Population Pharmacodynamic Models of Risperidone on PANSS Total Scores and Prolactin Levels in Schizophrenia
Abstract
:1. Introduction
2. Results
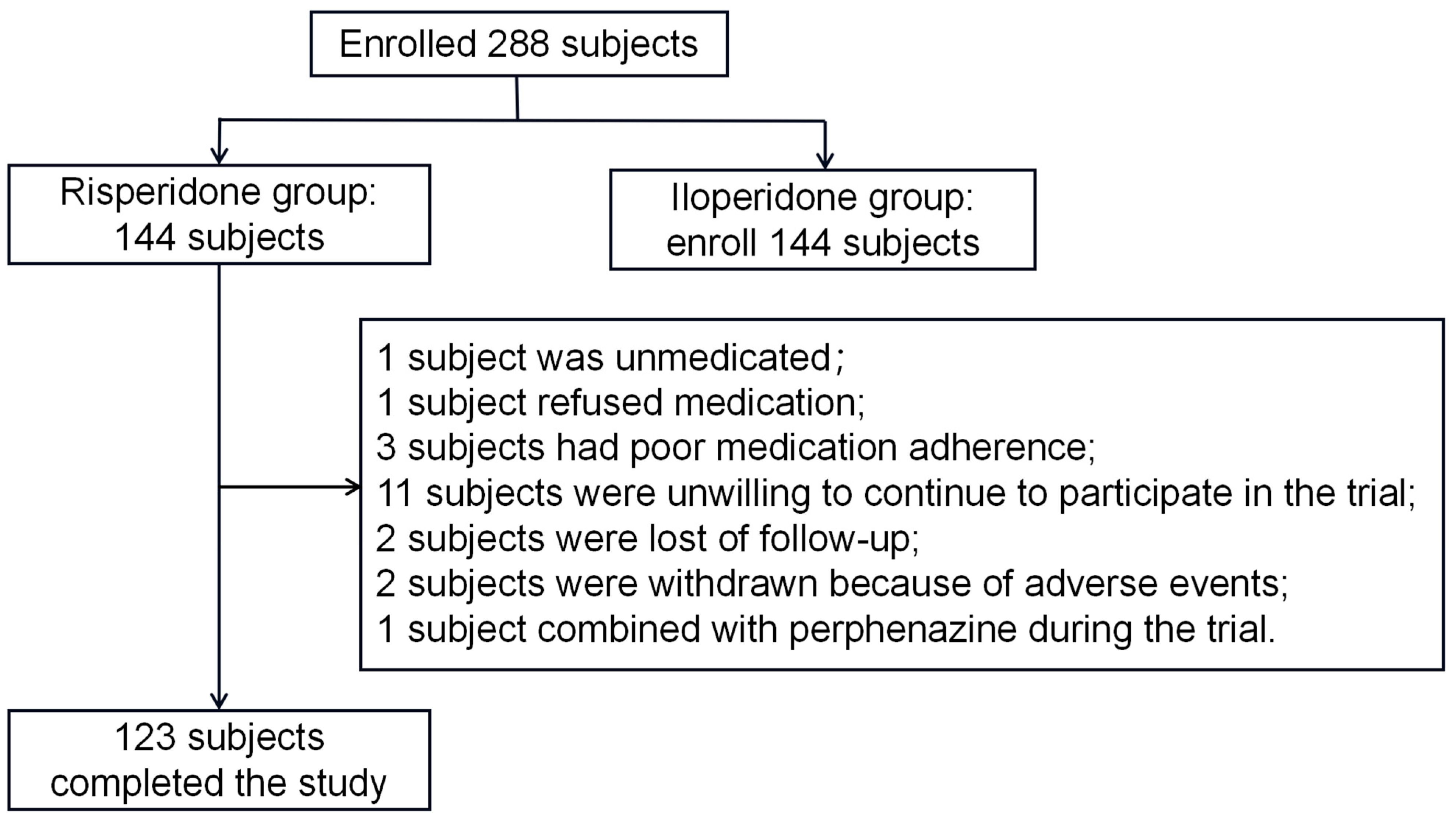
2.1. Demographic Data
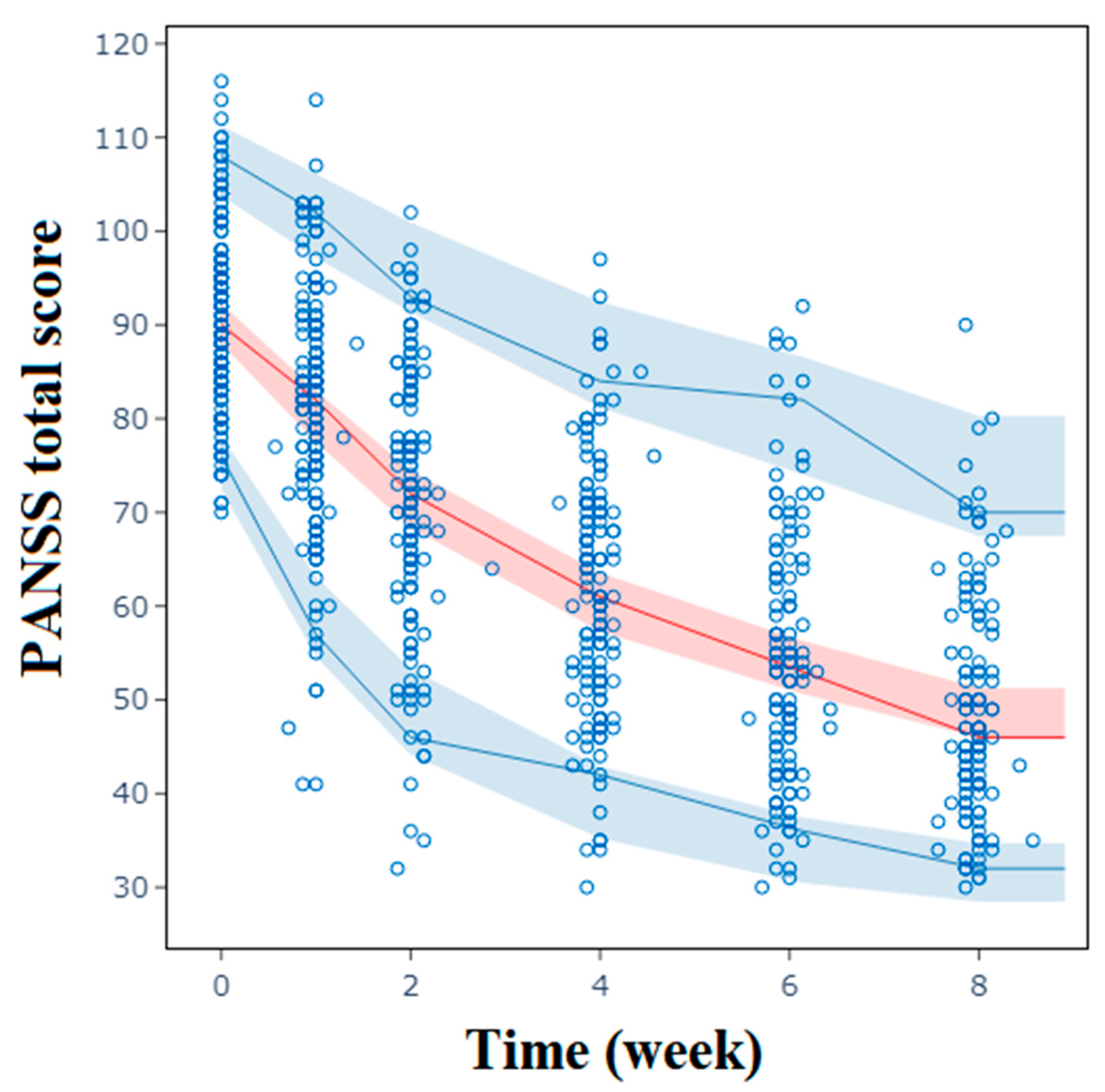
2.2. Population Pharmacodynamic Analysis of PANSS Total Scores
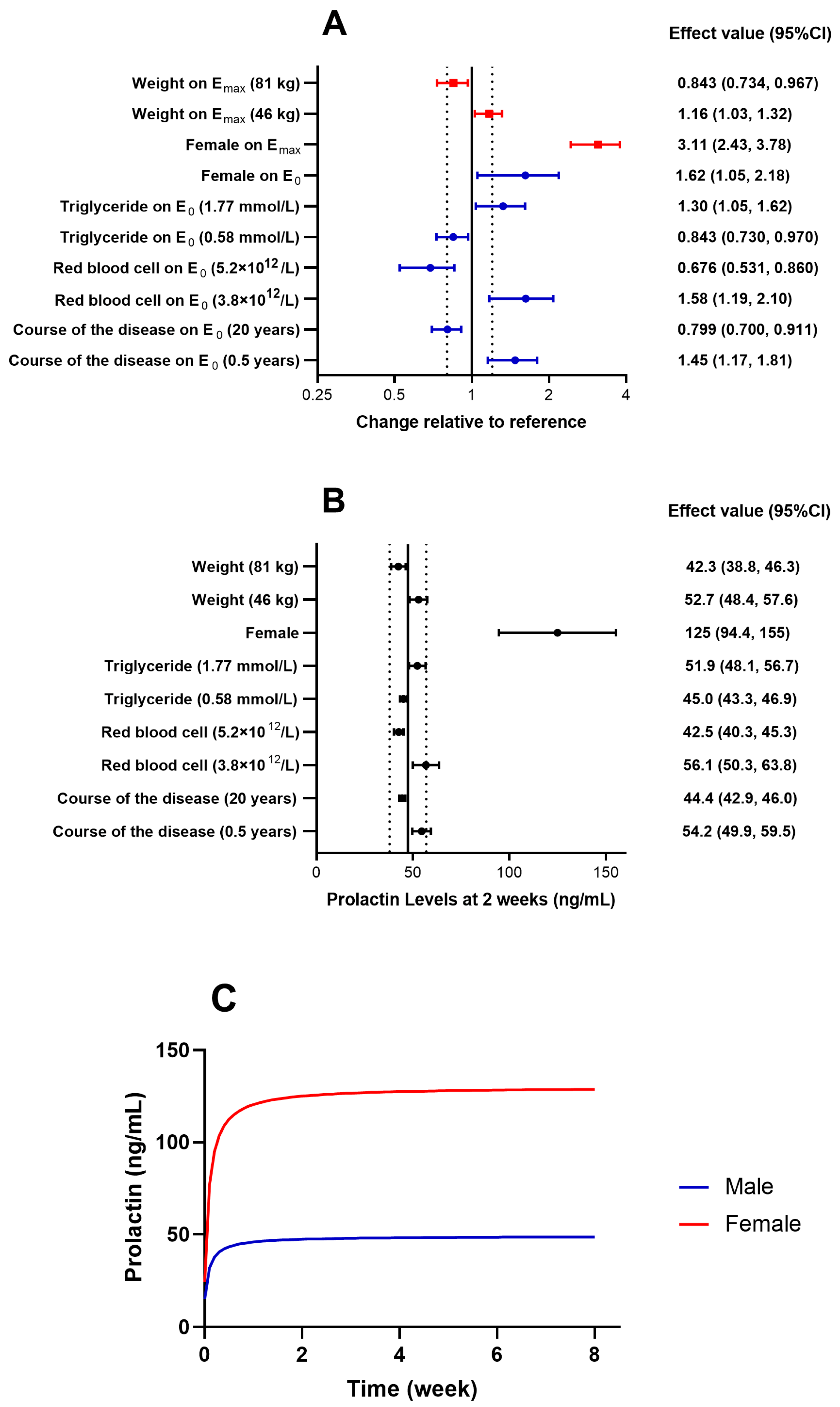
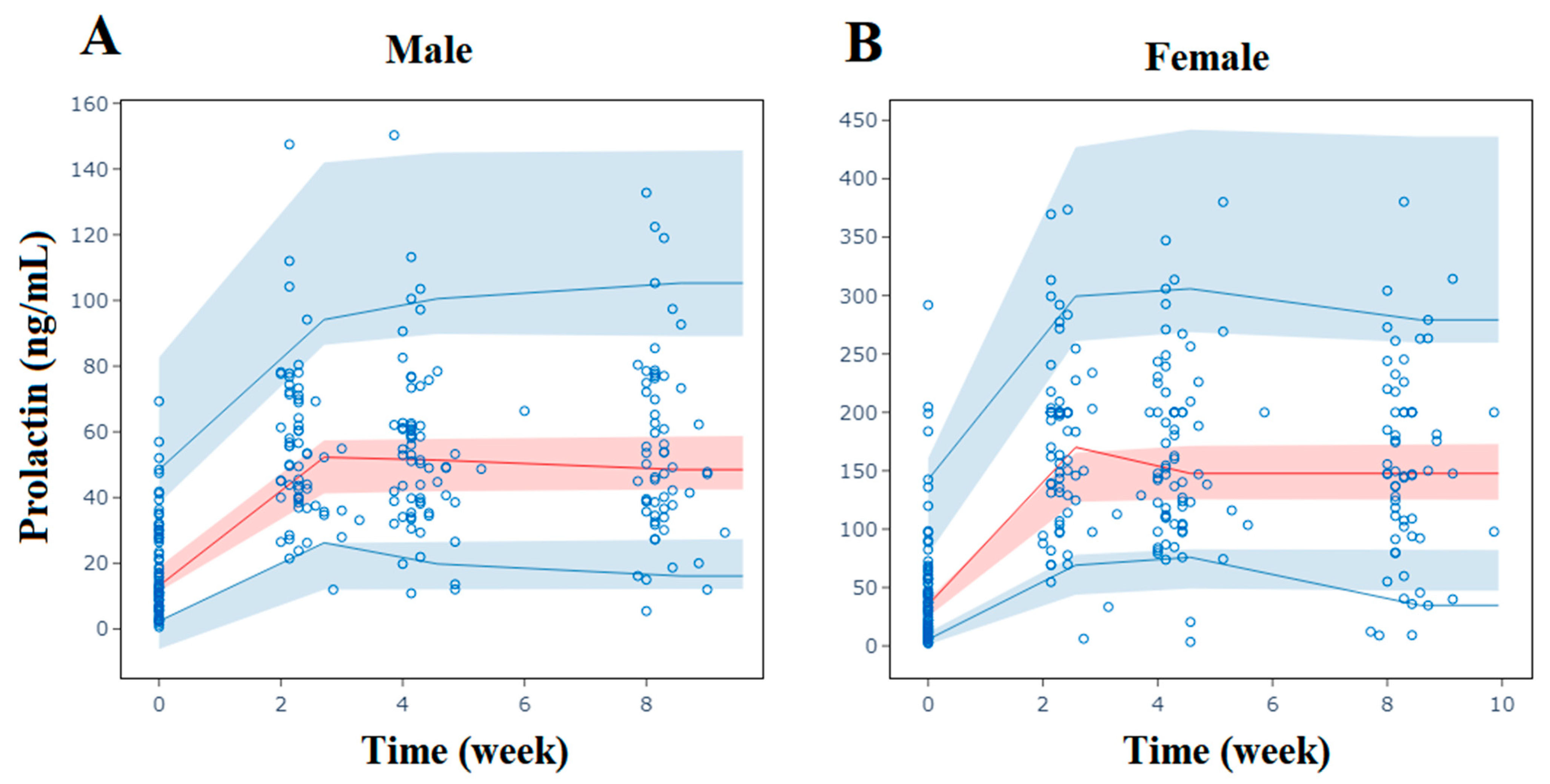
2.3. Population Pharmacodynamic Analysis of Prolactin
2.4. Model Evaluation
2.5. Predictions
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical Data
4.3. Modeling Approach
4.4. Model Evaluation
4.5. Predictions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grubor, M.; Zivkovic, M.; Sagud, M.; Nikolac, P.M.; Mihaljevic-Peles, A.; Pivac, N.; Muck-Seler, D.; Svob, S.D. HTR1A, HTR1B, HTR2A, HTR2C and HTR6 Gene Polymorphisms and Extrapyramidal Side Effects in Haloperidol-Treated Patients with Schizophrenia. Int. J. Mol. Sci. 2020, 21, 2345. [Google Scholar] [CrossRef]
- Marder, S.R.; Umbricht, D. Negative symptoms in schizophrenia: Newly emerging measurements, pathways, and treatments. Schizophr. Res. 2023, 258, 71–77. [Google Scholar] [CrossRef]
- McGlashan, T.H.; Fenton, W.S. Classical subtypes for schizophrenia: Literature review for DSM-IV. Schizophr. Bull. 1991, 17, 609–632. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Han, L.; Hu, Y.; Si, L.; Shao, X.; Lu, F.; Zhang, L. Effect of H2H management mode on blood sugar control and living ability in patients with schizophrenia and type 2 diabetes mellitus. Am. J. Transl. Res. 2023, 15, 223–232. [Google Scholar]
- Garcinuno, S.; Aranaz, I.; Civera, C.; Arias, C.; Acosta, N. Evaluating Non-Conventional Chitosan Sources for Controlled Release of Risperidone. Polymers 2022, 14, 1355. [Google Scholar] [CrossRef]
- Ricciardi, L.; Pringsheim, T.; Barnes, T.; Martino, D.; Gardner, D.; Remington, G.; Addington, D.; Morgante, F.; Poole, N.; Carson, A.; et al. Treatment Recommendations for Tardive Dyskinesia. Can. J. Psychiatry 2019, 64, 388–399. [Google Scholar] [CrossRef]
- Kumar, P.B.S.; Pandey, R.S.; Thirthalli, J.; Kumar, P.T.S.; Kumar, C.N. A Comparative Study of Short Term Efficacy of Aripiprazole and Risperidone in Schizophrenia. Curr. Neuropharmacol. 2017, 15, 1073–1084. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Huhn, M.; Rothe, P.; Krause, M.; Bighelli, I.; Schneider-Thoma, J.; Leucht, S. How well do patients with a first episode of schizophrenia respond to antipsychotics: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2017, 27, 835–844. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Chen, Z.; Liao, Y.; Wu, Y.; He, Z.; Yang, Z.; Zhang, Q. Rapid cycling bipolar disorder is associated with antithyroid antibodies, instead of thyroid dysfunction. BMC Psychiatry 2019, 19, 378. [Google Scholar] [CrossRef]
- Deligeoroglou, E.; Tsimaris, P. Menstrual disturbances in puberty. Best. Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 157–171. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef]
- Bostwick, J.R.; Guthrie, S.K.; Ellingrod, V.L. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy 2009, 29, 64–73. [Google Scholar] [CrossRef]
- Holt, R.I.; Peveler, R.C. Antipsychotics and hyperprolactinaemia: Mechanisms, consequences and management. Clin. Endocrinol. 2011, 74, 141–147. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.D.; Li, Z.P. Time course and dose effect of metformin on weight in patients with different disease states. Expert. Rev. Clin. Pharmacol. 2020, 13, 1169–1177. [Google Scholar] [CrossRef]
- Liu, X.; Jones, B.L.; Roberts, J.K.; Sherwin, C.M. Population pharmacokinetic/pharmacodynamic modeling of histamine response measured by histamine iontophoresis laser Doppler. J. Pharmacokinet. Pharmacodyn. 2016, 43, 385–393. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Marks, M.J.; Meinerz, N.M.; Brown, R.W.; Collins, A.C. 86Rb+ efflux mediated by alpha4beta2*-nicotinic acetylcholine receptors with high and low-sensitivity to stimulation by acetylcholine display similar agonist-induced desensitization. Biochem. Pharmacol. 2010, 80, 1238–1251. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liao, J.; Jiang, S.; Yan, J.; Yue, W.; Zhang, D.; Yan, H. Progressive Grey Matter Volume Changes in Patients with Schizophrenia over 6 Weeks of Antipsychotic Treatment and Their Relationship to Clinical Improvement. Neurosci. Bull. 2018, 34, 816–826. [Google Scholar] [CrossRef]
- Iraji, A.; Faghiri, A.; Fu, Z.; Rachakonda, S.; Kochunov, P.; Belger, A.; Ford, J.M.; Mcewen, S.; Mathalon, D.H.; Mueller, B.A.; et al. Multi-spatial-scale dynamic interactions between functional sources reveal sex-specific changes in schizophrenia. Netw. Neurosci. 2022, 6, 357–381. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium signalling and psychiatric disease: Bipolar disorder and schizophrenia. Cell Tissue Res. 2014, 357, 477–492. [Google Scholar] [CrossRef]
- Mallinger, A.G.; Thase, M.E.; Haskett, R.; Buttenfield, J.; Luckenbaugh, D.A.; Frank, E.; Kupfer, D.J.; Manji, H.K. Verapamil augmentation of lithium treatment improves outcome in mania unresponsive to lithium alone: Preliminary findings and a discussion of therapeutic mechanisms. Bipolar Disord. 2008, 10, 856–866. [Google Scholar] [CrossRef]
- Pazzaglia, P.J.; Post, R.M.; Ketter, T.A.; George, M.S.; Marangell, L.B. Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res. 1993, 49, 257–272. [Google Scholar] [CrossRef]
- Burdick, K.E.; Perez-Rodriguez, M.; Birnbaum, R.; Shanahan, M.; Larsen, E.; Harper, C.; Poskus, J.; Sklar, P. A molecular approach to treating cognition in schizophrenia by calcium channel blockade: An open-label pilot study of the calcium-channel antagonist isradipine. Schizophr. Res. Cogn. 2020, 21, 100180. [Google Scholar] [CrossRef]
- Vahdani, B.; Armani Kian, A.; Esmaeilzadeh, A.; Zenoozian, S.; Yousefi, V.; Mazloomzadeh, S. Adjunctive Raloxifene and Isradipine Improve Cognitive Functioning in Patients with Schizophrenia: A Pilot Study. J. Clin. Psychopharmacol. 2020, 40, 457–463. [Google Scholar] [CrossRef]
- Taylor, P.M.; Sachlos, E.; Dreger, S.A.; Chester, A.H.; Czernuszka, J.T.; Yacoub, M.H. Interaction of human valve interstitial cells with collagen matrices manufactured using rapid prototyping. Biomaterials 2006, 27, 2733–2737. [Google Scholar] [CrossRef]
- Ershova, E.S.; Jestkova, E.M.; Martynov, A.V.; Shmarina, G.V.; Umriukhin, P.E.; Bravve, L.V.; Zakharova, N.V.; Kostyuk, G.P.; Saveliev, D.V.; Orlova, M.D.; et al. Accumulation of Circulating Cell-Free CpG-Enriched Ribosomal DNA Fragments on the Background of High Endonuclease Activity of Blood Plasma in Schizophrenic Patients. Int. J. Genom. 2019, 2019, 8390585. [Google Scholar] [CrossRef]
- Giné-Servén, E.; Martinez-Ramirez, M.; Boix-Quintana, E.; Davi-Loscos, E.; Guanyabens, N.; Casado, V.; Muriana, D.; Torres-Rivas, C.; Crespo-Facorro, B.; Labad, J. Routine cerebrospinal fluid parameters as biomarkers in first-episode psychosis: A prospective observational study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 112, 110424. [Google Scholar] [CrossRef]
- Ziegler, T.E.; Prudom, S.L.; Zahed, S.R.; Parlow, A.F.; Wegner, F. Prolactin’s mediative role in male parenting in parentally experienced marmosets (Callithrix jacchus). Horm. Behav. 2009, 56, 436–443. [Google Scholar] [CrossRef]
- Socolovsky, M.; Fallon, A.E.; Lodish, H.F. The prolactin receptor rescues EpoR-/- erythroid progenitors and replaces EpoR in a synergistic interaction with c-kit. Blood 1998, 92, 1491–1496. [Google Scholar] [CrossRef]
- Yang, L.; Kuo, C.B.; Liu, Y.; Coss, D.; Xu, X.; Chen, C.; Oster-Granite, M.L.; Walker, A.M. Administration of unmodified prolactin (U-PRL) and a molecular mimic of phosphorylated prolactin (PP-PRL) during rat pregnancy provides evidence that the U-PRL:PP-PRL ratio is crucial to the normal development of pup tissues. J. Endocrinol. 2001, 168, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Kryzhanovskaya, L.; Schulz, S.C.; Mcdougle, C.; Frazier, J.; Dittmann, R.; Robertson-Plouch, C.; Bauer, T.; Xu, W.; Wang, W.; Carlson, J.; et al. Olanzapine versus placebo in adolescents with schizophrenia: A 6-week, randomized, double-blind, placebo-controlled trial. J. Am. Acad. Child. Adolesc. Psychiatr. 2009, 48, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mendiratta, G.; Singal, R.; Sharma, N.S. Plasma Prolactin and Total Lipid Levels and Subsequent Risk of Breast Cancer in Pre- and Postmenopausal Women: Experience from an Indian Rural Centre. Maedica 2017, 12, 258–266. [Google Scholar] [PubMed]
- Glintborg, D.; Altinok, M.; Mumm, H.; Buch, K.; Ravn, P.; Andersen, M. Prolactin is associated with metabolic risk and cortisol in 1007 women with polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1773–1779. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Zhang, L.; Fu, J.; An, Y.; Meng, H.; Wang, G. Increased Prolactin is an Adaptive Response to Protect Against Metabolic Disorders in Obesity. Endocr. Pract. 2021, 27, 728–735. [Google Scholar] [CrossRef]

| Median (Interquartile Range) | Min–Max | |
|---|---|---|
| Male (%) | 49.3 | |
| Age (years) | 33.3 (25.2–45.2) | 18.3–63.4 |
| Height (cm) | 165 (160–172) | 150–183 |
| Weight (kg) | 59.3 (52.0–70.0) | 37.0–106.0 |
| Baseline Prolactin (ng/mL) | 21.6 (10.2–42.6) | 0.596–292 |
| Course of Disease (Years) | 5.00 (1.00–8.00) | 0.500–38.0 |
| Baseline PANSS Total Scores | 90.0 (84.0–97.0) | 70.0–116.0 |
| Parameter | NONMEM | Bootstrap | ||
|---|---|---|---|---|
| Estimate | 95% CI | Median | 95% CI | |
| Fixed Effect | ||||
| E0 | 90.1 | 88.5, 91.7 | 90.1 | 88.5, 91.7 |
| γ | 1.31 | 1.18, 1.44 | 1.31 | 1.17, 1.45 |
| ET50 | 5.37 | 4.43, 6.31 | 5.42 | 4.46, 6.60 |
| Emax | 0.661 | 0.631, 0.691 | 0.663 | 0.630, 0.711 |
| Covariate Effect | ||||
| Plasma Calcium on γ | 2.56 | 1.12, 4.00 | 2.56 | 0.880, 4.01 |
| Course of Disease on ET50 | 0.267 | 0.180, 0.354 | 0.262 | 0.169, 0.355 |
| Sex on ET50 | 0.726 | 0.569, 0.883 | 0.725 | 0.571, 0.913 |
| Lactate Dehydrogenase on Emax | 0.191 | 0.0689, 0.313 | 0.197 | 0.0410, 0.347 |
| Inter-Individual Variability | ||||
| E0 (%) | 10.1 | 8.91, 11.3 | 10.1 | 8.84, 11.3 |
| γ (%) | 40.7 | 31.9, 49.5 | 40.3 | 31.5, 50.0 |
| ET50 (%) | 62.2 | 51.4, 73.0 | 60.9 | 50.6, 73.1 |
| ρ(E0, γ) | 0.389 | 0.154, 0.624 | 0.396 | 0.205, 0.455 |
| Residual Error | ||||
| Additive Error | 3.78 | 3.43, 4.13 | 3.77 | 3.41, 4.11 |
| Parameter | NONMEM | Bootstrap | ||
|---|---|---|---|---|
| Estimate | 95% CI | Median | 95% CI | |
| Fixed Effect | ||||
| E0 | 15.0 | 11.6, 18.3 | 14.9 | 11.9, 18.5 |
| ET50 | 0.100 FIX | |||
| Emax | 34.0 | 28.8, 39.2 | 34.2 | 28.9, 39.4 |
| Covariate Effect | ||||
| Course of Disease on E0 | −0.162 | −0.257, −0.0675 | −0.163 | −0.266, −0.0592 |
| Red Blood Cells on E0 | −2.71 | −4.38, −1.04 | −2.75 | −4.29, −1.09 |
| Triglycerides on E0 | 0.392 | 0.0685, 0.716 | 0.405 | 0.0819, 0.750 |
| Sex on E0 | 1.62 | 1.05, 2.18 | 1.63 | 1.15, 2.26 |
| Sex on Emax | 3.11 | 2.43, 3.78 | 3.09 | 2.48, 3.87 |
| Weight on Emax | −0.570 | −1.03, −0.112 | −0.566 | −1.10, −0.105 |
| Inter-Individual Variability | ||||
| E0 (%) | 70.0 | 56.3, 81.4 | 68.4 | 56.2, 82.9 |
| Emax (%) | 49.7 | 38.4, 58.9 | 48.8 | 38.7, 59.8 |
| Residual Error | ||||
| Proportional Error (%) | 25.0 | 18.5, 29.4 | 25.0 | 19.5, 29.1 |
| Additive Error (ng/mL) | 6.70 | 2.79, 9.06 | 6.67 | 3.35, 9.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhang, L.; Li, Y.; Yu, Y.; Shen, Y.; Sun, X.; Lou, K.; Luo, H.; Meng, Z.; Li, H.; et al. Population Pharmacodynamic Models of Risperidone on PANSS Total Scores and Prolactin Levels in Schizophrenia. Pharmaceuticals 2024, 17, 148. https://doi.org/10.3390/ph17020148
Huang Z, Zhang L, Li Y, Yu Y, Shen Y, Sun X, Lou K, Luo H, Meng Z, Li H, et al. Population Pharmacodynamic Models of Risperidone on PANSS Total Scores and Prolactin Levels in Schizophrenia. Pharmaceuticals. 2024; 17(2):148. https://doi.org/10.3390/ph17020148
Chicago/Turabian StyleHuang, Zhiwei, Lei Zhang, Yan Li, Yimin Yu, Yifeng Shen, Xiujia Sun, Kun Lou, Hongmei Luo, Zhibin Meng, Huafang Li, and et al. 2024. "Population Pharmacodynamic Models of Risperidone on PANSS Total Scores and Prolactin Levels in Schizophrenia" Pharmaceuticals 17, no. 2: 148. https://doi.org/10.3390/ph17020148






