Synthetic Derivatives against Wild-Type and Non-Wild-Type Sporothrix brasiliensis: In Vitro and In Silico Analyses
Abstract
:1. Introduction
2. Results
2.1. Clinical Epidemiological Data
2.2. Antifungal Susceptibility Assay
2.3. The In Silico Toxicological Profile and Pharmacokinetics
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates
4.2. Growth Conditions
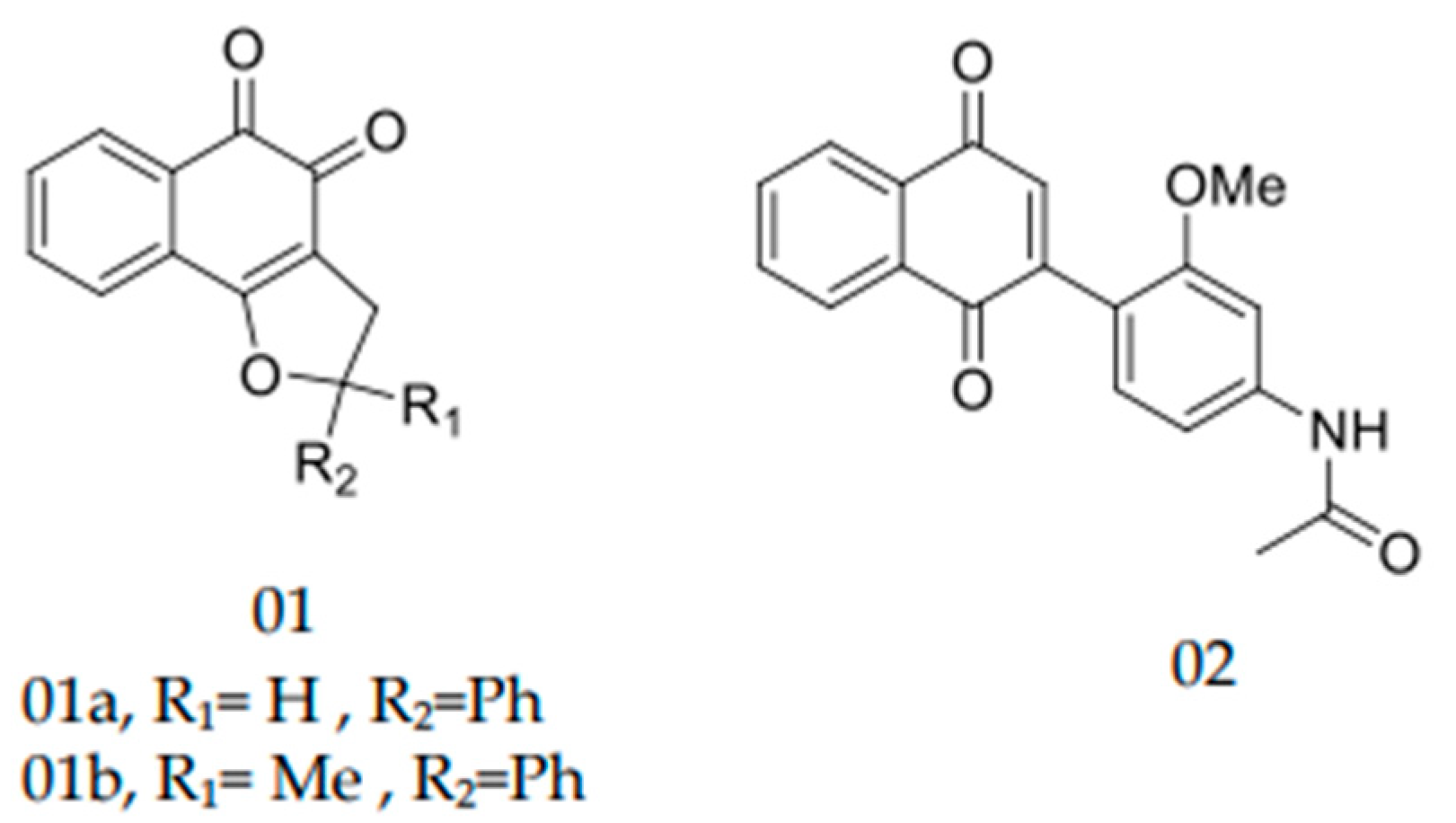
4.3. Synthetic Derivatives
4.4. Antifungal Susceptibility Assays
4.5. In Silico Toxicity and Pharmacological Profiles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gremião, I.D.F.; Miranda, L.H.M.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Rocha, E.M.D.S.D.; Montenegro, H.; Carneiro, A.J.B.; Xavier, M.O.; de Farias, M.R.; Monti, F.; Mansho, W.; Pereira, R.H.D.M.A.; Pereira, S.A.; et al. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz. J. Microbiol. 2021, 52, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Macêdo-Sales, P.A.; Souto, S.R.L.S.; Destefani, C.A.; Lucena, R.P.; Machado, R.L.D.; Pinto, M.R.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Rocha, E.M.S.; Baptista, A.R.S. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: A comparison between infected and non-infected populations. BMC Vet. Res. 2018, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etchecopaz, A.; Scarpa, M.; Mas, J.; Cuestas, M.L. Sporothrix brasiliensis: A growing hazard in the Northern area of Buenos Aires Province? Rev. Argent. Microbiol. 2020, 52, 350–351. [Google Scholar] [CrossRef]
- Etchecopaz, A.N.; Lanza, N.; Toscanini, M.A.; Devoto, T.B.; Pola, S.J.; Daneri, G.L.; Iovannitti, C.A.; Cuestas, M.L. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: Case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J. Mycol. Med. 2020, 30, 100908. [Google Scholar] [CrossRef] [PubMed]
- Etchecopaz, A.; Toscanini, M.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.; Bendezú, K.; Nusblat, A.; Iachini, R.; Cuestas, M. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.E.; Suarez, J.; Moreno, J.; Vallee, J.; Moreno, J.P. Zoonotic Sporotrichosis Related to Cat Contact: First Case Report from Panama in Central America. Cureus 2018, 10, e2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García Duarte, J.M.; Wattiez Acosta, V.R.; Fornerón Viera, P.M.L.; Aldama Caballero, A.; Gorostiaga Matiauda, G.A.; Rivelli de Oddone, V.B.; Pereira Brunell, J.G. Esporotricosis trasmitida por gato doméstico. Reporte de un caso familiar. Rev. Nac. 2017, 9, 67–76. [Google Scholar] [CrossRef]
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Ferreira Gremião, I.D.; Pereira, S.A. A One Health Approach to Combatting Sporothrix brasiliensis: Narrative Review of an Emerging Zoonotic Fungal Pathogen in South America. J. Fungi 2020, 6, 247. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Oliveira, M.M.E.; De Miranda, L.H.M.; Freitas, D.F.S.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Brilhante, R.S.N.; Rodrigues, A.M.; Sidrim, J.J.C.; Rocha, M.F.G.; Pereira, S.A.; Gremião, I.D.F.; Schubach, T.M.P.; de Camargo, Z.P. In vitrosusceptibility of antifungal drugs againstSporothrix brasiliensisrecovered from cats with sporotrichosis in Brazil: Table 1. Med. Mycol. 2016, 54, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Nakasu, C.C.T.; Waller, S.B.; Ripoll, M.K.; Ferreira, M.R.A.; Conceição, F.R.; Gomes, A.D.R.; Osório, L.D.G.; de Faria, R.O.; Cleff, M.B. Feline sporotrichosis: A case series of itraconazole-resistant Sporothrix brasiliensis infection. Braz. J. Microbiol. 2021, 52, 163–171. [Google Scholar] [CrossRef]
- Pereira, S.A.; Schubach, T.M.P.; Gremião, I.D.F.; Da Silva, D.T.; Figueiredo, F.B.; De Assis, N.V.; Passos, S.R.L. Aspectos terapêuticos da esporotricose felina. Acta Sci. Vet. 2018, 37, 311–321. [Google Scholar] [CrossRef]
- Waller, S.B.; Ripoll, M.K.; Madrid, I.M.; Acunha, T.; Cleff, M.B.; Chaves, F.C.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Susceptibility and resistance of Sporothrix brasiliensis to branded and compounded itraconazole formulations. Braz. J. Microbiol. 2021, 52, 155–162. [Google Scholar] [CrossRef]
- Waller, S.B.; Lana, D.F.D.; Quatrin, P.M.; Ferreira, M.R.A.; Fuentefria, A.M.; Mezzari, A. Antifungal resistance on Sporothrix species: An overview. Braz. J. Microbiol. 2021, 52, 73–80. [Google Scholar] [CrossRef]
- Borba-Santos, L.P.; Rodrigues, A.M.; Gagini, T.B.; Fernandes, G.F.; Castro, R.; de Carmargo, Z.P.; Nucci, M.; Lopes-Bezerra, L.M.; Ishida, K.; Rozental, S. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med. Mycol. 2015, 53, 178–188. [Google Scholar] [CrossRef]
- Macêdo-Sales, P.A.; Souza, L.O.P.; Della-Terra, P.P.; Lozoya-Pérez, N.E.; Machado, R.L.D.; Rocha, E.M.D.S.D.; Lopes-Bezerra, L.M.; Guimarães, A.J.; Rodrigues, A.M.; Mora-Montes, H.M.; et al. Coinfection of domestic felines by distinct Sporothrix brasiliensis in the Brazilian sporotrichosis hyperendemic area. Fungal Genet. Biol. 2020, 140, 103397. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Cássia Pires, D.; Brihante, R.S.N.; da Costa Sidrim, J.J.; Gadelha, M.F.; Colombo, A.L.; de Camargo, Z.P. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect. Dis. 2014, 14, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchotene, K.O.; Brandolt, T.M.; Klafke, G.B.; Poester, V.R.; Xavier, M.O. In vitro susceptibility of Sporothrix brasiliensis: Comparison of yeast and mycelial phases. Med. Mycol. 2017, 55, 869–876. [Google Scholar] [CrossRef]
- Forezi, L.; Borba-Santos, L.P.; Cardoso, M.F.C.; Ferreira, V.F.; Rozental, S.; Silva, F.D.C.D. Synthesis and Antifungal Activity of Coumarins Derivatives against Sporothrix spp. Curr. Top. Med. Chem. 2018, 18, 164–171. [Google Scholar] [CrossRef]
- Garcia Ferreira, P.; Pereira Borba-Santos, L.; Noronha, L.L.; Deckman Nicoletti, C.; de Sá Haddad Queiroz, M.; de Carvalho da Silva, F.; Rozental, S.; Omena Futuro, D.; Francisco Ferreira, V. Synthesis, Stability Studies, and Antifungal Evaluation of Substituted α- and β-2,3-Dihydrofuranaphthoquinones against Sporothrix brasiliensis and Sporothrix schenckii. Molecules 2019, 24, 930. [Google Scholar] [CrossRef] [Green Version]
- Mathias, L.; Almeida, J.; Passoni, L.; Gossani, C.; Taveira, G.; Gomes, V.; Motta, O. Antifungal activity of silver salts of Keggin-type heteropolyacids against Sporothrix spp. J. Microbiol. Biotechnol. 2020, 30, 540–551. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Abreu, D.P.B.; Almeida-Paes, R.; Brilhante, R.S.N.; Chakrabarti, A.; Chowdhary, A.; Hagen, F.; Córdoba, S.; Gonzalez, G.M.; Govender, N.P.; et al. Multicenter, International Study of MIC/MEC Distributions for Definition of Epidemiological Cutoff Values for Sporothrix Species Identified by Molecular Methods. Antimicrob. Agents Chemother. 2017, 61, e01057-17. [Google Scholar] [CrossRef] [Green Version]
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests, NCCLS document M2-A8, Approved Standard—Eighth ed.; NCCLS: Wayne, PA, USA, 2003; ISBN 1-56238-485-6. [Google Scholar]
- Almeida-Paes, R.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; Machado, A.C.S.; Oliveira, M.M.E.; Pereira, S.A.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem. Inst. Oswaldo Cruz 2017, 112, 376–381. [Google Scholar] [CrossRef]
- Riffel, A.; Medina, L.; Stefani, V.; Santos, R.; Bizani, D.; Brandelli, A. In vitro antimicrobial activity of a new series of 1,4-naphthoquinones. Braz. J. Med Biol. Res. 2002, 35, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.K.; Chhor, R.B.; Singh, R.V.; Rai, S.; Yadav, D.B. Design, synthesis and evaluation of novel 1,4-naphthoquinone derivatives as antifungal and anticancer agents. Bioorganic Med. Chem. Lett. 2004, 14, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Louvis, A.D.R.; Silva, N.A.A.; Semaan, F.S.; Silva, F.D.C.D.; Saramago, G.; de Souza, L.C.S.V.; Ferreira, B.L.A.; Castro, H.C.; Salles, J.P.; Souza, A.L.A.; et al. Synthesis, characterization and biological activities of 3-aryl-1,4-naphthoquinones—Green palladium-catalysed Suzuki cross coupling. New J. Chem. 2016, 40, 7643–7656. [Google Scholar] [CrossRef]
- Tandon, V.K.; Maurya, H.K.; Mishra, N.N.; Shukla, P.K. Micelles catalyzed chemoselective synthesis ‘in water’ and biological evaluation of oxygen containing hetero-1,4-naphthoquinones as potential antifungal agents. Bioorganic Med. Chem. Lett. 2011, 21, 6398–6403. [Google Scholar] [CrossRef]
- Tandon, V.K.; Yadav, D.B.; Maurya, H.K.; Chaturvedi, A.K.; Shukla, P.K. Design, synthesis, and biological evaluation of 1,2,3-trisubstituted-1,4-dihydrobenzo[g]quinoxaline-5,10-diones and related compounds as antifungal and antibacterial agents. Bioorg. Med. Chem. 2006, 14, 6120–6126. [Google Scholar] [CrossRef]
- Janeczko, M.; Kubiński, K.; Martyna, A.; Muzyczka, A.; Boguszewska-Czubara, A.; Czernik, S.; Tokarska-Rodak, M.; Chwedczuk, M.; Demchuk, O.M.; Golczyk, H.; et al. 1,4-Naphthoquinone derivatives potently suppress Candida Albicans growth, inhibit formation of hyphae and show no toxicity toward zebrafish embryos. J. Med. Microbiol. 2018, 67, 598–609. [Google Scholar] [CrossRef]
- Chandra, S.; Vandana; Kumar, S. Synthesis, spectroscopic, anticancer, antibacterial and antifungal studies of Ni(II) and Cu(II) complexes with hydrazine carboxamide, 2-[3-methyl-2-thienyl methylene]. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 356–363. [Google Scholar] [CrossRef]
- Casanova, B.B.; Muniz, M.N.; De Oliveira, T.; De Oliveira, L.F.; Machado, M.M.; Fuentefria, A.M.; Gosmann, G.; Gnoatto, S.C.B. Synthesis and Biological Evaluation of Hydrazone Derivatives as Antifungal Agents. Molecules 2015, 20, 9229–9241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, R.D.A.; de Melo, C.V.S.; Marques, F.J.D.F.; Serpa, R.; Evangelista, A.J.D.J.; Caetano, E.P.; Mafezoli, J.; Oliveira, M.D.C.F.D.; da Silva, M.R.; Bandeira, T.D.J.P.G.; et al. Synthesis and in vitro antifungal activity of isoniazid-derived hydrazones against Coccidioides posadasii. Microb. Pathog. 2016, 98, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Secci, D.; Bizzarri, B.; Bolasco, A.; Carradori, S.; D’Ascenzio, M.; Rivanera, D.; Mari, E.; Polletta, L.; Zicari, A. Synthesis, anti-Candida activity, and cytotoxicity of new (4-(4-iodophenyl)thiazol-2-yl)hydrazine derivatives. Eur. J. Med. Chem. 2012, 53, 246–253. [Google Scholar] [CrossRef]
- Carvalho, P.H.D.A.; Duval, A.R.; Leite, F.R.M.; Nedel, F.; Cunico, W.; Lund, R.G. (7-Chloroquinolin-4-yl)arylhydrazones: C andida albicans enzymatic repression and cytotoxicity evaluation, Part 2. J. Enzym. Inhib. Med. Chem. 2015, 31, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Abu-Melha, S.; Gomha, S.; Abouzied, A.; Edrees, M.; Dena, A.A.; Muhammad, Z. Microwave-Assisted One Pot Three-Component Synthesis of Novel Bioactive Thiazolyl-Pyridazinediones as Potential Antimicrobial Agents against Antibiotic-Resistant Bacteria. Molecules 2021, 26, 4260. [Google Scholar] [CrossRef]
- Hadni, H.; Elhallaoui, M. 3D-QSAR, docking and ADMET properties of aurone analogues as antimalarial agents. Heliyon 2020, 6, e03580. [Google Scholar] [CrossRef]
- Ortiz, C.L.; Completo, G.C.; Nacario, R.C.; Nellas, R.B. Potential Inhibitors of Galactofuranosyltransferase 2 (GlfT2): Molecular Docking, 3D-QSAR, and In Silico ADMETox Studies. Sci. Rep. 2019, 9, 17096. [Google Scholar] [CrossRef] [PubMed]
- Larregieu, C.A.; Benet, L.Z. Drug Discovery and Regulatory Considerations for Improving In Silico and In Vitro Predictions that Use Caco-2 as a Surrogate for Human Intestinal Permeability Measurements. AAPS J. 2013, 15, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.K.; Al-Attraqchi, O.; Prasad, M.R.; Tekade, R.K. Protein and Tissue Binding: Implication on Pharmacokinetic Parameters. In Dosage Form Design Considerations; Elsevier: Amsterdam, The Netherlands, 2018; Volume I, ISBN 9780128144244. [Google Scholar]
- Mikov, M.; Đanić, M.; Pavlović, N.; Stanimirov, B.; Golocorbin-Kon, S.; Stankov, K.; Al-Salami, H. The Role of Drug Metabolites in the Inhibition of Cytochrome P450 Enzymes. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 881–890. [Google Scholar] [CrossRef]
- Correia, M.A.; Monteflano, P.R.O. Inhibition of cytochrome P450 enzymes. In Cytochrome P450; Ortiz de Montellano, P.R., Ed.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-12107-9. [Google Scholar]
- de Oliveira Moraes, A.D.T.; de Miranda, M.D.S.; Jacob, Í.T.T.; da Cruz Amorim, C.A.; de Moura, R.O.; da Silva, S.Â.S.; Soares, M.B.P.; de Almeida, S.M.V.; de Lima Souza, T.R.C.; de Oliveira, J.F.; et al. Synthesis, in vitro and in vivo biological evaluation, COX-1/2 inhibition and molecular docking study of indole-N-acylhydrazone derivatives. Bioorg. Med. Chem. 2018, 26, 5388–5396. [Google Scholar] [CrossRef]
- Nakajima, M.; Yoshida, R.; Shimada, N.; Yamazaki, H.; Yokoi, T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab. Dispos. 2001, 29, 1110–1113. [Google Scholar]
- Hu, B.; Joseph, J.; Geng, X.; Wu, Y.; Suleiman, M.R.; Liu, X.; Shi, J.; Wang, X.; He, Z.; Wang, J.; et al. Refined pharmacophore features for virtual screening of human thromboxane A2 receptor antagonists. Comput. Biol. Chem. 2020, 86, 107249. [Google Scholar] [CrossRef]
- Björnsson, E.S. Hepatotoxicity by Drugs: The Most Common Implicated Agents. Int. J. Mol. Sci. 2016, 17, 224. [Google Scholar] [CrossRef] [Green Version]
- Vedani, A.; Smieško, M. In Silico Toxicology in Drug Discovery—Concepts Based on Three-dimensional Models. Altern. Lab. Anim. 2009, 37, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.; Siramshetty, V.B.; Drwal, M.N.; Preissner, R. Computational methods for prediction of in vitro effects of new chemical structures. J. Cheminform. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Oliveira, D.S. de Avaliação Clínico-Epidemiológica e Perfil de Sensibilidade a Antifúngicos de Sporothrix brasiliensis Isolados a Partir de Felinos Domésticos do Estado do Rio de Janeiro. Master’s Thesis, Universidade Federal Fluminense, Faculdade de Medicina Veterinária, Rio de Janerio, Brazil, 2016. [Google Scholar]
- Novais, J.S.; Rosandiski, A.C.; De Carvalho, C.M.; Silva, L.S.D.S.; Souza, L.C.D.S.V.D.; Santana, M.V.; Martins, N.R.C.; Castro, H.C.; Ferreira, V.F.; Gonzaga, D.T.G.; et al. Efficient Synthesis and Antibacterial Profile of Bis(2-hydroxynaphthalene- 1,4-dione). Curr. Top. Med. Chem. 2020, 20, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; Von Korff, M.; Reich, J.R.; Rufener, C. OSIRIS, an Entirely in-House Developed Drug Discovery Informatics System. J. Chem. Inf. Model. 2009, 49, 232–246. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]



| Clinical/Epidemiological | Wild Type | Non-Wild Type | ||||
|---|---|---|---|---|---|---|
| WT1 | WT2 | WT3 | NWT1 | NWT2 | NWT3 | |
| Age (years) | 10 | 5 | 2.5 | 2 | 1 | 1 |
| Sex | M | F | M | M | M | F |
| Castration | Yes | No | No | Yes | No | Yes |
| Free roaming | No | Yes | Yes | Yes | Yes | Yes |
| Anatomical site | Abdomen, head, chest and pelvic limb | Head and neck | Head, thoracic and pelvic limb | Base of tail and paw | Head | Pelvic limb |
| Relapse | No | No | Yes | Yes | No | No |
| Duration of ITC treatment (months) | 60 | NI | 8 | >120 | NI | 2 |
| Azole/Hydrazones | ATCC/Clinical Isolates | |||||||
|---|---|---|---|---|---|---|---|---|
| S. bra | WT 1 | WT 2 | WT 3 | NWT 1 | NWT 2 | NWT 3 | ||
| ITC | MIC | 2 | 1 | 4 | 1 | 32 | 16 | 8 |
| MFC | 16 | 8 | 32 | 8 | >128 | 128 | 128 | |
| H1 | MIC | 8 | 2 | 4 | 2 | 1 | 8 | 8 |
| MFC | 4 | 2 | 8 | 2 | 2 | 16 | 8 | |
| H2 | MIC | 8 | 16 | 16 | 8 | 8 | 16 | 8 |
| MFC | 8 | 16 | 16 | 8 | 16 | 16 | 16 | |
| H3 | MIC | 4 | 2 | 4 | 16 | 1 | 16 | 1 |
| MFC | 4 | 4 | 8 | 16 | 1 | 16 | 1 | |
| Azole/Hydrazones | ATCC/Clinical Isolates | |||
|---|---|---|---|---|
| S. bra | WT GM | NWT GM | ||
| ITC | MIC | 2 | 2 | 18.6 |
| MFC | 16 | 16 | 128 | |
| H1 | MIC | 8 | 2.7 | 5.7 |
| MFC | 4 | 4 | 8.7 | |
| H2 | MIC | 8 | 13.3 | 10.7 |
| MFC | 8 | 13.3 | 16 | |
| H3 | MIC | 4 | 7.3 | 6 |
| MFC | 4 | 9.3 | 6 | |
| Azole/Quinones | ATCC/Clinical Isolates | |||||||
|---|---|---|---|---|---|---|---|---|
| S. bra | WT 1 | WT 2 | WT 3 | NWT 1 | NWT 2 | NWT 3 | ||
| Itraconazole | MIC | 2 | 1 | 4 | 1 | 32 | 16 | 8 |
| MFC | 16 | 8 | 32 | 8 | >128 | 128 | 128 | |
| Q1 | MIC | >128 | 32 | >128 | NA | 128 | NA | 64 |
| MFC | >128 | 32 | >128 | NA | 128 | NA | 64 | |
| Q2 | MIC | >128 | 64 | NA | NA | >128 | NA | 64 |
| MFC | >128 | 64 | NA | NA | >128 | NA | 128 | |
| Q3 | MIC | >128 | 64 | NA | NA | >128 | NA | 128 |
| MFC | >128 | 128 | NA | NA | >128 | NA | 128 | |
| Q4 | MIC | >128 | 128 | NA | NA | >128 | >128 | 128 |
| MFC | >128 | 128 | NA | NA | >128 | >128 | 128 | |
| Q5 | MIC | >128 | NA | NA | NA | NA | NA | 128 |
| MFC | >128 | NA | NA | NA | NA | NA | 128 | |
| Q6 | MIC | NA | 128 | NA | NA | NA | NA | 64 |
| MFC | NA | 128 | NA | NA | NA | NA | 64 | |
| Q7 | MIC | NA | 128 | NA | NA | NA | NA | 64 |
| MFC | NA | 128 | NA | NA | NA | NA | 64 | |
| Q8 | MIC | 32 | 128 | >128 | NA | 32 | >128 | 64 |
| MFC | 64 | 128 | >128 | NA | 32 | >128 | 64 | |
| Q9 | MIC | >128 | 128 | NA | NA | NA | NA | 128 |
| MFC | >128 | 128 | NA | NA | NA | NA | 128 | |
| Q10 | MIC | 128 | 128 | NA | NA | NA | NA | 128 |
| MFC | 128 | 128 | NA | NA | NA | NA | 128 | |
| Q11 | MIC | 128 | 64 | NA | NA | NA | NA | 128 |
| MFC | 128 | 64 | NA | NA | NA | NA | 128 | |
| Compounds | Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oral Rat Acute Toxicity (LD50) | Oral Rat Chronic Toxicity (LOAEL) | Minnow Toxicity | HERG I | HERG II | Hepatotoxicity | Toxicological End Points | ||||
| Immunotoxicity | Carcinogenicity | Cytotoxicity | Mutagenicity | |||||||
| Numeric (mol/kg) | Numeric (log mg/kg_bw/day) | Numeric (log LC 50) | Categorical (Yes/No) | Categorical (Active/Inactive) | ||||||
| Itraconazole | 2.966 | 0.055 | −4.446 | No | Yes | Yes | Yes | No | No | No |
| Q1 | 1.952 | 2.322 | −2.223 | No | Yes | Yes | No | No | No | No |
| Q2 | 2.098 | 2.398 | −2.889 | No | Yes | Yes | No | No | No | No |
| Q3 | 2.245 | 2.381 | −2.098 | No | Yes | Yes | No | No | No | No |
| Q4 | 2.580 | 1.549 | −1.846 | No | Yes | Yes | Yes | No | No | Yes |
| Q5 | 2.577 | 1.581 | −1.211 | No | Yes | Yes | No | No | No | Yes |
| Q6 | 2.972 | 1.221 | 0.919 | No | Yes | No | No | No | No | No |
| Q7 | 2.965 | 2.426 | 0.274 | No | Yes | No | No | No | No | No |
| Q8 | 2.711 | 2.974 | −6.407 | No | Yes | Yes | No | No | No | No |
| Q9 | 2.844 | 1.724 | −0.550 | No | No | Yes | No | No | No | No |
| Q10 | 2.905 | 1.578 | −1.852 | No | Yes | Yes | No | No | No | No |
| Q11 | 2.247 | 2.597 | −2.126 | No | Yes | Yes | Yes | No | No | No |
| H1 | 2.596 | 1.380 | 0.167 | No | No | No | No | No | No | Yes |
| H2 | 2.549 | 1.340 | 0.644 | No | No | Yes | No | No | No | No |
| H3 | 2.984 | 1.238 | −0.396 | No | No | No | No | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, L.C.d.S.V.; Alcântara, L.M.; de Macêdo-Sales, P.A.; Reis, N.F.; de Oliveira, D.S.; Machado, R.L.D.; Geraldo, R.B.; dos Santos, A.L.S.; Ferreira, V.F.; Gonzaga, D.T.G.; et al. Synthetic Derivatives against Wild-Type and Non-Wild-Type Sporothrix brasiliensis: In Vitro and In Silico Analyses. Pharmaceuticals 2022, 15, 55. https://doi.org/10.3390/ph15010055
de Souza LCdSV, Alcântara LM, de Macêdo-Sales PA, Reis NF, de Oliveira DS, Machado RLD, Geraldo RB, dos Santos ALS, Ferreira VF, Gonzaga DTG, et al. Synthetic Derivatives against Wild-Type and Non-Wild-Type Sporothrix brasiliensis: In Vitro and In Silico Analyses. Pharmaceuticals. 2022; 15(1):55. https://doi.org/10.3390/ph15010055
Chicago/Turabian Stylede Souza, Lais Cavalcanti dos Santos Velasco, Lucas Martins Alcântara, Pãmella Antunes de Macêdo-Sales, Nathália Faria Reis, Débora Sena de Oliveira, Ricardo Luiz Dantas Machado, Reinaldo Barros Geraldo, André Luis Souza dos Santos, Vítor Francisco Ferreira, Daniel Tadeu Gomes Gonzaga, and et al. 2022. "Synthetic Derivatives against Wild-Type and Non-Wild-Type Sporothrix brasiliensis: In Vitro and In Silico Analyses" Pharmaceuticals 15, no. 1: 55. https://doi.org/10.3390/ph15010055







