Application of Microfluidics for Bacterial Identification
Abstract
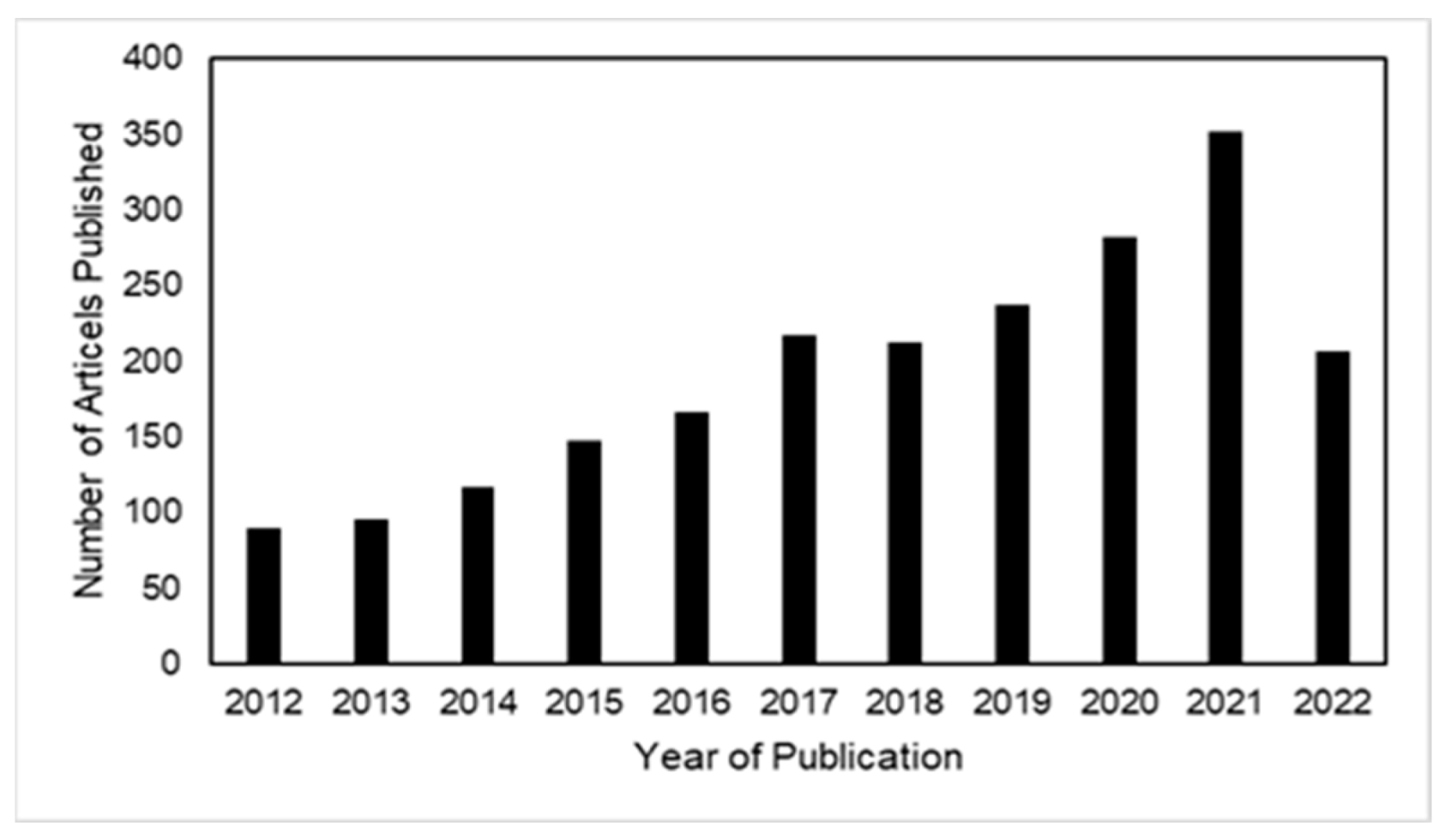
:1. Introduction
2. Enabling Microfluidics
2.1. Microfluidics with PCR
2.2. Microfluidics with LAMP
2.3. Microfluidics with Mass Spectrometry
3. Raman Spectroscopy Based Methods
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Surveillance for foodborne disease outbreaks—United States, 2009–2010. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 41–47. [Google Scholar]
- Na, W.; Nam, D.; Lee, H.; Shin, S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens. Bioelectron. 2018, 108, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Zhang, W.; Li, N.; Pu, Q.; Lin, J.-M. Recent progress on microfluidic biosensors for rapid detection of pathogenic bacteria. Chin. Chem. Lett. 2022, 33, 1743–1751. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Rothman, R.E. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet. Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [Green Version]
- Abayasekara, L.M.; Perera, J.; Chandrasekharan, V.; Gnanam, V.S.; Udunuwara, N.A.; Liyanage, D.S.; Bulathsinhala, N.E.; Adikary, S.; Aluthmuhandiram, J.V.S.; Thanaseelan, C.S.; et al. Detection of bacterial pathogens from clinical specimens using conventional microbial culture and 16S metagenomics: A comparative study. BMC Infect. Dis. 2017, 17, 631. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.-A.; Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Toner, M.; Irimia, D. Blood-on-a-chip. Annu. Rev. Biomed. Eng. 2005, 7, 77–103. [Google Scholar] [CrossRef] [Green Version]
- Iyer, V.; Yang, Z.; Ko, J.; Weissleder, R.; Issadore, D. Advancing microfluidic diagnostic chips into clinical use: A review of current challenges and opportunities. Lab Chip 2022, 22, 3110–3121. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, J.; Song, Y.; Zhou, L.; Zhu, Z.; Yang, C.J. Distance-based microfluidic quantitative detection methods for point-of-care testing. Lab Chip 2016, 16, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.; Quake, S. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 3110–3121. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Lazcka, O.; Del Campo, F.J.; Munoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef]
- Shanholtzer, C.; Schaper, P.; Peterson, L. Concentrated gram stain smears prepared with a cytospin centrifuge. J. Clin. Microbiol. 1982, 16, 1052–1056. [Google Scholar] [CrossRef] [Green Version]
- Gorgannezhad, L.; Stratton, H.; Nguyen, N.-T. Microfluidic-Based Nucleic Acid Amplification Systems in Microbiology. Micromachines 2019, 10, 408. [Google Scholar] [CrossRef] [Green Version]
- Hofer, U. The majority is uncultured. Nat. Rev. Microbiol. 2018, 16, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Erdeiné Kis, Á.; Laczi, K.; Bende, G.; Szilágyi, Á.; Kovács, T.; Perei, K.; Rákhely, G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Bio/Technol. 2020, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, R.M.; Goodacre, R. Characterisation and identification of bacteria using SERS. Chem. Soc. Rev. 2008, 37, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Arshavsky-Graham, S.; Segal, E. Lab-on-a-Chip Devices for Point-of-Care Medical Diagnostics; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lim, Y.C.; Kouzani, A.Z.; Duan, W. Lab-on-a-chip: A component view. Microsyst. Technol. 2010, 16, 1995–2015. [Google Scholar] [CrossRef]
- Bridle, H.; Miller, B.; Desmulliez, M.P. Application of microfluidics in waterborne pathogen monitoring: A review. Water Res. 2014, 55, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Foudeh, A.M.; Didar, T.F.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef]
- Kant, K.; Shahbazi, M.-A.; Dave, V.P.; Ngo, T.A.; Chidambara, V.A.; Than, L.Q.; Bang, D.D.; Wolff, A. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol. Adv. 2018, 36, 1003–1024. [Google Scholar] [CrossRef] [Green Version]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef]
- Zhou, W.; Le, J.; Chen, Y.; Cai, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic devices for bacteria and fungus research. TrAC Trends Anal. Chem. 2019, 112, 175–195. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, H.; Liu, B.; Qiao, L. Detection of pathogenic microorganisms by microfluidics based analytical methods. Anal. Chem. 2018, 90, 5512–5520. [Google Scholar] [CrossRef]
- Zhao, X.; Li, M.; Liu, Y. Microfluidic-based approaches for foodborne pathogen detection. Microorganisms 2019, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic systems for pathogen sensing: A review. Sensors 2009, 9, 4804–4823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, W.; Wang, L.; Lin, J.; Liu, Y. Magnetic Bead Chain-Based Continuous-Flow DNA Extraction for Microfluidic PCR Detection of Salmonella. Micromachines 2021, 12, 384. [Google Scholar] [CrossRef]
- Fang, Y.-L.; Wang, C.-H.; Chen, Y.-S.; Chien, C.-C.; Kuo, F.-C.; You, H.-L.; Lee, M.S.; Lee, G.-B. An integrated microfluidic system for early detection of sepsis-inducing bacteria. Lab Chip 2021, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Zhang, C.; Xing, D. Segmented continuous-flow multiplex polymerase chain reaction microfluidics for high-throughput and rapid foodborne pathogen detection. Anal. Chim. Acta 2014, 826, 51–60. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chang, J.-R.; Hung, S.-C.; Dou, H.-Y.; Lee, G.-B. Rapid molecular diagnosis of live Mycobacterium tuberculosis on an integrated microfluidic system. Sens. Actuators B Chem. 2022, 365, 131968. [Google Scholar] [CrossRef]
- Berger, J.; Aydin, M.Y.; Stavins, R.; Heredia, J.; Mostafa, A.; Ganguli, A.; Valera, E.; Bashir, R.; King, W.P. Portable Pathogen Diagnostics Using Microfluidic Cartridges Made from Continuous Liquid Interface Production Additive Manufacturing. Anal. Chem. 2021, 93, 10048–10055. [Google Scholar] [CrossRef]
- Lin, P.-H.; Li, B.-R. Passively driven microfluidic device with simple operation in the development of nanolitre droplet assay in nucleic acid detection. Sci. Rep. 2021, 11, 21019. [Google Scholar] [CrossRef]
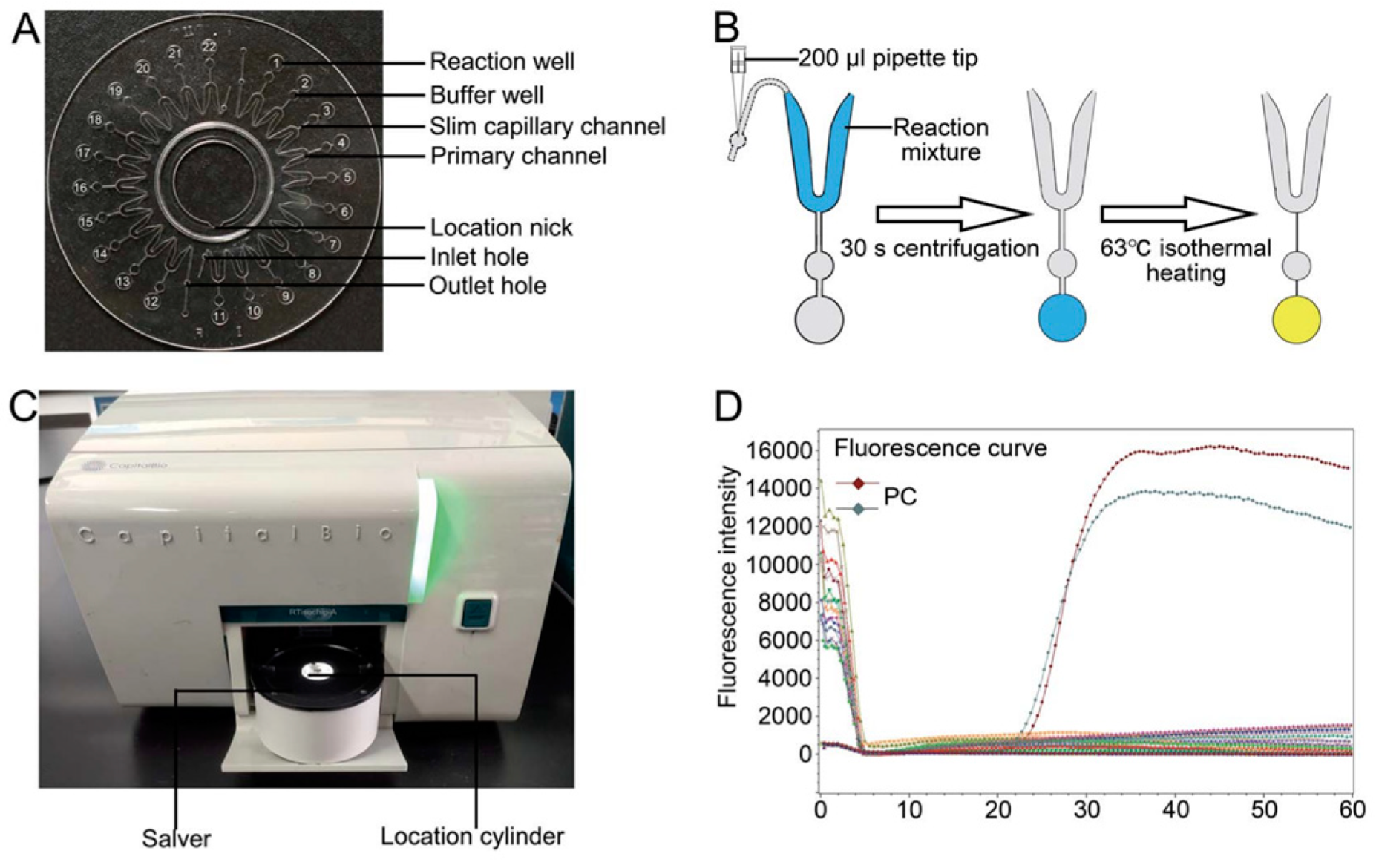
- Seo, J.H.; Park, B.H.; Oh, S.J.; Choi, G.; Kim, D.H.; Lee, E.Y.; Seo, T.S. Development of a high-throughput centrifugal loop-mediated isothermal amplification microdevice for multiplex foodborne pathogenic bacteria detection. Sens. Actuators B Chem. 2017, 246, 146–153. [Google Scholar] [CrossRef]
- Tourlousse, D.M.; Ahmad, F.; Stedtfeld, R.D.; Seyrig, G.; Tiedje, J.M.; Hashsham, S.A. A polymer microfluidic chip for quantitative detection of multiple water- and foodborne pathogens using real-time fluorogenic loop-mediated isothermal amplification. Biomed. Microdevices 2012, 14, 769–778. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Zhao, X.; Du, W.; Feng, X.; Liu, B.-F. A self-contained microfluidic in-gel loop-mediated isothermal amplification for multiplexed pathogen detection. Sens. Actuators B Chem. 2017, 239, 1–8. [Google Scholar] [CrossRef]
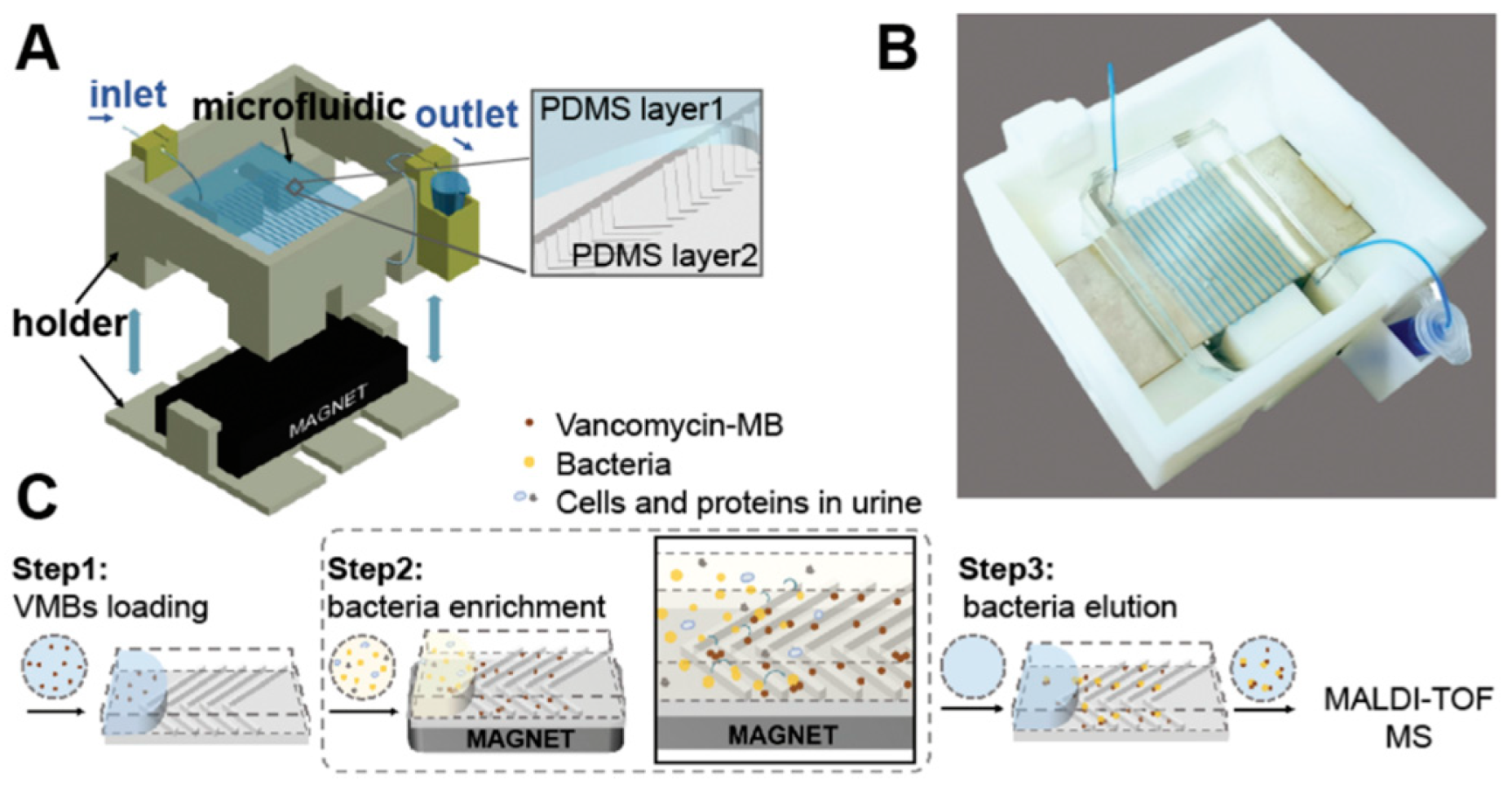
- Shen, Y.; Yi, J.; Song, M.; Li, D.; Wu, Y.; Liu, Y.-J.; Yang, M.; Qiao, L. Highly efficient enrichment and identification of pathogens using a herringbone microfluidic chip and by MALDI-TOF mass spectrometry. Analyst 2021, 146, 4146–4153. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Wu, J. Capture and detection of urine bacteria using a microchannel silicon nanowire microfluidic chip coupled with MALDI-TOF MS. Analyst 2021, 146, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-C.; Chen, Y.-Z.; Lin, S.-J.; Cheng, H.-W.; Wang, J.-K.; Wang, Y.-L.; Han, Y.-Y.; Huang, N.-T. A microfluidic microwell device operated by the automated microfluidic control system for surface-enhanced Raman scattering-based antimicrobial susceptibility testing. Biosens. Bioelectron. 2021, 191, 113483. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-W.; Cheng, H.-W.; Shiue, J.; Wang, J.-K.; Wang, Y.-L.; Huang, N.-T. Antibiotic Susceptibility Test with Surface-Enhanced Raman Scattering in a Microfluidic System. Anal. Chem. 2019, 91, 10988–10995. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lorenzo, L.; Garrido-Maestu, A.; Bhunia, A.K.; Espiña, B.; Prado, M.; Dieguez, L.; Abalde-Cela, S. Gold nanostars for the detection of foodborne pathogens via surface-enhanced Raman scattering combined with microfluidics. ACS Appl. Nano Mater. 2019, 2, 6081–6086. [Google Scholar] [CrossRef]
- Prakash, S.; Yeom, J. Nanofluidics and Microfluidics: Systems and Applications; William Andrew: Norwich, NY, USA, 2014. [Google Scholar]
- Funano, S.-i.; Ota, N.; Tanaka, Y. A simple and reversible glass–glass bonding method to construct a microfluidic device and its application for cell recovery. Lab Chip 2021, 21, 2244–2254. [Google Scholar] [CrossRef]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.; Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 2012, 6, 016505. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Tian, F.; Chang, H.; Zhang, J.; Wang, C.; Rao, W.; Hu, H. Interactions of Bacteria with Monolithic Lateral Silicon Nanospikes Inside a Microfluidic Channel. Front. Chem. 2019, 7, 483. [Google Scholar] [CrossRef] [Green Version]
- Dochow, S.; Beleites, C.; Henkel, T.; Mayer, G.; Albert, J.; Clement, J.; Krafft, C.; Popp, J. Quartz microfluidic chip for tumour cell identification by Raman spectroscopy in combination with optical traps. Anal. Bioanal. Chem. 2013, 405, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Fernández, R.; García, P.; García, J.V.; García, M.; Gamero-Sandemetrio, E.; Reviakine, I.; Arnau, A.; Jiménez, Y. A Multichannel Microfluidic Sensing Cartridge for Bioanalytical Applications of Monolithic Quartz Crystal Microbalance. Biosensors 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Weisgrab, G.; Ovsianikov, A.; Costa, P.F. Functional 3D Printing for Microfluidic Chips. Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar] [CrossRef] [Green Version]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef] [Green Version]
- Morales Navarrete, P.; Yuan, J. A single-layer PDMS chamber for on-chip bacteria culture. Micromachines 2020, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Krafft, B.; Tycova, A.; Urban, R.D.; Dusny, C.; Belder, D. Microfluidic device for concentration and SERS-based detection of bacteria in drinking water. Electrophoresis 2021, 42, 86–94. [Google Scholar] [CrossRef]
- Nam, Y.-H.; Lee, S.-K.; Kim, J.-H.; Park, J.-H. PDMS membrane filter with nano-slit array fabricated using three-dimensional silicon mold for the concentration of particles with bacterial size range. Microelectron. Eng. 2019, 215, 111008. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
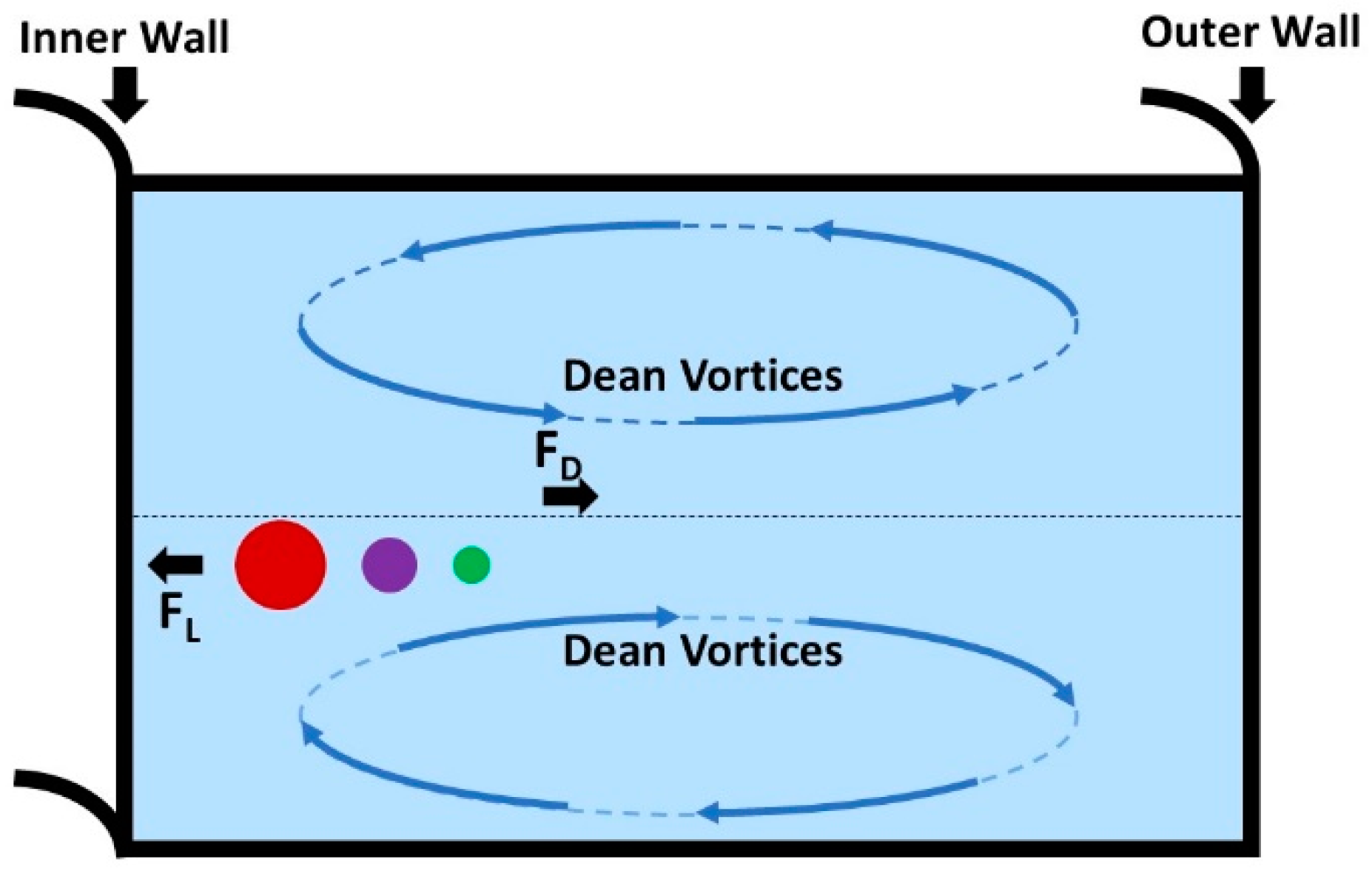
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef]
- Segre, G.; Silberberg, A. Radial particle displacements in Poiseuille flow of suspensions. Nature 1961, 189, 209–210. [Google Scholar] [CrossRef]
- Bretherton, F.P. The motion of rigid particles in a shear flow at low Reynolds number. J. Fluid Mech. 1962, 14, 284–304. [Google Scholar] [CrossRef]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, H.; Ardabili, S.; Faridi, A.M.; Gantelius, J.; Kowalewski, J.M.; Mårtensson, G.; Russom, A. Dean flow-coupled inertial focusing in curved channels. Biomicrofluidics 2014, 8, 034117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-L.; Yao, D.-J. The separation of microalgae using Dean flow in a spiral microfluidic device. Inventions 2018, 3, 40. [Google Scholar] [CrossRef] [Green Version]
- Condina, M.R.; Dilmetz, B.A.; Razavi Bazaz, S.; Meneses, J.; Ebrahimi Warkiani, M.; Hoffmann, P. Rapid separation and identification of beer spoilage bacteria by inertial microfluidics and MALDI-TOF mass spectrometry. Lab Chip 2019, 19, 1961–1970. [Google Scholar] [CrossRef] [Green Version]
- Ahrberg, C.D.; Manz, A.; Chung, B.G. Polymerase chain reaction in microfluidic devices. Lab Chip 2016, 16, 3866–3884. [Google Scholar] [CrossRef] [Green Version]
- Salman, A.; Carney, H.; Bateson, S.; Ali, Z. Shunting microfluidic PCR device for rapid bacterial detection. Talanta 2020, 207, 120303. [Google Scholar] [CrossRef]
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Li, K.T. Analysis of PCR Kinetics inside a Microfluidic DNA Amplification System. Micromachines 2018, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Zhang, Y.; Lin, S.; Wang, T.-H.; Yang, S. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol. Adv. 2011, 29, 830–839. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Xing, D. Fast identification of foodborne pathogenic viruses using continuous-flow reverse transcription-PCR with fluorescence detection. Microfluid. Nanofluidics 2011, 10, 367–380. [Google Scholar] [CrossRef]
- Thomas, S.; Orozco, R.L.; Ameel, T. Thermal gradient continuous-flow PCR: A guide to design. Microfluid. Nanofluidics 2014, 17, 1039–1051. [Google Scholar] [CrossRef]
- Erlich, H.A.; Gelfand, D.; Sninsky, J.J. Recent advances in the polymerase chain reaction. Science 1991, 252, 1643–1651. [Google Scholar] [CrossRef]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef]
- Homann, A.R.; Niebling, L.; Zehnle, S.; Beutler, M.; Delamotte, L.; Rothmund, M.-C.; Czurratis, D.; Beller, K.-D.; Zengerle, R.; Hoffmann, H. A microfluidic cartridge for fast and accurate diagnosis of Mycobacterium tuberculosis infections on standard laboratory equipment. Lab Chip 2021, 21, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Wang, Y.; Yang, N.; Shu, B.; Zhang, G.; Liu, D. A fully automated microfluidic PCR-array system for rapid detection of multiple respiratory tract infection pathogens. Anal. Bioanal. Chem. 2021, 413, 1787–1798. [Google Scholar] [CrossRef]
- Bae, N.H.; Lim, S.Y.; Song, Y.; Jeong, S.W.; Shin, S.Y.; Kim, Y.T.; Lee, T.J.; Lee, K.G.; Lee, S.J.; Oh, Y.-J.; et al. A Disposable and Multi-Chamber Film-Based PCR Chip for Detection of Foodborne Pathogen. Sensors 2018, 18, 3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshiki, M.; Miura, T.; Kazama, S.; Segawa, T.; Ishii, S.; Hatamoto, M.; Yamaguchi, T.; Kubota, K.; Iguchi, A.; Tagawa, T.; et al. Microfluidic PCR Amplification and MiSeq Amplicon Sequencing Techniques for High-Throughput Detection and Genotyping of Human Pathogenic RNA Viruses in Human Feces, Sewage, and Oysters. Front. Microbiol. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Voldman, J. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Eng. 2006, 8, 425–454. [Google Scholar] [CrossRef] [Green Version]
- Braff, W.A.; Willner, D.; Hugenholtz, P.; Rabaey, K.; Buie, C.R. Dielectrophoresis-based discrimination of bacteria at the strain level based on their surface properties. PLoS ONE 2013, 8, e76751. [Google Scholar] [CrossRef]
- Li, Z.; Ju, R.; Sekine, S.; Zhang, D.; Zhuang, S.; Yamaguchi, Y. All-in-one microfluidic device for on-site diagnosis of pathogens based on integrated continuous flow PCR and electrophoresis biochip. Lab Chip 2019, 19, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, P.; Li, Z.; Tao, C.; You, Q.; Sekine, S.; Zhuang, S.; Zhang, D.; Yamaguchi, Y. A continuous flow PCR array microfluidic chip applied for simultaneous amplification of target genes of periodontal pathogens. Lab Chip 2022, 22, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, X.; Xie, X.; Bahnemann, J.; Lin, X.; Wu, X.; Wang, S.; Hoffmann, M.R. Propidium monoazide pretreatment on a 3D-printed microfluidic device for efficient PCR determination of ‘live versus dead’ microbial cells. Environ. Sci. Water Res. Technol. 2018, 4, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Madadelahi, M.; Ghazimirsaeed, E.; Shamloo, A. Design and fabrication of a two-phase diamond nanoparticle aided fast PCR device. Anal. Chim. Acta 2019, 1068, 28–40. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2018, 60, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Cong, H.; Hassan, J.; Gonzalez, G.; Gilchrist, M.D.; Zhang, N. Pathogen detection on microfluidic platforms: Recent advances, challenges, and prospects. Biosens. Bioelectron. X 2022, 10, 100134. [Google Scholar] [CrossRef]
- Wang, X.; Hong, X.-Z.; Li, Y.-W.; Li, Y.; Wang, J.; Chen, P.; Liu, B.-F. Microfluidics-based strategies for molecular diagnostics of infectious diseases. Mil. Med. Res. 2022, 9, 11. [Google Scholar] [CrossRef]
- Azizi, M.; Zaferani, M.; Cheong, S.H.; Abbaspourrad, A. Pathogenic Bacteria Detection Using RNA-Based Loop-Mediated Isothermal-Amplification-Assisted Nucleic Acid Amplification via Droplet Microfluidics. ACS Sens. 2019, 4, 841–848. [Google Scholar] [CrossRef]
- Rane, T.D.; Chen, L.; Zec, H.C.; Wang, T.-H. Microfluidic continuous flow digital loop-mediated isothermal amplification (LAMP). Lab Chip 2015, 15, 776–782. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Guo, X.; Mao, P.; Ren, Y.; Li, Z.; You, M.; Hu, J.; Tian, M.; Yao, C.; Li, F.; et al. A Portable Digital Loop-Mediated Isothermal Amplification Platform Based on Microgel Array and Hand-Held Reader. ACS Sens. 2021, 6, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, Y.; Fang, X.; Liu, Y.; Du, M.; Lu, X.; Li, Q.; Sun, Y.; Ma, J.; Lan, T. Microfluidic-RT-LAMP chip for the point-of-care detection of emerging and re-emerging enteric coronaviruses in swine. Anal. Chim. Acta 2020, 1125, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.; Hu, C.; Wang, Y.; Wang, L.; Peng, F.; Geng, P.; Guan, M. Recent advancements in microfluidic chip biosensor detection of foodborne pathogenic bacteria: A review. Anal. Bioanal. Chem. 2022, 414, 2883–2902. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Duan, L.; Fu, J.; Chai, F.; Zhou, Q.; Wang, Y.; Shao, X.; Wang, L.; Yan, M.; Su, X.; et al. A real-time LAMP-based dual-sample microfluidic chip for rapid and simultaneous detection of multiple waterborne pathogenic bacteria from coastal waters. Anal. Methods 2021, 13, 2710–2721. [Google Scholar] [CrossRef]
- Jiang, X.; Jing, W.; Sun, X.; Liu, Q.; Yang, C.; Liu, S.; Qin, K.; Sui, G. High-Throughput Microfluidic Device for LAMP Analysis of Airborne Bacteria. ACS Sens. 2016, 1, 958–962. [Google Scholar] [CrossRef]
- Papagiannopoulou, C.; Parchen, R.; Rubbens, P.; Waegeman, W. Fast Pathogen Identification Using Single-Cell Matrix-Assisted Laser Desorption/Ionization-Aerosol Time-of-Flight Mass Spectrometry Data and Deep Learning Methods. Anal. Chem. 2020, 92, 7523–7531. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Yin, F.; Qin, Q.; Bi, H.; Liu, B.; Qiao, L. Microfluidic filter device coupled mass spectrometry for rapid bacterial antimicrobial resistance analysis. Analyst 2020, 146. [Google Scholar] [CrossRef]
- Feucherolles, M.; Poppert, S.; Utzinger, J.; Becker, S.L. MALDI-TOF mass spectrometry as a diagnostic tool in human and veterinary helminthology: A systematic review. Parasites Vectors 2019, 12, 245. [Google Scholar] [CrossRef]
- Ha, N.S.; de Raad, M.; Han, L.Z.; Golini, A.; Petzold, C.J.; Northen, T.R. Faster, better, and cheaper: Harnessing microfluidics and mass spectrometry for biotechnology. RSC Chem. Biol. 2021, 2, 1331–1351. [Google Scholar] [CrossRef]
- Rubakhin, S.S.; Greenough, W.T.; Sweedler, J.V. Spatial profiling with MALDI MS: Distribution of neuropeptides within single neurons. Anal. Chem. 2003, 75, 5374–5380. [Google Scholar] [CrossRef]
- Si, T.; Li, B.; Comi, T.J.; Wu, Y.; Hu, P.; Wu, Y.; Min, Y.; Mitchell, D.A.; Zhao, H.; Sweedler, J.V. Profiling of microbial colonies for high-throughput engineering of multistep enzymatic reactions via optically guided matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Chem. Soc. 2017, 139, 12466–12473. [Google Scholar] [CrossRef]
- Hama, B.; Mahajan, G.; Fodor, P.S.; Kaufman, M.; Kothapalli, C.R. Evolution of mixing in a microfluidic reverse-staggered herringbone micromixer. Microfluid. Nanofluidics 2018, 22, 54. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [Green Version]
- Bumbrah, G.S.; Sharma, R.M. Raman spectroscopy–Basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egypt. J. Forensic Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; He, P.; Wang, Z.; Li, G.; Majed, N.; Gu, A.Z. Advances in single cell Raman spectroscopy technologies for biological and environmental applications. Curr. Opin. Biotechnol. 2020, 64, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, U.; Rösch, P.; Popp, J. Raman spectroscopy towards clinical application: Drug monitoring and pathogen identification. Int. J. Antimicrob. Agents 2015, 46, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A. Analytical applications of Raman spectroscopy. Talanta 2008, 76, 1–8. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Z.; Yang, D.; Xie, S.; Jiang, Z.; Niessner, R.; Haisch, C.; Zhou, H.; Sun, P. Bacteria Detection: From Powerful SERS to Its Advanced Compatible Techniques. Adv. Sci. 2020, 7, 2001739. [Google Scholar] [CrossRef]
- Xia, L.; Li, G. Recent progress of microfluidics in surface-enhanced Raman spectroscopic analysis. J. Sep. Sci. 2021, 44, 1752–1768. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Shi, L.; Shao, L.; Fu, P.; Wang, K.; Xiao, R.; Wang, S.; Gu, B. Rapid identification and antibiotic susceptibility test of pathogens in blood based on magnetic separation and surface-enhanced Raman scattering. Microchim. Acta 2019, 186, 475. [Google Scholar] [CrossRef]
- Yang, F.; Chen, L.; Li, D.; Xu, Y.; Li, S.; Wang, L. Printer-assisted array flexible surface-enhanced Raman spectroscopy chip preparation for rapid and label-free detection of bacteria. J. Raman Spectrosc. 2020, 51, 932–940. [Google Scholar] [CrossRef]
- Hou, D.; Maheshwari, S.; Chang, H.-C. Rapid bioparticle concentration and detection by combining a discharge driven vortex with surface enhanced Raman scattering. Biomicrofluidics 2007, 1, 014106. [Google Scholar] [CrossRef] [Green Version]
- Dina, N.E.; Colniță, A.; Marconi, D.; Gherman, A.M.R. Microfluidic Portable Device for Pathogens’ Rapid SERS Detection. Proceedings 2020, 60, 2. [Google Scholar] [CrossRef]
- Escoriza, M.F.; Vanbriesen, J.M.; Stewart, S.; Maier, J. Studying bacterial metabolic states using Raman spectroscopy. Appl. Spectrosc. 2006, 60, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, E.; Niciński, K.; Korsak, D.; Szymborski, T.; Kamińska, A. Sources of variability in SERS spectra of bacteria: Comprehensive analysis of interactions between selected bacteria and plasmonic nanostructures. Anal. Bioanal. Chem. 2019, 411, 2001–2017. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, C.; Chhallani, M.; Bocklitz, T.; Rösch, P.; Popp, J. Simulation of Transportation and Storage and Their Influence on Raman Spectra of Bacteria. Anal. Chem. 2019, 91, 13688–13694. [Google Scholar] [CrossRef]
- Ho, C.-S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 2019, 10, 4927. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.-S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef]
- Wu, J.-M.; Tsai, C.-J.; Ho, T.-W.; Lai, F.; Tai, H.-C.; Lin, M.-T. A Unified Framework for Automatic Detection of Wound Infection with Artificial Intelligence. Appl. Sci. 2020, 10, 5353. [Google Scholar] [CrossRef]
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. 2019, 28, 73–81. [Google Scholar] [CrossRef]
- Zieliński, B.; Plichta, A.; Misztal, K.; Spurek, P.; Brzychczy-Włoch, M.; Ochońska, D. Deep learning approach to bacterial colony classification. PLoS ONE 2017, 12, e0184554. [Google Scholar] [CrossRef]
- Singh, A.; Thakur, N.; Sharma, A. A review of supervised machine learning algorithms. In Proceedings of the 2016 3rd International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 16–18 March 2016; pp. 1310–1315. [Google Scholar]
- Lu, W.; Chen, X.; Wang, L.; Li, H.; Fu, Y.V. Combination of an Artificial Intelligence Approach and Laser Tweezers Raman Spectroscopy for Microbial Identification. Anal. Chem. 2020, 92, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B.; Ali, N.; Bocklitz, T.; Rösch, P.; Popp, J. Discrimination between pathogenic and non-pathogenic E. coli strains by means of Raman microspectroscopy. Anal. Bioanal. Chem. 2020, 412, 8241–8247. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, R.; Klautau, A. In defense of one-vs-all classification. J. Mach. Learn. Res. 2004, 5, 101–141. [Google Scholar]
- Chauhan, V.K.; Dahiya, K.; Sharma, A. Problem formulations and solvers in linear SVM: A review. Artif. Intell. Rev. 2019, 52, 803–855. [Google Scholar] [CrossRef]
- Rahman, A.; Kang, S.; Wang, W.; Huang, Q.; Kim, I.; Vikesland, P.J. Lectin-Modified Bacterial Cellulose Nanocrystals Decorated with Au Nanoparticles for Selective Detection of Bacteria Using Surface-Enhanced Raman Scattering Coupled with Machine Learning. ACS Appl. Nano Mater. 2022, 5, 259–268. [Google Scholar] [CrossRef]
- Abdiansah, A.; Wardoyo, R. Time complexity analysis of support vector machines (SVM) in LibSVM. Int. J. Comput. Appl. 2015, 128, 28–34. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Kanno, N.; Kato, S.; Ohkuma, M.; Matsui, M.; Iwasaki, W.; Shigeto, S. Machine learning-assisted single-cell Raman fingerprinting for in situ and nondestructive classification of prokaryotes. iScience 2021, 24, 102975. [Google Scholar] [CrossRef]
- Ren, Y.; Ji, Y.; Teng, L.; Zhang, H. Using Raman spectroscopy and chemometrics to identify the growth phase of Lactobacillus casei Zhang during batch culture at the single-cell level. Microb. Cell Factories 2017, 16, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobotics 2013, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Liu, W.; Tang, J.-W.; Lyu, J.-W.; Wang, J.-J.; Pan, Y.-C.; Shi, X.-Y.; Liu, Q.-H.; Zhang, X.; Gu, B.; Wang, L. Discrimination between carbapenem-resistant and carbapenem-sensitive Klebsiella pneumoniae strains through computational analysis of surface-enhanced raman spectra: A pilot study. Microbiol. Spectr. 2022, 10, e02409–e02421. [Google Scholar] [CrossRef]
- Tang, J.-W.; Liu, Q.-H.; Yin, X.-C.; Pan, Y.-C.; Wen, P.-B.; Liu, X.; Kang, X.-X.; Gu, B.; Zhu, Z.-B.; Wang, L. Comparative analysis of machine learning algorithms on surface enhanced raman spectra of clinical Staphylococcus species. Front. Microbiol. 2021, 12, 696921. [Google Scholar] [CrossRef]
- Ciloglu, F.U.; Saridag, A.M.; Kilic, I.H.; Tokmakci, M.; Kahraman, M.; Aydin, O. Identification of methicillin-resistant Staphylococcus aureus bacteria using surface-enhanced Raman spectroscopy and machine learning techniques. Analyst 2020, 145, 7559–7570. [Google Scholar] [CrossRef]
- Taunk, K.; De, S.; Verma, S.; Swetapadma, A. A brief review of nearest neighbor algorithm for learning and classification. In Proceedings of the 2019 International Conference on Intelligent Computing and Control Systems (ICCS), Madurai, India, 15–17 May 2019; pp. 1255–1260. [Google Scholar]
- Krogh, A. What are artificial neural networks? Nat. Biotechnol. 2008, 26, 195–197. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, S.; Athaiya, A. Activation functions in neural networks. Towards Data Sci. 2017, 6, 310–316. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, B.; Topatana, W.; Cao, J.; Zhu, H.; Juengpanich, S.; Mao, Q.; Yu, H.; Cai, X. Classification and mutation prediction based on histopathology H&E images in liver cancer using deep learning. NPJ Precis. Oncol. 2020, 4, 14. [Google Scholar]
- Salehinejad, H.; Sankar, S.; Barfett, J.; Colak, E.; Valaee, S. Recent advances in recurrent neural networks. arXiv 2017, arXiv:1801.01078. [Google Scholar]
- Gadkar, V.J.; Goldfarb, D.M.; Gantt, S.; Tilley, P.A.G. Real-time Detection and Monitoring of Loop Mediated Amplification (LAMP) Reaction Using Self-quenching and De-quenching Fluorogenic Probes. Sci. Rep. 2018, 8, 5548. [Google Scholar] [CrossRef] [PubMed]


| Identification Technique | Device Details | Target Organism | Limit of Detection (LoD) | Reaction Time | Sample Input and Volume | Reference |
|---|---|---|---|---|---|---|
| PCR | PDMS multiplex microfluidic PCR chip | Salmonella | 100 CFU/mL | ~47 min | Extracted DNA–84 μL | [34] |
| Integrated PDMS microfluidic chip with membrane-based filteration module, bacterial-capture module utilizing a micro-mixer with FcMBL-coated magnetic beads, and multiplex PCR module | Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus saprophyticus | 1–5 CFU/mL | 4 h | Bacteria inoculated human blood samples–5.4 mL | [35] | |
| Coiled PTFE capillary tube with multiplex segmented continuous-flow PCR | Salmonella enterica, Listeria monocytogenes, Escherichia coli O157:H7, Staphylococcus aureus | 100 gene copies/mL | ~19 min | Extracted DNA from artifically contaminated food samples–25 μL | [36] | |
| Integrated PDMS microfluidic chip with 12 singleplex reaction chambers | Mycobacterium tuberculosis | 100 CFU | 90 min | Bacteria inoculated ddH2O, 1xPBS, normal saline (0.9%NaCl), sputum, and whole blood–20 μL | [37] | |
| LAMP | Continuous liquid interface production (CLIP) -based AM PTFE capillary cartridge with integrated LAMP | Escherichia coli | 50 CFU/mL | 40–50 min | Bacteria inoculated whole blood samples–8 μL | [38] |
| PDMS and capillary channel-based microfluidic chip with singleplex LAMP integration | Escherichia coli malB | 1 pg/mL | ~60 min | Extracted DNA–60 nL | [39] | |
| PMMA spiral microchannel with 24 multiplex LAMP reaction chambers | Escherichia coli O157:H7, Salmonella typhimurium, Vibrio parahaemolyticus | 500 gene copies/reaction | ~60 min | Extracted DNA–75 mL | [40] | |
| Cyclo olefin polymer (COP)-based chip containing a straight microchannel connected by 15 multiplex LAMP reaction wells | Salmonella, Campylobacter jejuni, Shigella, Vibrio cholerae | 10–100 genomes/mL | ~20 min | Extracted DNA–15 μL | [41] | |
| PDMS channel for sample delivery to mutiplex LAMP reaction chambers | Escherichia coli, Proteus hauseri, Vibrio parahaemolyticus, Salmonella subsp. Enterica | ~3 copies/mL | ~120 min | Bacteria inoculated solution–600 nL | [42] | |
| MALDI-ToF MS | Repetitive PDMS herringbone channel containing vancomycin modified magnetic beads with off-chip MALDI-ToF MS | Staphylococcus aureus, Staphylococcus hominis, Staphylococcus epidermidis, Enterococcus gallinarum | 104–105 CFU/mL | 90 min | Bacteria inoculated solution–250 μL | [43] |
| Microchannel silicon nanowire (McSiNW) microfluidic chip with off-chip MALDI-ToF MS | Escherichia coli (cultured and uncultured) | 103–106 CFU/mL | 60 min | Bacteria inoculated urine–500 μL | [44] | |
| Raman Spectroscopy | PDMS microfluidic microwell device with bonded SERS substrate | Escherichia coli | 108 CFU/mL | 3.5 hrs | Bacteria inoculated solution–5 mL | [45] |
| Membrane filtration-based PMMA microfluidic with SERS-active substrate | Escherichia coli, Staphylococcus aureus | 103 CFU/mL | 30 min | Bacteria inoculated solution–10 mL | [46] | |
| PDMS microchannel with SERS functionalized components | Listeria monocytogenes, Listeria innocua | 105 CFU/mL | 30 min | Combined mixture of bacteria and SERS-tagged gold nanostars | [47] |
| AI Algorithm | Target Organism | Accuracy | Methodology | Considerations | Reference |
|---|---|---|---|---|---|
| Support Vector Machince (SVM) | Escherichia coli | 81.1% | Use hyperplane optimization to demarcate between class data | Not inherently designed for multi-class (2+) classification | [126,128] |
| Random Forests (RFs) | 3 bacterial and 3 archaeal species | 98.9% | Average of multiple decision trees trained on random subsets of training data | Lack of interpretability and tendency to overfit model | [133,134] |
| k-nearest-neighbors (KNN) | 10 methicillin-resistant S. aureus, 6 methicillin-sensitive S. aureus, and 6 L. pneumophila isolates | 97.8% | Maps high dimensional data to a higher dimensional space and define class members based on proximity by a distance measure | Optimization of k along with computational complexity requires extended effort | [139,140] |
| Gradient Boosted Machines (GBM) | 15 strains of Klebsiella pneumoniae based on Carbapenem resistance | 99.40% | Apply loss function to a base learner (decision tree, regression model, etc.) and repeat training until loss function reaches minima | Computational complexity due to number of iterations needed to minimize loss function | [137] |
| Convolutional Neural Networks (CNN) | 30 species and strains of various bacteria | 89.1% | Model neuronal connections based on activation function for input classification | Complex theory behind neural networks requires expert knowledge before use | [119] |
| Identification Technique | Advantages | Disadvantages |
|---|---|---|
| PCR | Small amount of biomass required for amplification and detection [73], Portability enables rapid PoC testing [74,75] | Complex fabrication due to thermocycler utilization [90,91], prior sample preparation often required, potential for false positive results [85], inefficient discrimination between viable and nonviable cells [84] |
| LAMP | Small amount of biomass required for amplification and detection, high operating speed, eliminates the necessity for a thermocycler [90,91] | Difficult thermoregulation [88,89], prior sample preparation often required [145], inaccurate fluorescent dye detection creates potential for false positive results [145] |
| MALDI-ToF MS | Does not require amplification of genetic material [6], high specificity for identification [98] | Large amount of biomass required for detection [98], Difficult to integrate on chip [101] |
| Raman Spectroscopy | Non-destructive to samples, real-time acquisition without need for extensive sample manipulation, acquisition in confined spaces | Low efficiency of Raman scattering makes measurements harder [109], application hindered by variable bacterial growth conditions [116], causing metabolomic changes in bacteria that can result in variations in spectral reading |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, F.; Kesterson, D.; Lei, K.; Hord, C.; Patel, A.; Kaffenes, A.; Congivaram, H.; Prakash, S. Application of Microfluidics for Bacterial Identification. Pharmaceuticals 2022, 15, 1531. https://doi.org/10.3390/ph15121531
Daniel F, Kesterson D, Lei K, Hord C, Patel A, Kaffenes A, Congivaram H, Prakash S. Application of Microfluidics for Bacterial Identification. Pharmaceuticals. 2022; 15(12):1531. https://doi.org/10.3390/ph15121531
Chicago/Turabian StyleDaniel, Fraser, Delaney Kesterson, Kevin Lei, Catherine Hord, Aarti Patel, Anastasia Kaffenes, Harrshavasan Congivaram, and Shaurya Prakash. 2022. "Application of Microfluidics for Bacterial Identification" Pharmaceuticals 15, no. 12: 1531. https://doi.org/10.3390/ph15121531





