 0.0 |
| p-Coumaric Acid (700 µg per well) | 0.0 ± 0.0 |
| Phloretin (700 µg per well) | 0.0 ± 0.0 |
| Pen/Strep (200U/200 µg per well) | 33.5 ± 2.2 |
| Methylglyoxal (800 µg per well) | 31.8 ± 1.7 |
| Negative Control (Methanol–Water, 20:80) | 0.0 ± 0.0 |
| Author | Year | Main Findings | References |
|---|---|---|---|
| Schneider et al. | 2013 | This study compared locally produced Portobello honey (PBH) with Manuka honey (MH) in fighting three bacteria causing wound infections. Both honeys demonstrated significant inhibition at 75% and 50%, although PBH at 10% had slightly lower activity than MH (p ≤ 0.001). | [38] |
| Kirkpatrick et al. | 2017 | This study compared the antibacterial, phenol content, and antioxidant abilities of MSY, MGO, and PLA from Manuka honey. Antioxidant capacity was tested with ABTS or IRAC, while antibacterial effects were measured against E. coli, B. subtilis, or S. aureus using a disc diffusion assay. MGO and PLA showed antibacterial effects but lacked noticeable antioxidant or phenol traits. | [47] |
| Lou et al. | 2011 | This study evaluated PCA’s antibacterial activity and mechanism against bacteria, finding effective inhibition of bacterial growth at MIC values from 10 to 80 mg/mL. The results suggest that PCA acts by disrupting cell membranes and binding to bacterial DNA, leading to cell death. | [27] |
| Elamine et al. | 2021 | Zantaz honey’s antibacterial effects on E. coli, P. aeruginosa, and S. aureus were assessed using chemometric tools. Polyphenols, particularly epicatechin, 4-coumaric acid, methylsyringate, and PCA, showed a strong positive correlation with antibacterial activity. | [48] |
| Kot et al. | 2015 | This study evaluated phytochemicals as potential alternative antimicrobials for preventing and deactivating E. coli biofilms on urinary catheters. Phytochemicals could serve as significant sources of antibiofilm agents with preventive capabilities against E. coli biofilm formation on urinary catheters. | [49] |
| Ning et al. | 2017 | This study investigated PLA’s antibacterial mechanism against L. monocytogenes and E. coli. Flow cytometry using propidium iodide (PI) showed PLA’s ability to damage L. monocytogenes’ membrane but not E. coli’s. A fluorescence assays indicated PLA’s interaction with bacterial DNA through intercalation, suggesting that it targets both membrane and genomic DNA. | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassym, L.; Kussainova, A.; Semenova, Y.; McLoone, P. Antimicrobial Effect of Honey Phenolic Compounds against E. coli—An In Vitro Study. Pharmaceuticals 2024, 17, 560. https://doi.org/10.3390/ph17050560
Kassym L, Kussainova A, Semenova Y, McLoone P. Antimicrobial Effect of Honey Phenolic Compounds against E. coli—An In Vitro Study. Pharmaceuticals. 2024; 17(5):560. https://doi.org/10.3390/ph17050560
Chicago/Turabian StyleKassym, Laura, Assiya Kussainova, Yuliya Semenova, and Pauline McLoone. 2024. "Antimicrobial Effect of Honey Phenolic Compounds against E. coli—An In Vitro Study" Pharmaceuticals 17, no. 5: 560. https://doi.org/10.3390/ph17050560