Baricitinib Lipid-Based Nanosystems as a Topical Alternative for Atopic Dermatitis Treatment
Abstract
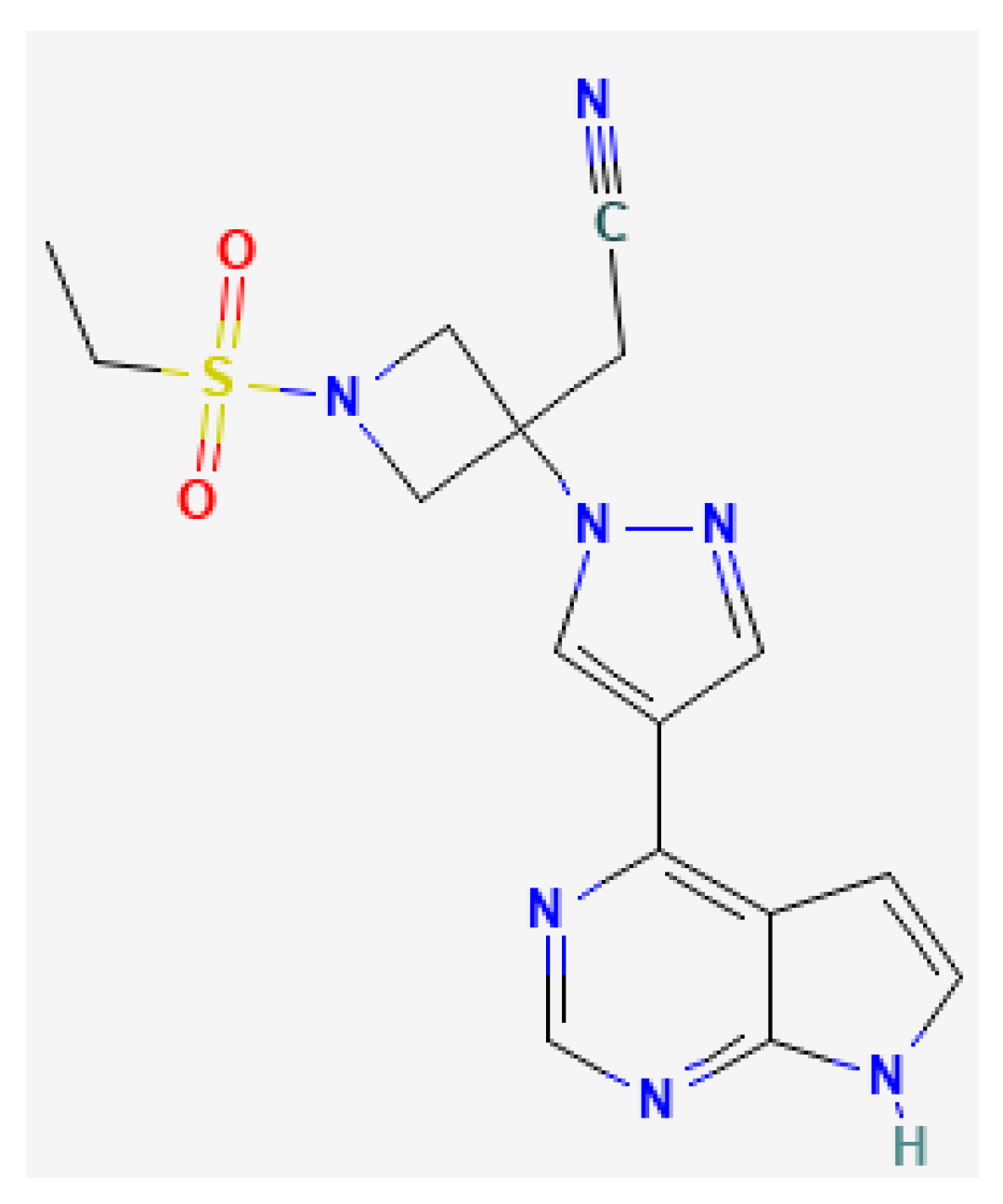
:1. Introduction
2. Results
2.1. Liposomes Physicochemical Characterization
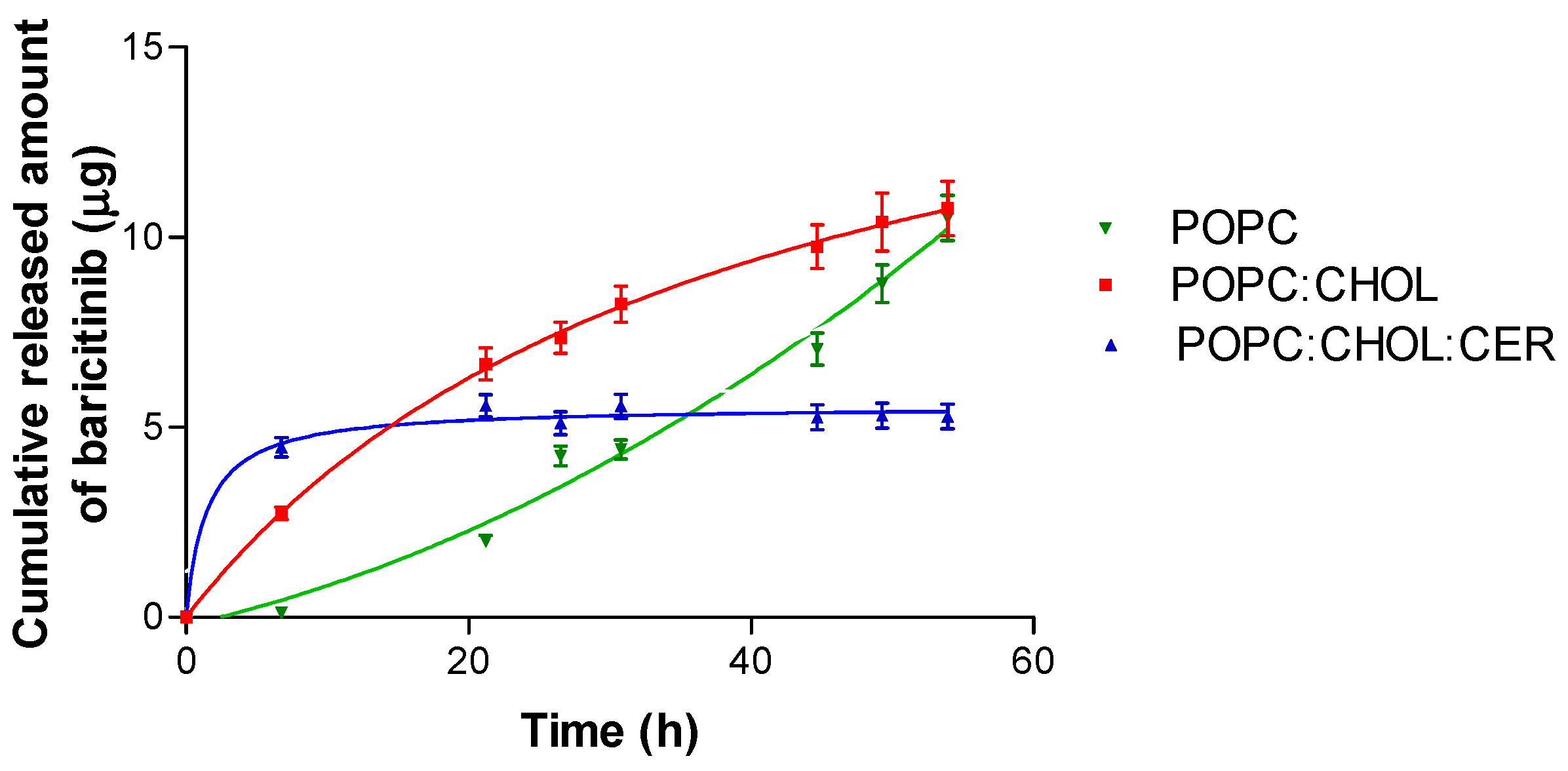
2.2. In Vitro Drug Release Study
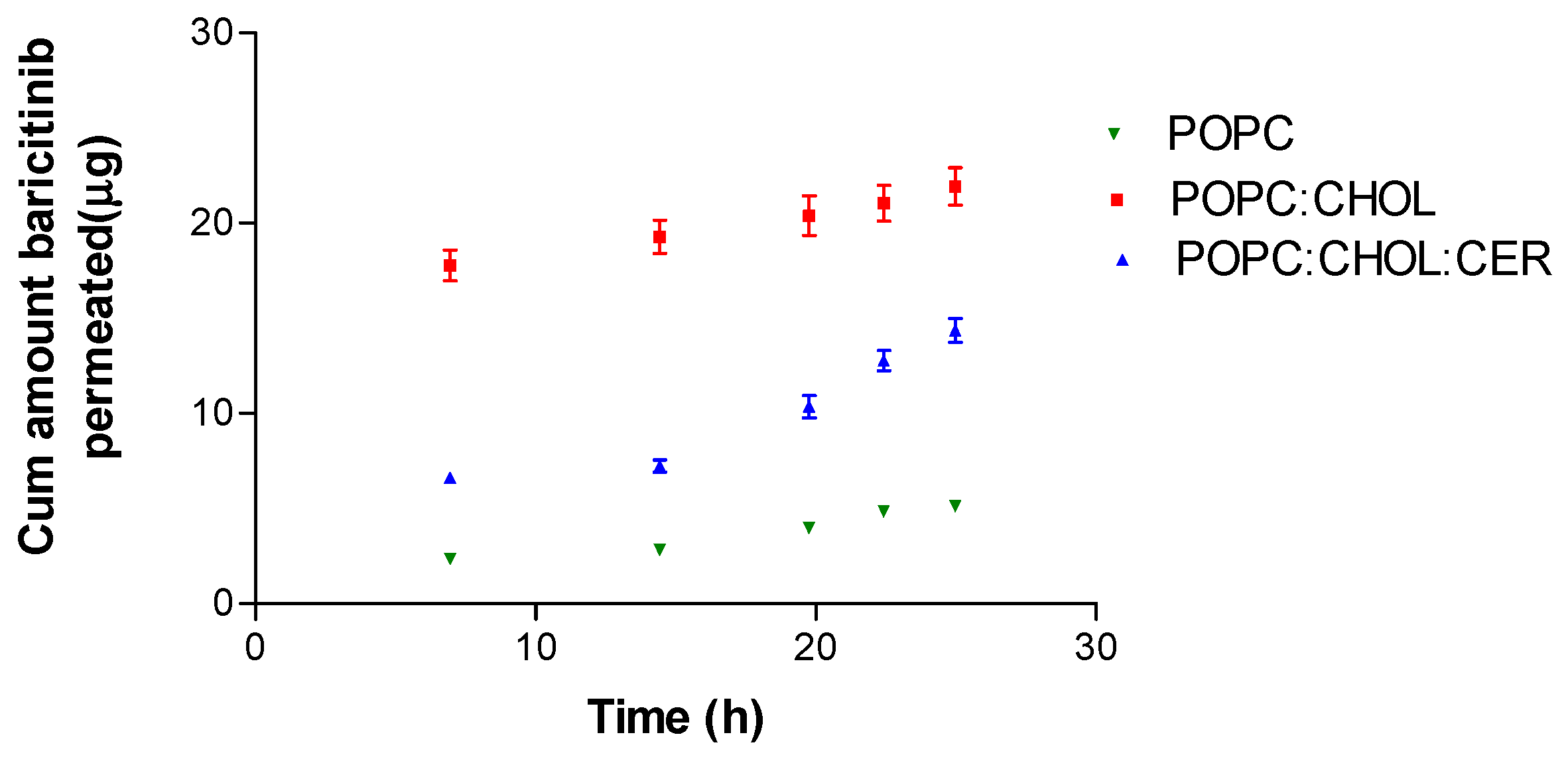
2.3. Ex Vivo Permeation Study
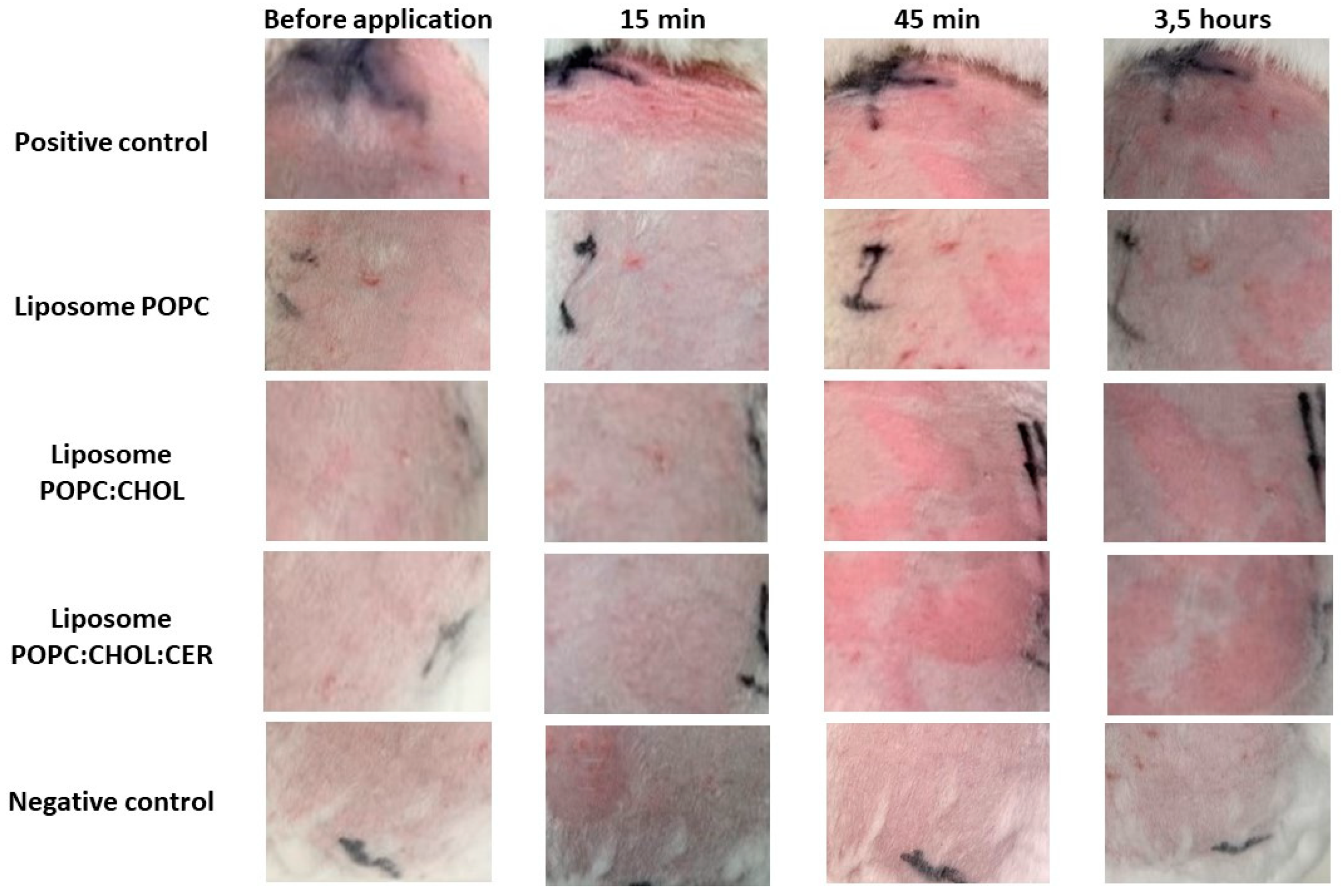
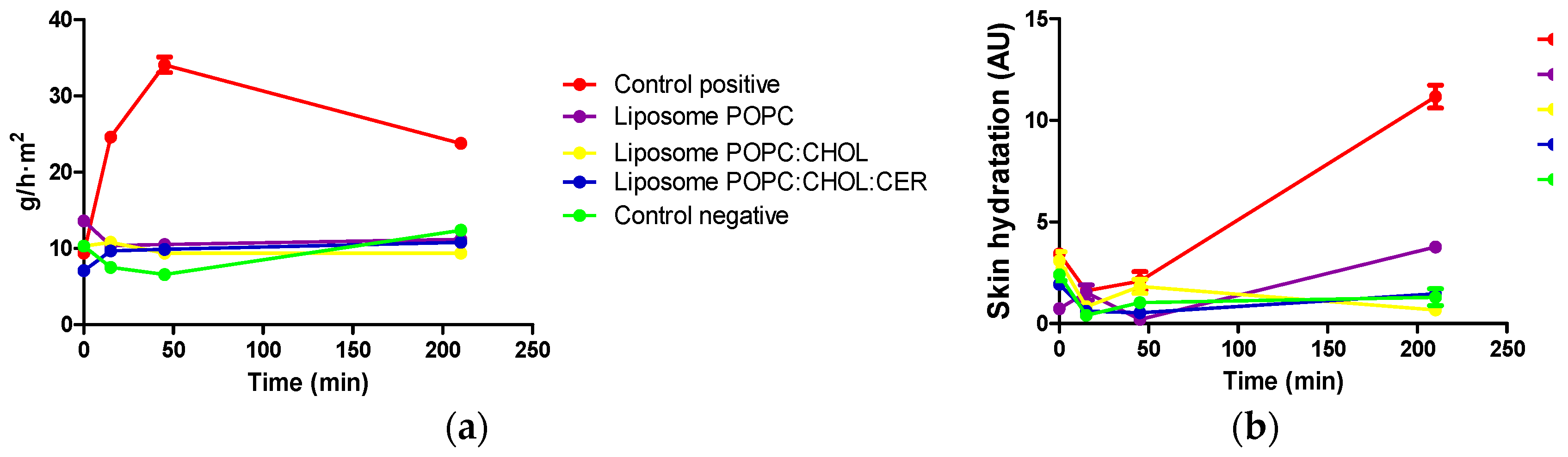
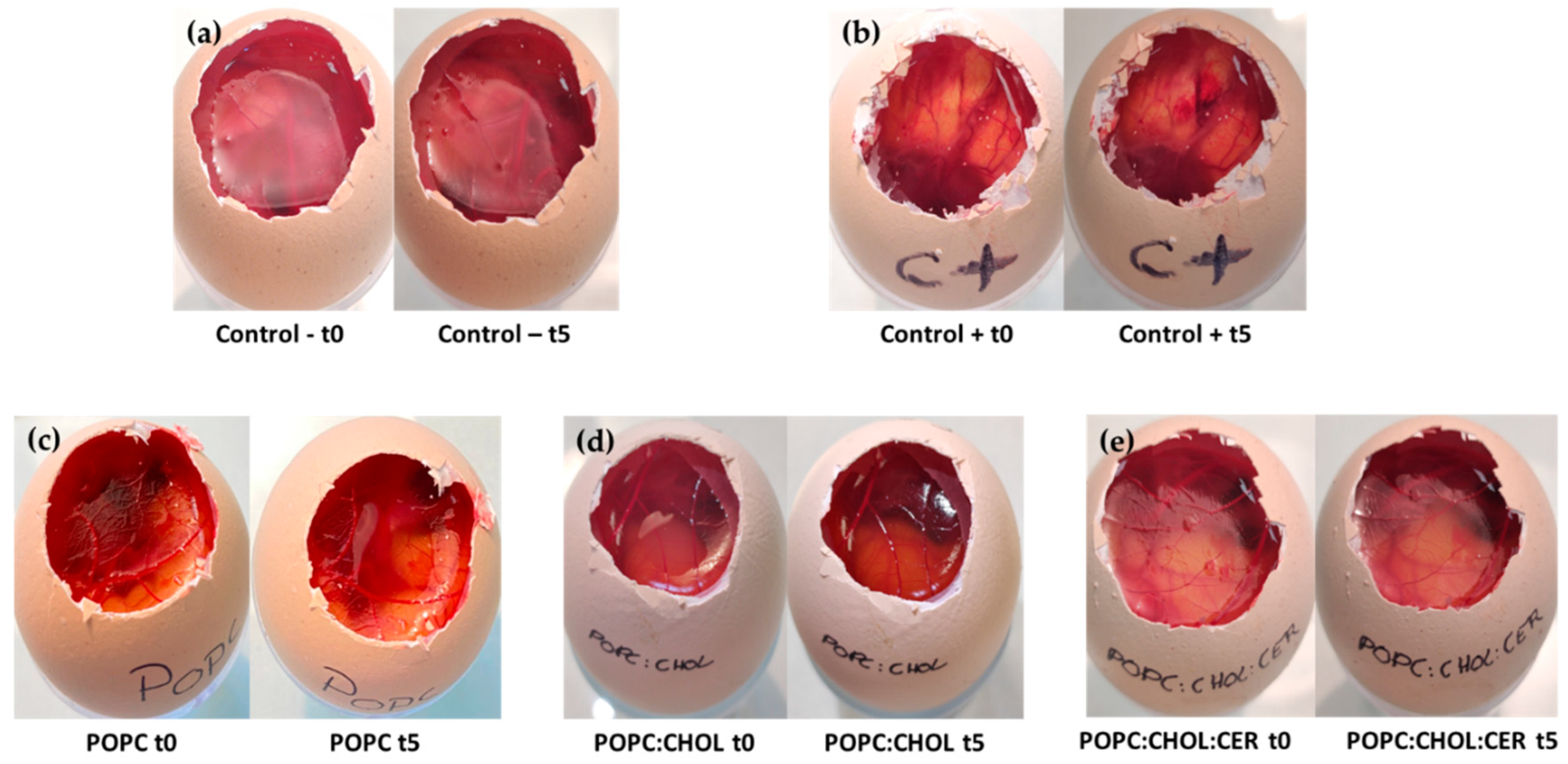
2.4. Tolerance Study and Histological Analysis
2.4.1. In Vivo Tolerance Study
2.4.2. Histological Analysis
2.4.3. In Vitro Tolerance of the Liposomal Formulations for Periocular Application
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Biological Materials
4.3. Methods
4.3.1. Preparation of the Liposomes
4.3.2. Liposomes’ Physicochemical Characterization
4.3.3. In Vitro Drug Release Study
4.3.4. Ex Vivo Permeation Study
4.3.5. Baricitinib Determination by HPLC
4.3.6. Tolerance Study and Histological Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Tabriz Univ. Med. Sci. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Michniak-Kohn, B. Preparation and Characterization of Lipid Based Nanosystems for Topical Delivery of Quercetin. Eur. J. Pharm. Sci. 2013, 48, 442–452. [Google Scholar] [CrossRef]
- Sguizzato, M.; Esposito, E.; Cortesi, R.; Terrinoni, A. Molecular Sciences Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Florence, A.T. Liposomes in Drug Delivery. Drugs 1993, 45, 15–28. [Google Scholar] [CrossRef]
- Coderch, L.; López, O.; De La Maza, A.; Parra, J.L. Ceramides and Skin Function. Am. J. Clin. Derm. 2003, 4, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Boncheva, M. The Physical Chemistry of the Stratum Corneum Lipids. Int. J. Cosmet. Sci. 2014, 36, 505–515. [Google Scholar] [CrossRef]
- Danso, M.; Boiten, W.; van Drongelen, V.; Gmelig Meijling, K.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered Expression of Epidermal Lipid Bio-Synthesis Enzymes in Atopic Dermatitis Skin Is Accompanied by Changes in Stratum Corneum Lipid Composition. J. Derm. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M. Lipid Abnormalities and Lipid-Based Repair Strategies in Atopic Dermatitis. Biochim. Et Biophys. Acta.-Mol. Cell Biol. Lipids 2014, 1841, 323–330. [Google Scholar] [CrossRef] [Green Version]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The Important Role of Stratum Corneum Lipids for the Cutaneous Barrier Function. Biochim. Et Biophys. Acta.-Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Lowe, A.J.; Matheson, M.C.; Burgess, J.A.; Allen, K.J.; Abramson, M.J. Atopic Dermatitis and the Atopic March Revisited. Allergy 2014, 69, 17–27. [Google Scholar] [CrossRef]
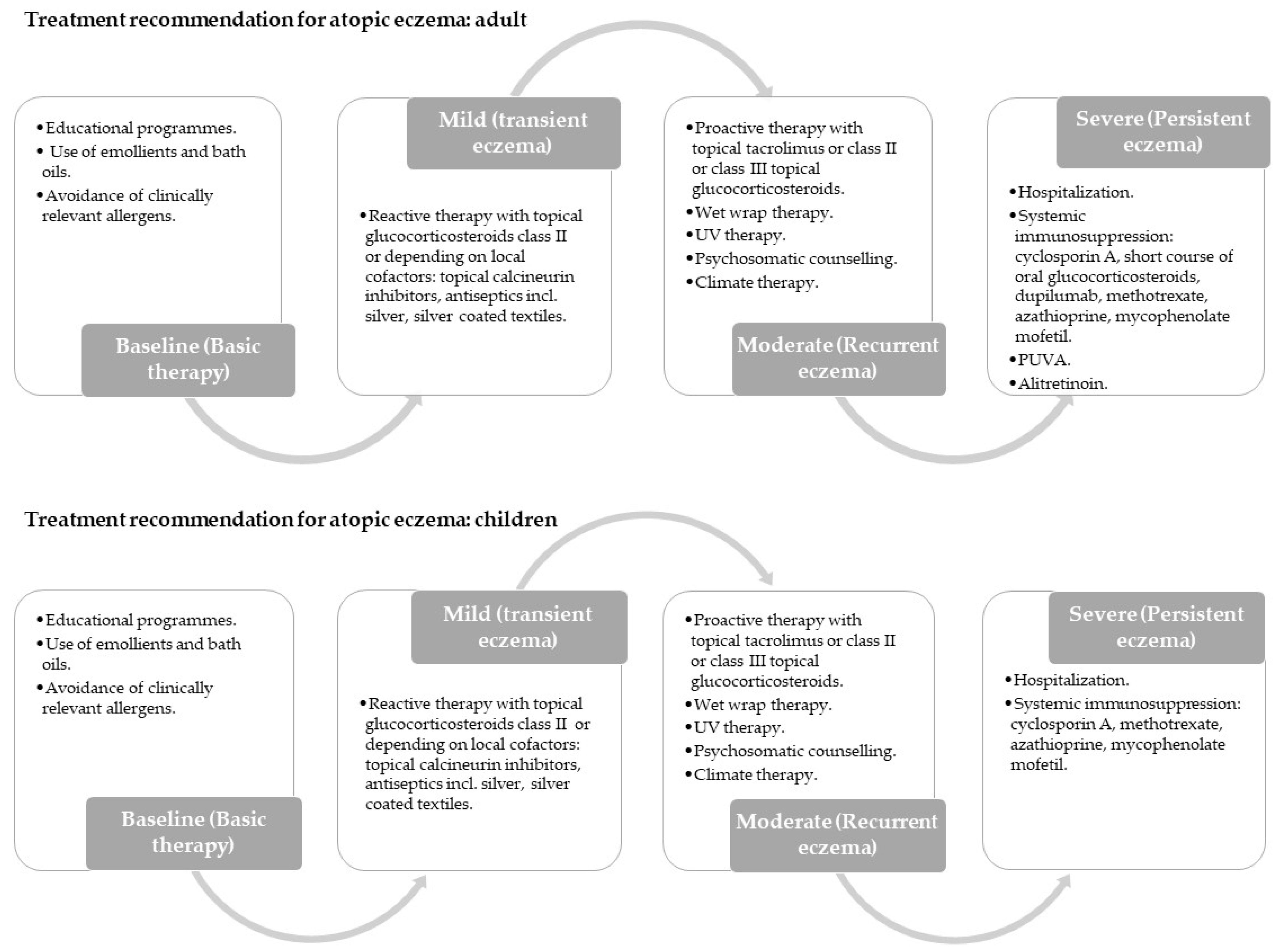
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-Based European Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis) in Adults and Children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oranje, A.P. Practical Issues on Interpretation of Scoring Atopic Dermatitis: SCORAD Index, Objective SCORAD, Patient-Oriented SCORAD and Three-Item Severity Score. Curr. Probl. Dermatol. 2011, 41, 149–155. [Google Scholar] [PubMed]
- Jorgensen, S.C.J.; Tse, C.L.Y.; Burry, L.; Dresser, L.D. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacother. J. Hum. Pharmacol. Drug. Ther. 2020, 40, 843–856. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CHMP). Olumiant-Assessment Report; Committee for Medicinal Products for Human Use (CHMP): London, UK, 2020. [Google Scholar]
- Saeki, H.; Ohya, Y.; Furuta, J.; Arakawa, H.; Ichiyama, S.; Katsunuma, T.; Katoh, N.; Tanaka, A.; Tsunemi, Y.; Nakahara, T.; et al. Executive Summary: Japanese Guidelines for Atopic Dermatitis (ADGL) 2021. Allergol. Int. 2022, 71, 448–458. [Google Scholar] [CrossRef]
- National Library of Medicine (US); National Center for Biotechnology Information PubChem Compound Summary for CID 5497103, 1-Palmitoyl-2-Oleoyl-Sn-Glycero-3-Phosphocholine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (accessed on 27 September 2022).
- Jurecek, L.; Rajcigelova, T.; Kozarova, A.; Werner, T.; Vormann, J.; Kolisek, M. Beneficial Effects of an Alkaline Topical Treatment in Patients with Mild Atopic Dermatitis. J. Cosmet. Derm. 2021, 20, 2824–2831. [Google Scholar] [CrossRef]
- Mallandrich, M.; Calpena, A.C.; Clares, B.; Parra, A.; García, M.L.; Soriano, J.L.; Fernández-Campos, F. Nano-Engineering of Ketorolac Tromethamine Platforms for Ocular Treatment of Inflammatory Disorders. Nanomedicine 2021, 16, 401–414. [Google Scholar] [CrossRef]
- González-Rodríguez, M.; Rabasco, A. Charged Liposomes as Carriers to Enhance the Permeation through the Skin. Expert Opin. Drug. Deliv. 2011, 8, 857–871. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.; Kost, J.; Barenholz, Y. Ultrasound, Liposomes, and Drug Delivery: Principles for Using Ultrasound to Control the Release of Drugs from Liposomes. Chem. Phys. Lipids 2009, 162, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.F.; Calpena Campmany, A.C.; Delgado, G.R.; Serrano, O.L.; Naveros, B.C. Development and Characterization of a Novel Nystatin-Loaded Nanoemulsion for the Buccal Treatment of Candidosis: Ultrastructural Effects and Release Studies. J. Pharm. Sci. 2012, 101, 3739–3752. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Villegas, V.; Clares-Naveros, B.; García-López, M.L.; Calpena-Campmany, A.C.; Bustos-Zagal, P.; Garduño-Ramírez, M.L. Development and Characterization of Two Nano-Structured Systems for Topical Application of Flavanones Isolated from Eysenhardtia Platycarpa. Colloids Surf. B Biointerfaces 2014, 116, 183–192. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pappas, A. Epidermal Surface Lipids. Dermatoendocrinol 2009, 1, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinico, C.; Fadda, A.M. Vesicular Carriers for Dermal Drug Delivery. Expert Opin. Drug. Deliv. 2009, 6, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel Chemical Permeation Enhancers for Transdermal Drug Delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.G.; Chen, X.; Lee, F.; Emm, T.; Scherle, P.A.; Lo, Y.; Punwani, N.; Williams, W.V.; Yeleswaram, S. The Pharmacokinetics, Pharmacodynamics, and Safety of Baricitinib, an Oral JAK 1/2 Inhibitor, in Healthy Volunteers. J. Clin. Pharm. 2014, 54, 1354–1361. [Google Scholar] [CrossRef]
- Rins, M.; Diez, I.; Calpena, A.C.; Obach, R. Skin Density in the Hairless Rat. Evidence of Regional Differences. Eur. J. Drug. Metab. Pharm. 1991, Spec No 3, 456–457. [Google Scholar]
- Medi, B.M.; Singh, J. Skin Targeted DNA Vaccine Delivery Using Electroporation in Rabbits: II. Safety. Int. J. Pharm. 2006, 308, 61–68. [Google Scholar] [CrossRef]
- Anigbogu, A.; Patil, S.; Singh, P.; Liu, P.; Dinh, S.; Maibach, H. An in Vivo Investigation of the Rabbit Skin Responses to Transdermal Iontophoresis. Int. J. Pharm. 2000, 200, 195–206. [Google Scholar] [CrossRef]
- Vázquez-González, M.L.; Botet-Carreras, A.; Domènech, Ò.; Teresa Montero, M.; Borrell, J.H. Planar Lipid Bilayers Formed from Thermodynamically-Optimized Liposomes as New Featured Carriers for Drug Delivery Systems through Human Skin. Int. J. Pharm. 2019, 563, 1–8. [Google Scholar] [CrossRef]
- Moussaoui, S.E.; Abo-horan, I.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-ramírez, M.L.; Clares, B.; Soriano, J.L.; Calpena, A.C.; Fernández-campos, F.; et al. Polymeric Nanoparticles and Chitosan Gel Loading Ketorolac Tromethamine to Alleviate Pain Associated with Condyloma Acuminata during the Pre- and Post-ablation. Pharmaceutics 2021, 13, 1784. [Google Scholar] [CrossRef]
- Moussaoui, S.E.; Fernández-Campos, F.; Alonso, C.; Limón, D.; Halbaut, L.; Garduño-Ramirez, M.L.; Calpena, A.C.; Mallandrich, M. Topical Mucoadhesive Alginate-Based Hydrogel Loading Ketorolac for Pain Management after Pharmacotherapy, Ablation, or Surgical Removal in Condyloma Acuminata. Gels 2021, 7, 8. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurakula, M.; Srinivas, C.; Kasturi, N.; Diwan, P. V Formulation and Evaluation of Prednisolone Proliposomal Gel for Effective Topical Pharmacotherapy. Int. J. Pharm. Sci. Drug. Res. 2012, 4, 35–43. [Google Scholar]
- Pérez-González, N.; Bozal-De Febrer, N.; Calpena-Campmany, A.C.; Nardi-Ricart, A.; Rodríguez-Lagunas, M.J.; Morales-Molina, J.A.; Soriano-Ruiz, J.L.; Fernández-Campos, F.; Clares-Naveros, B.; Dinu, V. New Formulations Loading Caspofungin for Topical Therapy of Vulvovaginal Candidiasis. Gel 2021, 7, 259. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, J.-H.; Lim, K.-M. Alternatives to In Vivo Draize Rabbit Eye and Skin Irritation Tests with a Focus on 3D Reconstructed Human Cornea—Like Epithelium and Epidermis Models. Toxicol. Res. 2017, 33, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.K. Chapter 12-Toxicological Testing: In Vivo Systems. In Fundamentals of Toxicology; Academic Press: Cambridge , MA, USA, 2016; pp. 131–150. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J. Dermal Toxicity and Microscopic Alterations by JP-8 Jet Fuel Components in vivo in Rabbit. Env. Toxicol. Pharm. 2004, 16, 153–161. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K.; Elmaradny, H.A. Tailored Limonene-Based Nanosized Microemulsion: Formulation, Physicochemical Characterization and In Vivo Skin Irritation Assessment. Adv. Pharm. Bull. 2020, 11, 274–285. [Google Scholar] [CrossRef]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-Inflammatory Activity of Nanocrystalline Silver-Derived Solutions in Porcine Contact Dermatitis. J. Inflamm. 2010, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Cao, X.; Li, X.; Ding, H. Topical Application with Conjugated Linoleic Acid Ameliorates 2, 4-dinitrofluorobenzene-induced Atopic Dermatitis-like Lesions in BALB/c Mice. Exp. Derm. 2021, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.L.; Steiner, S.; Havens, M.; Tramposch, K.M. Mouse Skin Inflammation Induced by Multiple Topical Applications of 12-O-Tetradecanoylphorbol-13-Acetate. Ski. Pharm. Physiol. 1991, 4, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Sévrain, S.; Bonté, F. Skin Hydration: A Review on Its Molecular Mechanisms. J. Cosmet. Derm. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netzlaff, F.; Kostka, K.H.; Lehr, C.M.; Schaefer, U.F. TEWL Measurements as a Routine Method for Evaluating the Integrity of Epidermis Sheets in Static Franz Type Diffusion Cells in Vitro. Limitations Shown by Transport Data Testing. Eur. J. Pharm. Biopharm. 2006, 63, 44–50. [Google Scholar] [CrossRef]
- Parra-Coca, A.; Boix-Montañés, A.; Calpena-Campmany, A.C.; Colom, H. In Vivo Pharmacokinetic Evaluation of Carprofen Delivery from Intra-Articular Nanoparticles in Rabbits: A Population Modelling Approach. Res. Vet. Sci. 2021, 137, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Garrós, N.; Mallandrich, M.; Beirampour, N.; Mohammadi, R.; Domènech, Ò.; Rodríguez-Lagunas, M.J.; Clares, B.; Colom, H. Baricitinib Liposomes as a New Approach for the Treatment of Sjögren’s Syndrome. Pharmaceutics 2022, 14, 1895. [Google Scholar] [CrossRef]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). Test Method Protocol: Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) Test Method; Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM): Bethesda, MD, USA, 2010.



| Composition | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|
| 1 Day | 1 Month | 1 Day | 1 Month | 1 Day | 1 Month | |
| POPC | 86.0 ± 1.4 | 86.9 ± 1.1 | 0.120 | 0.119 | −14.2 ± 0.3 | −13.9 ± 0.8 |
| POPC:CHOL (8:2, mol/mol) | 53.5 ± 0.9 | 55.4 ± 1.3 | 0.174 | 0.172 | −13.2 ± 1.3 | −13.5 ± 0.9 |
| POPC:CHOL:CER (3.6:2.4:4.0 mol/mol/mol) | 64.1 ± 0.3 | 65.0 ± 0.7 | 0.120 | 0.117 | −18.3 ± 1.9 | −18.1 ± 1.3 |
| Liposome | Equation | Best-Fit Values According to the Equation | ||||
|---|---|---|---|---|---|---|
| A | B | C | Bmax (μg) | Kd (h) | ||
| POPC | Polynominal: Second Order y = A + Bx + Cx2 | 0.21 | 0.08 | 0.002 | - | - |
| POPC:CHOL | One site binding (hyperbola) y = Bmaxx/(Kd + x) | - | - | - | 18.37 | 38.34 |
| POPC:CHOL:CER | One site binding (hyperbola) y = Bmaxx/(Kd + x) | - | - | - | 5.55 | 1.45 |
| AP25h (µg) | Jss (µg/h) | Kp (10−4 cm/h) | Css (ng/mL) | |
|---|---|---|---|---|
| POPC | 5.13 ± 0.52 | 0.22 ± 0.03 | 1.14 ± 0.16 | 0.36 ± 0.05 |
| POPC:CHOL | 21.93 ± 2.20 | 0.29 ± 0.03 | 1.52 ± 0.16 | 0.48 ± 0.05 |
| POPC:CHOL:CER | 14.36 ± 1.40 | 0.77 ± 0.07 | 4.01 ± 0.37 | 1.28 ± 0.12 |
| Chemicals | Before Induced Er and Ed | 15 min | 45 min | 3 h | ||||
|---|---|---|---|---|---|---|---|---|
| Erythema | Edema | Erythema | Edema | Erythema | Edema | Erythema | Edema | |
| Negative control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Liposome POPC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Liposome POPC:CHOL | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.00 ± 0.53 | 0.00 ± 0.00 | 0.65 ± 0.40 | 0.00 ± 0.00 |
| Liposome POPC:CHOL:CER | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.45 ± 0.42 | 0.00 ± 0.00 | 0.37 ± 0.03 | 0.00 ± 0.00 |
| Positive control (xylol) | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.54 ± 0.20 | 1.62 ± 0.41 | 3.50 ± 0.40 | 1.84 ± 0.13 | 2.40 ± 0.30 | 0.50 ± 0.02 |
| Formulation | |||||
|---|---|---|---|---|---|
| Negative control | Positive control (0.1 N NaOH) | POPC | POPC:CHOL | POPC:CHOL:CER | |
| Irritation score (IS) | 0.07 ± 0.00 | 16.10 ± 0.08 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrós, N.; Bustos-Salgados, P.; Domènech, Ò.; Rodríguez-Lagunas, M.J.; Beirampour, N.; Mohammadi-Meyabadi, R.; Mallandrich, M.; Calpena, A.C.; Colom, H. Baricitinib Lipid-Based Nanosystems as a Topical Alternative for Atopic Dermatitis Treatment. Pharmaceuticals 2023, 16, 894. https://doi.org/10.3390/ph16060894
Garrós N, Bustos-Salgados P, Domènech Ò, Rodríguez-Lagunas MJ, Beirampour N, Mohammadi-Meyabadi R, Mallandrich M, Calpena AC, Colom H. Baricitinib Lipid-Based Nanosystems as a Topical Alternative for Atopic Dermatitis Treatment. Pharmaceuticals. 2023; 16(6):894. https://doi.org/10.3390/ph16060894
Chicago/Turabian StyleGarrós, Núria, Paola Bustos-Salgados, Òscar Domènech, María José Rodríguez-Lagunas, Negar Beirampour, Roya Mohammadi-Meyabadi, Mireia Mallandrich, Ana C. Calpena, and Helena Colom. 2023. "Baricitinib Lipid-Based Nanosystems as a Topical Alternative for Atopic Dermatitis Treatment" Pharmaceuticals 16, no. 6: 894. https://doi.org/10.3390/ph16060894









