Catenin Alpha 2 May Be a Biomarker or Potential Drug Target in Psychiatric Disorders with Perseverative Negative Thinking
Abstract
:1. Introduction
2. Results
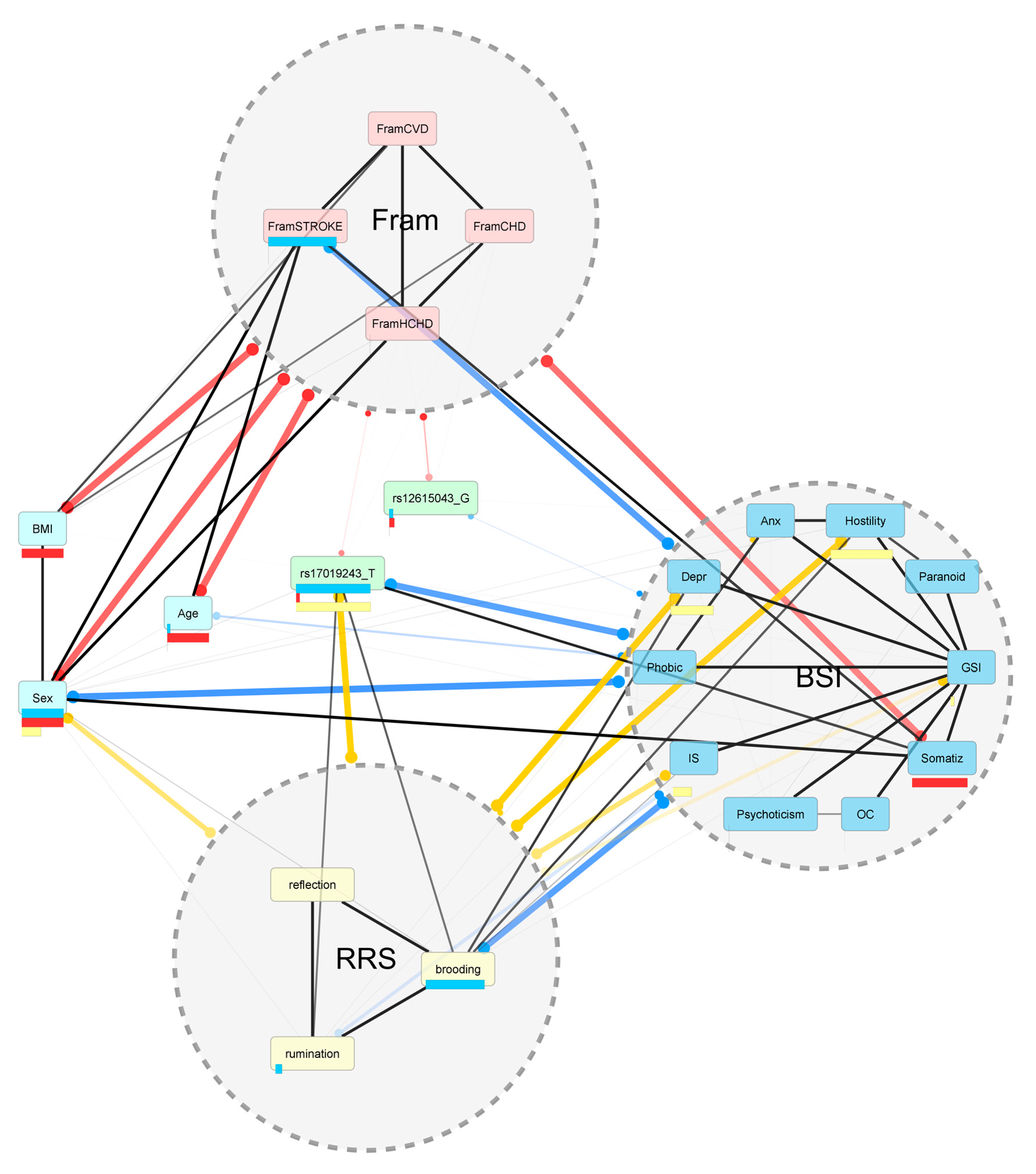
2.1. Associations between Rumination Score and Disease Risk Phenotypes
Associations between Other Ruminative Response Scale (RRS) Scores Brooding and Reflection and Disease Risk Phenotypes
2.2. Associations of CTNNA2 SNPs with Each of the Phenotypes, in FaST-LMM Regression Models
2.3. The Mediating Role of Rumination between CTNNA2 and Psychiatric Symptoms
The Mediating Role of Other RRS Scores Brooding and Reflection between CTNNA2 and Psychiatric Symptoms
3. Discussion
3.1. CTNNA2 Has Pleiotropic Effects on Cardiovascular Phenotypes and Rumination
3.2. CTNNA2 Effects on Divergent Psychiatric Symptoms Are Entirely Mediated by Rumination
3.3. Catenin Alpha 2 Protein (Encoded by CTNNA2) as a Potential Drug Target in Multiple Psychiatric Disorders or Multimorbid Conditions
3.4. Limitations
4. Materials and Methods
4.1. Participants
4.2. Measures
4.3. Genotyping and Quality Control
4.4. Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaffer, A.E.; Breuss, M.W.; Caglayan, A.O.; Al-Sanaa, N.; Al-Abdulwahed, H.Y.; Kaymakcalan, H.; Yilmaz, C.; Zaki, M.S.; Rosti, R.O.; Copeland, B.; et al. Biallelic loss of human CTNNA2, encoding alphaN-catenin, leads to ARP2/3 complex overactivity and disordered cortical neuronal migration. Nat. Genet. 2018, 50, 1093–1101. [Google Scholar] [CrossRef]
- Scott, L.J.; Muglia, P.; Kong, X.Q.; Guan, W.; Flickinger, M.; Upmanyu, R.; Tozzi, F.; Li, J.Z.; Burmeister, M.; Absher, D.; et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc. Natl. Acad. Sci. USA 2009, 106, 7501–7506. [Google Scholar] [CrossRef] [Green Version]
- Lesch, K.P.; Timmesfeld, N.; Renner, T.J.; Halperin, R.; Roser, C.; Nguyen, T.T.; Craig, D.W.; Romanos, J.; Heine, M.; Meyer, J.; et al. Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree linkage studies. J. Neural Transm. 2008, 115, 1573–1585. [Google Scholar] [CrossRef]
- Song, C.; Zhang, H. TARV: Tree-based analysis of rare variants identifying risk modifying variants in CTNNA2 and CNTNAP2 for alcohol addiction. Genet. Epidemiol. 2014, 38, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Smeland, O.B.; Frei, O.; Kauppi, K.; Hill, W.D.; Li, W.; Wang, Y.; Krull, F.; Bettella, F.; Eriksen, J.A.; Witoelar, A.; et al. Identification of Genetic Loci Jointly Influencing Schizophrenia Risk and the Cognitive Traits of Verbal-Numerical Reasoning, Reaction Time, and General Cognitive Function. JAMA Psychiatry 2017, 74, 1065–1075. [Google Scholar] [CrossRef]
- Uhl, G.R.; Liu, Q.R.; Drgon, T.; Johnson, C.; Walther, D.; Rose, J.E.; David, S.P.; Niaura, R.; Lerman, C. Molecular genetics of successful smoking cessation: Convergent genome-wide association study results. Arch. Gen. Psychiatry 2008, 65, 683–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Gizer, I.R.; Bizon, C.; Slutske, W.; Peng, Q.; Schork, N.J.; Wilhelmsen, K.C. Single nucleotide polymorphisms in the REG-CTNNA2 region of chromosome 2 and NEIL3 associated with impulsivity in a Native American sample. Genes Brain Behav. 2016, 15, 568–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodbourn, P.T.; Bosten, J.M.; Bargary, G.; Hogg, R.E.; Lawrance-Owen, A.J.; Mollon, J.D. Variants in the 1q21 risk region are associated with a visual endophenotype of autism and schizophrenia. Genes Brain Behav. 2014, 13, 144–151. [Google Scholar] [CrossRef]
- Simonson, M.A.; Wills, A.G.; Keller, M.C.; McQueen, M.B. Recent methods for polygenic analysis of genome-wide data implicate an important effect of common variants on cardiovascular disease risk. BMC Med. Genet. 2011, 12, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.W.; Kim, S.S.; Kim, Y. Genome-wide association study of orthostatic hypotension and supine-standing blood pressure changes in two korean populations. Genom. Inform. 2013, 11, 129–134. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Wisco, B.E.; Lyubomirsky, S. Rethinking Rumination. Perspect. Psychol. Sci. 2008, 3, 400–424. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Alloy, L.B. A roadmap to rumination: A review of the definition, assessment, and conceptualization of this multifaceted construct. Clin. Psychol. Rev. 2009, 29, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosschot, J.F.; Gerin, W.; Thayer, J.F. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J. Psychosom. Res. 2006, 60, 113–124. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Watkins, E.R. A Heuristic for Developing Transdiagnostic Models of Psychopathology: Explaining Multifinality and Divergent Trajectories. Perspect. Psychol. Sci. 2011, 6, 589–609. [Google Scholar] [CrossRef]
- Aldao, A.; Nolen-Hoeksema, S.; Schweizer, S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin. Psychol. Rev. 2010, 30, 217–237. [Google Scholar] [CrossRef]
- Silveira, E.d.M., Jr.; Anna, M.K.-S., Jr. Rumination in bipolar disorder: A systematic review. Braz. J. Psychiatry 2015, 37, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellings, T.M.B.; Alden, L.E. Cognitive processes in social anxiety: The effects of self-focus, rumination and anticipatory processing. Behav. Res. Ther. 2000, 38, 243–257. [Google Scholar] [CrossRef]
- Ehlers, A.; Mayou, R.A.; Bryant, B. Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. J. Abnorm. Psychol. 1998, 107, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Mayou, R.A.; Ehlers, A.; Bryant, B. Posttraumatic stress disorder after motor vehicle accidents: 3-year follow-up of a prospective longitudinal study. Behav. Res. Ther. 2002, 40, 665–675. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Stice, E.; Wade, E.; Bohon, C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. J. Abnorm. Psychol. 2007, 116, 198–207. [Google Scholar] [CrossRef]
- Caselli, G.; Ferretti, C.; Leoni, M.; Rebecchi, D.; Rovetto, F.; Spada, M.M. Rumination as a predictor of drinking behaviour in alcohol abusers: A prospective study. Addiction 2010, 105, 1041–1048. [Google Scholar] [CrossRef]
- Craner, J.R.; Sigmon, S.T.; Martinson, A.A.; McGillicuddy, M.L. Premenstrual disorders and rumination. J. Clin. Psychol. 2014, 70, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Maurage, P.; Philippot, P.; Grynberg, D.; Leleux, D.; Delatte, B.; Mangelinckx, C.; Belge, J.B.; Constant, E. Imbalance between abstract and concrete repetitive thinking modes in schizophrenia. Compr. Psychiatry 2017, 78, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.A.; Christenfeld, N.J. Cardiovascular disease and psychiatric comorbidity: The potential role of perseverative cognition. Cardiovasc. Psychiatry. Neurol. 2009, 2009, 791017. [Google Scholar] [CrossRef] [Green Version]
- Treynor, W.; Gonzalez, R.; Nolen-Hoeksema, S. Rumination reconsidered: A psychometric analysis. Cognit. Ther. Res. 2003, 27, 247–259. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Eszlari, N.; Millinghoffer, A.; Petschner, P.; Gonda, X.; Baksa, D.; Pulay, A.J.; Rethelyi, J.M.; Breen, G.; Deakin, J.F.W.; Antal, P.; et al. Genome-wide association analysis reveals KCTD12 and miR-383-binding genes in the background of rumination. Transl. Psychiatry 2019, 9, 019–0454. [Google Scholar] [CrossRef] [PubMed]
- Eszlari, N.; Petschner, P.; Gonda, X.; Baksa, D.; Elliott, R.; Anderson, I.M.; Deakin, J.F.W.; Bagdy, G.; Juhasz, G. Childhood Adversity Moderates the Effects of HTR2A Epigenetic Regulatory Polymorphisms on Rumination. Front. Psychiatry 2019, 10, 394. [Google Scholar] [CrossRef]
- Shaw, Z.A.; Hilt, L.M.; Starr, L.R. The developmental origins of ruminative response style: An integrative review. Clin. Psychol. Rev. 2019, 74, 101780. [Google Scholar] [CrossRef]
- Hilt, L.M.; Sander, L.C.; Nolen-Hoeksema, S.; Simen, A.A. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neurosci. Lett. 2007, 429, 12–16. [Google Scholar] [CrossRef]
- Juhasz, G.; Dunham, J.S.; McKie, S.; Thomas, E.; Downey, D.; Chase, D.; Lloyd-Williams, K.; Toth, Z.G.; Platt, H.; Mekli, K.; et al. The CREB1-BDNF-NTRK2 Pathway in Depression: Multiple Gene-Cognition-Environment Interactions. Biol. Psychiatry 2011, 69, 762–771. [Google Scholar] [CrossRef]
- Eszlari, N.; Kovacs, D.; Petschner, P.; Pap, D.; Gonda, X.; Elliott, R.; Anderson, I.M.; Deakin, J.F.W.; Bagdy, G.; Juhasz, G. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: A potential risk mechanism for depression. Transl. Psychiatry 2016, 6, e745. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.P.; Rhee, S.H.; Friedman, N.P.; Corley, R.P.; Munn-Chernoff, M.A.; Hewitt, J.K.; Whisman, M.A. A Twin Study Examining Rumination as a Transdiagnostic Correlate of Psychopathology. Clin. Psychol. Sci. 2016, 4, 971–987. [Google Scholar] [CrossRef] [Green Version]
- Chu, T.T.; Liu, Y. An integrated genomic analysis of gene-function correlation on schizophrenia susceptibility genes. J. Hum. Genet. 2010, 55, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Georgescu, I.A.; Popa, D.; Zagrean, L. The Anatomical and Functional Heterogeneity of the Mediodorsal Thalamus. Brain Sci. 2020, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Mexal, S.; Berger, R.; Pearce, L.; Barton, A.; Logel, J.; Adams, C.E.; Ross, R.G.; Freedman, R.; Leonard, S. Regulation of a novel alphaN-catenin splice variant in schizophrenic smokers. Am. J. Med Genetics. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2008, 147, 759–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, K.; Chisaka, O.; Van Roy, F.; Takeichi, M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat. Neurosci. 2004, 7, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Falls, W.; Finger, J.H.; Longo-Guess, C.M.; Ackerman, S.L. Deletion in Catna2, encoding alpha N-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nat. Genet. 2002, 31, 279–284. [Google Scholar] [CrossRef]
- Braff, D.L. Prepulse inhibition of the startle reflex: A window on the brain in schizophrenia. Curr. Top. Behav. Neurosci. 2010, 4, 349–371. [Google Scholar] [CrossRef]
- Bagyura, Z.; Kiss, L.; Edes, E.; Lux, A.; Polgar, L.; Soos, P.; Szenczi, O.; Szelid, Z.; Vadas, R.; Jozan, P.; et al. Cardiovascular screening programme in the Central Hungarian region. The Budakalasz Study. Orv. Hetil. 2014, 155, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, R.B.; Wolf, P.A.; Belanger, A.J.; Kannel, W.B. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke 1994, 25, 40–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derogatis, L.R. BSI: Brief. Symptom Inventory: Administration, Scoring, and Procedures Manual; National Computer Systems Pearson, Inc.: Minneapolis, MN, USA, 1993. [Google Scholar]
- Coleman, J.R.; Euesden, J.; Patel, H.; Folarin, A.A.; Newhouse, S.; Breen, G. Quality control, imputation and analysis of genome-wide genotyping data from the Illumina HumanCoreExome microarray. Brief. Funct. Genom. 2016, 15, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippert, C.; Listgarten, J.; Liu, Y.; Kadie, C.M.; Davidson, R.I.; Heckerman, D. FaST linear mixed models for genome-wide association studies. Nat. Methods 2011, 8, 833–835. [Google Scholar] [CrossRef]
- Gao, X.; Starmer, J.; Martin, E.R. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet. Epidemiol. 2008, 32, 361–369. [Google Scholar] [CrossRef]
- Gauderman, W.J. Sample size requirements for matched case-control studies of gene-environment interaction. Stat. Med. 2002, 21, 35–50. [Google Scholar] [CrossRef]
- Draper, D. Bayesian multilevel analysis and MCMC. In Handbook of Multilevel Analysis; Springer: New York, NY, USA, 2008; pp. 77–139. [Google Scholar]
- Antal, P.; Millinghoffer, A.; Hullám, G.; Szalai, C.; Falus, A. A Bayesian View of Challenges in Feature Selection: Feature Aggregation, Multiple Targets, Redundancy and Interaction. In Proceedings of the Workshop on New Challenges for Feature Selection in Data Mining and Knowledge Discovery at ECML/PKDD 2008, Antwerp, Belgium, 15 September 2008; pp. 74–89. [Google Scholar]
- Cooper, G.F.; Herskovits, E. A Bayesian Method for the Induction of Probabilistic Networks from Data. Mach. Learn. 1992, 9, 309–347. [Google Scholar] [CrossRef]

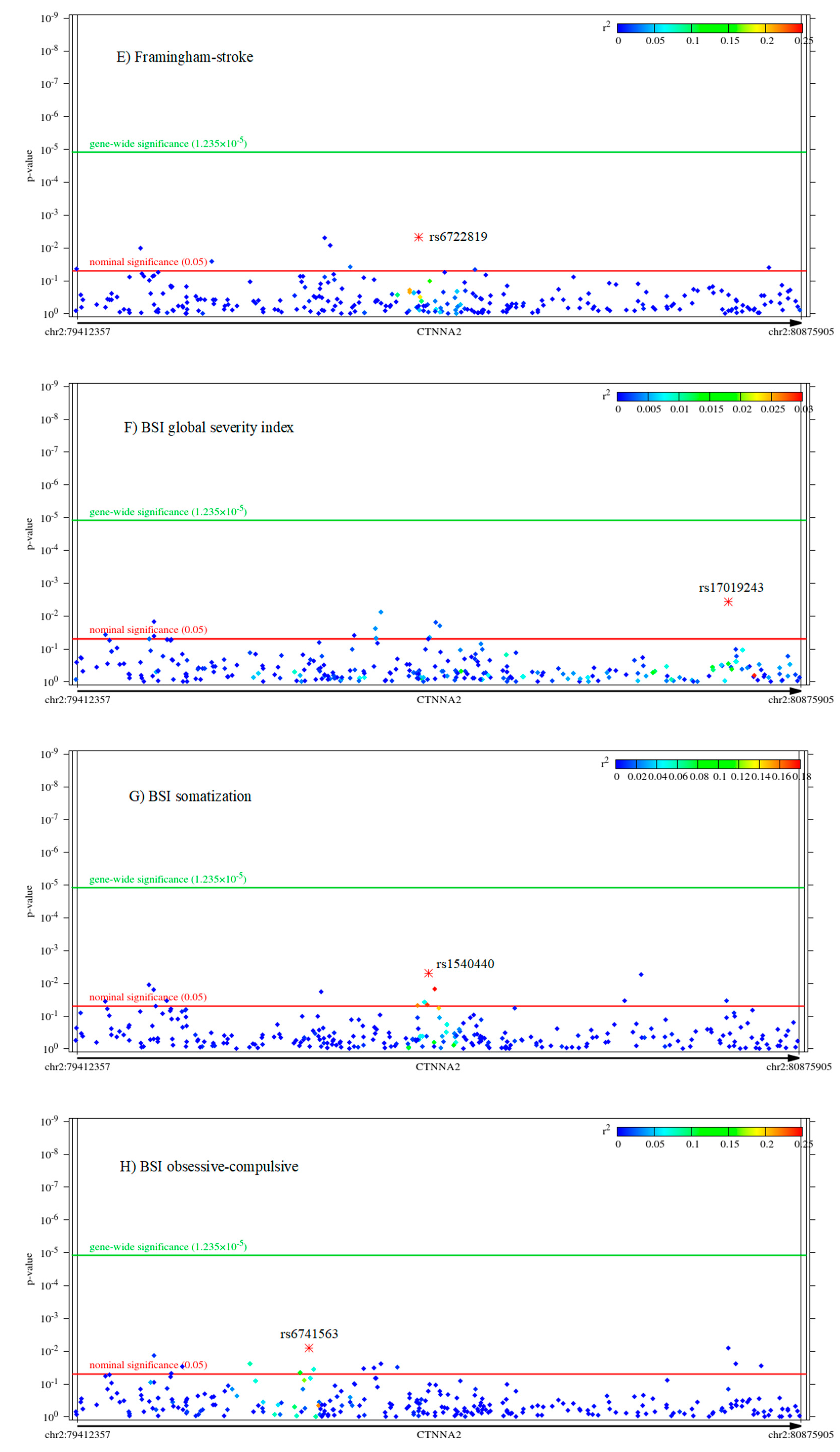
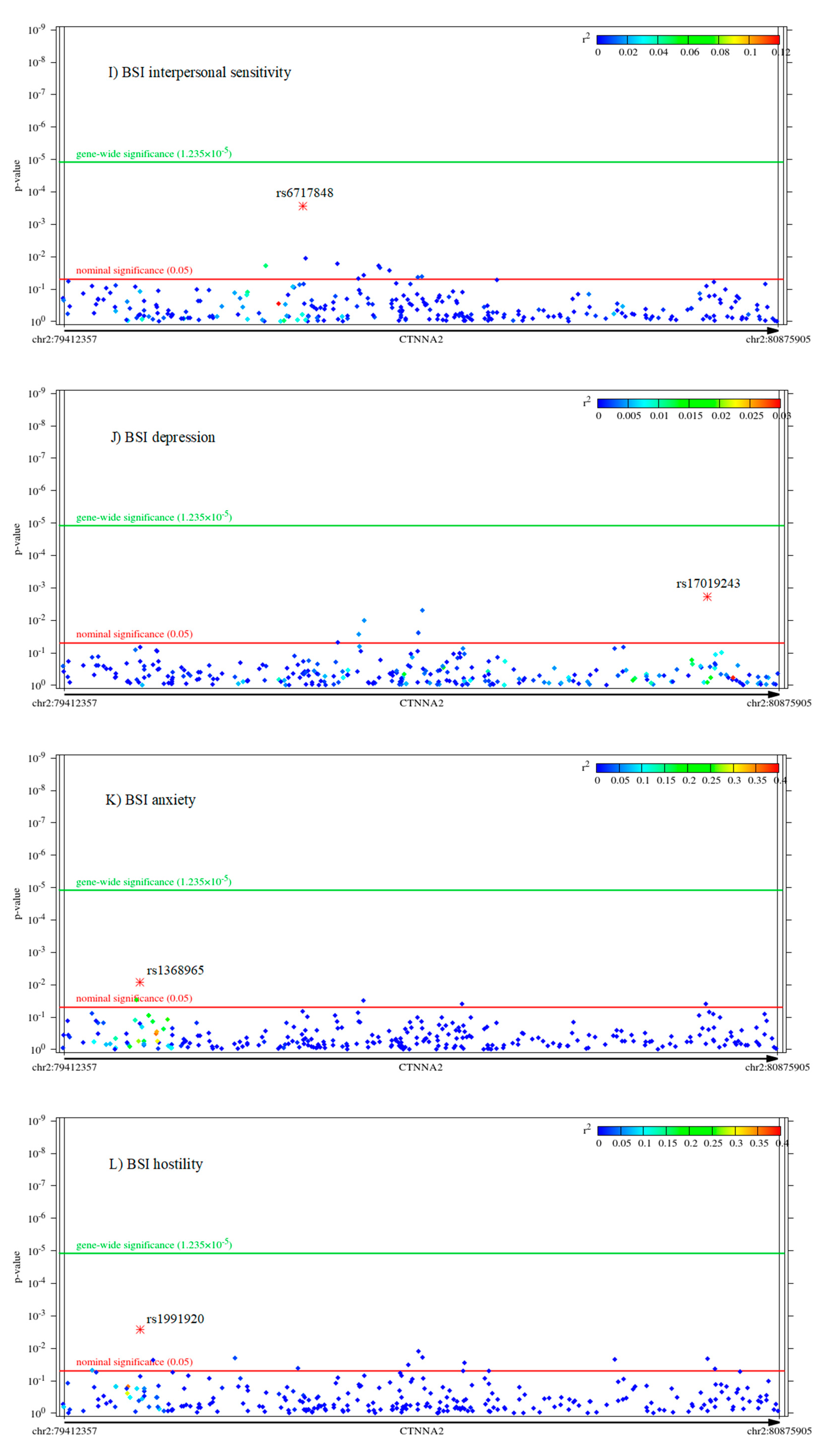
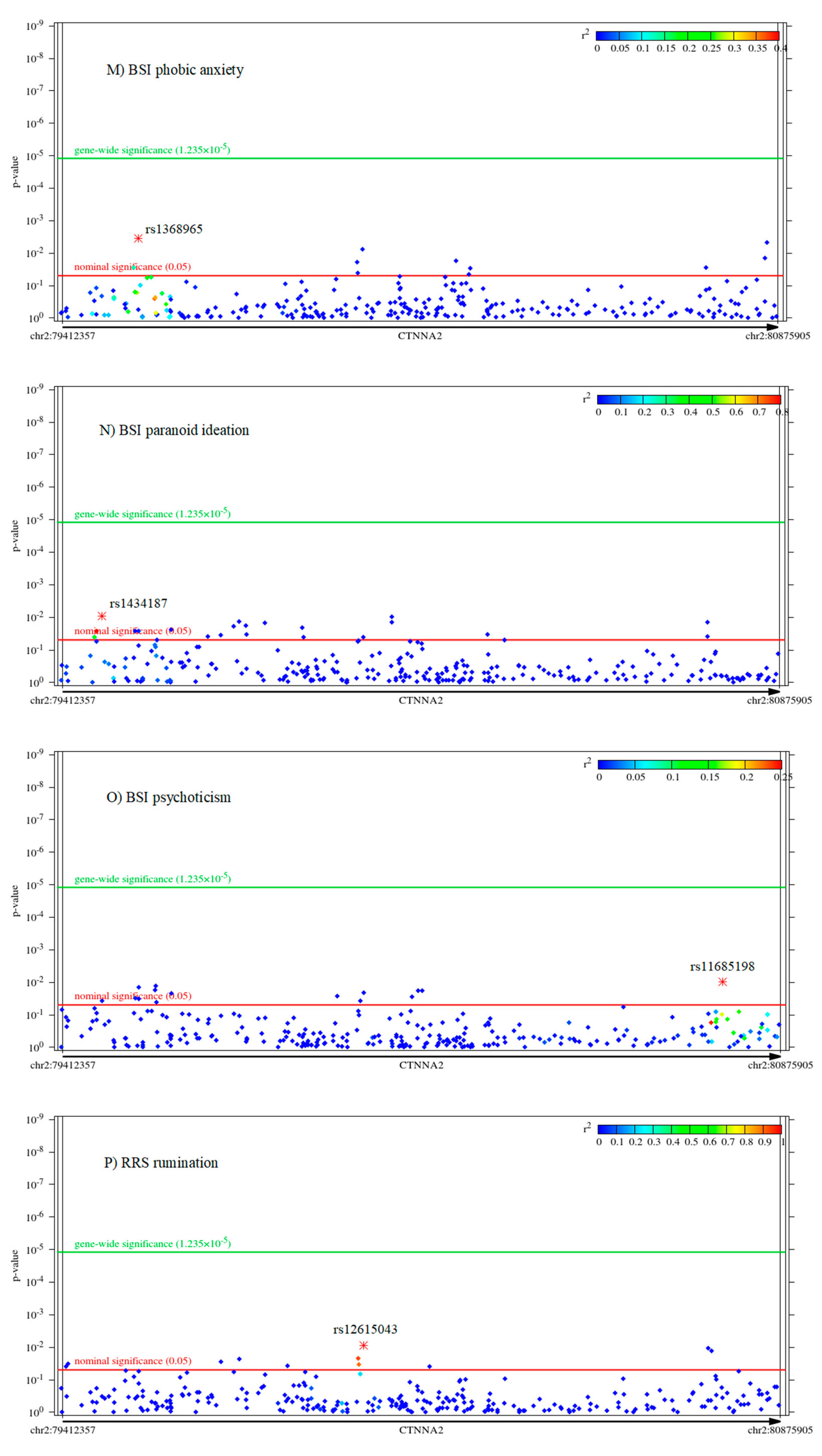
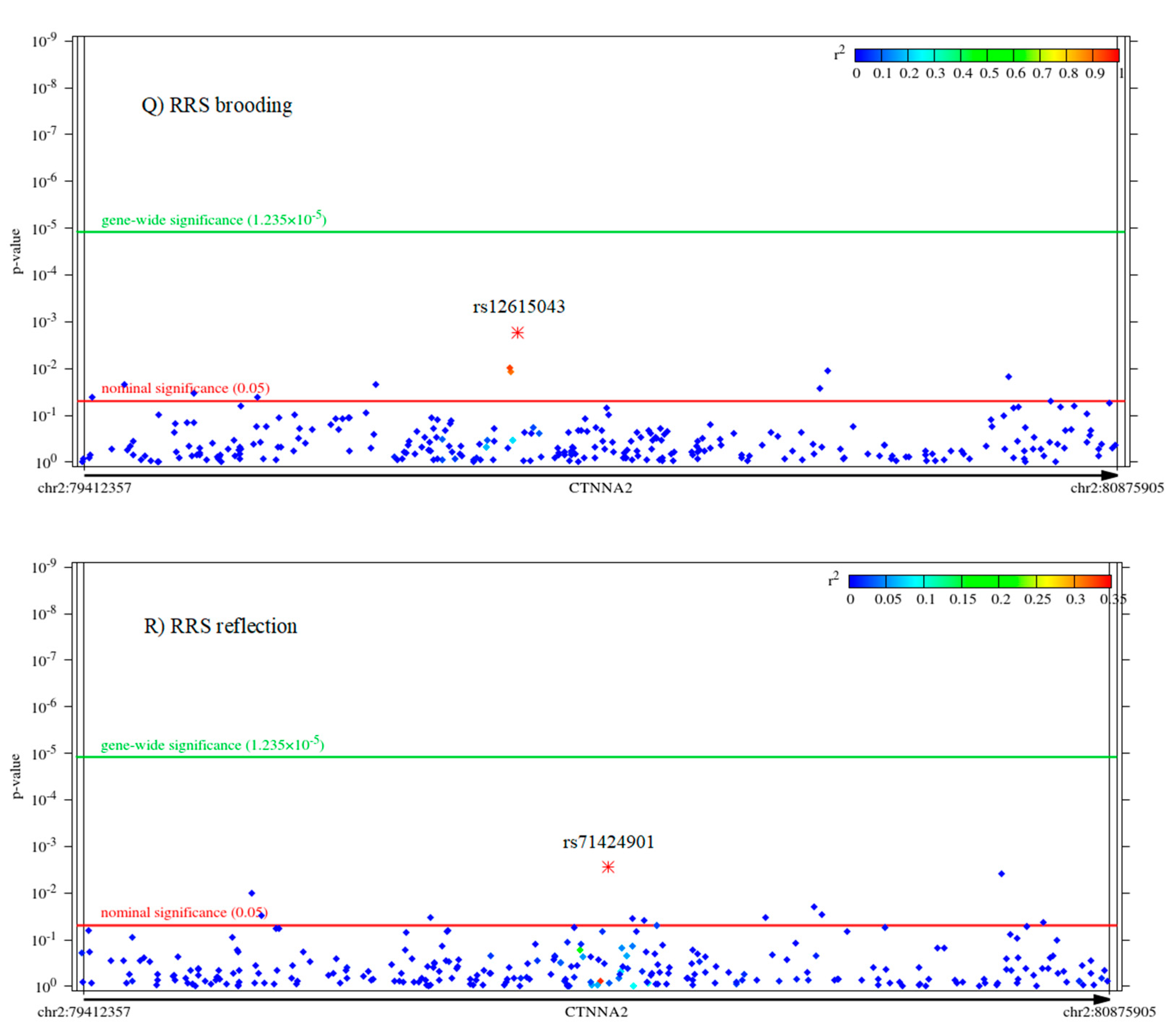

| Variable | Mean | Standard Error of Mean | Standard Deviation | Power to Detect CTNNA2 SNP Effect | Cronbach’s Alpha |
|---|---|---|---|---|---|
| Age at medical examination | 53.72 | 0.490 | 13.815 | ||
| Age at questionnaire filling | 54.85 | 0.500 | 14.109 | ||
| BMI | 27.39 | 0.179 | 5.034 | 5.05–99.99% | |
| Framingham-CVD | 13.78 | 0.426 | 11.998 | 5.01–76.98% | |
| Framingham-CHD | 8.59 | 0.277 | 7.821 | 5.02–98.58% | |
| Framingham-HCHD | 4.17 | 0.181 | 5.097 | 5.04–99.99% | |
| Framingham-stroke | 2.83 | 0.117 | 3.288 | 5.11–99.99% | |
| BSI global severity index | 0.55 | 0.018 | 0.508 | 9.58–99.99% | 0.957 |
| BSI somatization | 0.51 | 0.022 | 0.617 | 8.08–99.99% | 0.800 |
| BSI obsessive-compulsive | 0.70 | 0.025 | 0.703 | 7.37–99.99% | 0.828 |
| BSI interpersonal sensitivity | 0.94 | 0.025 | 0.695 | 7.42–99.99% | 0.643 |
| BSI depression | 0.48 | 0.021 | 0.606 | 8.20–99.99% | 0.862 |
| BSI anxiety | 0.54 | 0.024 | 0.668 | 7.62–99.99% | 0.824 |
| BSI hostility | 0.50 | 0.020 | 0.566 | 8.67–99.99% | 0.733 |
| BSI phobic anxiety | 0.32 | 0.021 | 0.584 | 8.45–99.99% | 0.800 |
| BSI paranoid ideation | 0.71 | 0.024 | 0.673 | 7.59–99.99% | 0.737 |
| BSI psychoticism | 0.42 | 0.020 | 0.564 | 8.70–99.99% | 0.675 |
| RRS rumination | 1.89 | 0.018 | 0.503 | 9.67–99.99% | 0.812 |
| RRS brooding | 1.91 | 0.020 | 0.570 | 8.62–99.99% | 0.759 |
| RRS reflection | 1.86 | 0.021 | 0.602 | 8.24–99.99% | 0.753 |
| Variable | RRS Rumination Score Residual | RRS Brooding Score Residual | RRS Reflection Score Residual | |
|---|---|---|---|---|
| RRS rumination score residual | Pearson correlation | 1 | 0.508 | 0.532 |
| p | 2.148 × 10−53 | 2.291 × 10−59 | ||
| RRS brooding score residual | Pearson correlation | 0.508 | 1 | −0.459 |
| p | 2.148 × 10−53 | 1.263 × 10−42 | ||
| RRS reflection score residual | Pearson correlation | 0.532 | −0.459 | 1 |
| p | 2.291 × 10−59 | 1.263 × 10−42 | ||
| BMI | Pearson correlation | −0.017 | 0.079 | −0.095 |
| p | 0.632 | 0.026 | 0.007 | |
| Framingham-CVD | Pearson correlation | 0.019 | 0.020 | −0.001 |
| p | 0.598 | 0.565 | 0.982 | |
| Framingham-CHD | Pearson correlation | 0.015 | 0.011 | 0.005 |
| p | 0.667 | 0.756 | 0.890 | |
| Framingham-HCHD | Pearson correlation | 0.027 | 0.017 | 0.011 |
| p | 0.451 | 0.636 | 0.754 | |
| Framingham-stroke | Pearson correlation | 0.024 | 0.020 | 0.005 |
| p | 0.502 | 0.568 | 0.896 | |
| BSI global severity index | Pearson correlation | 0.454 | 0.435 | 0.040 |
| p | 1.357 × 10−41 | 4.553 × 10−38 | 0.258 | |
| BSI somatization | Pearson correlation | 0.278 | 0.299 | −0.007 |
| p | 1.586 × 10−15 | 7.339 × 10−18 | 0.835 | |
| BSI obsessive-compulsive | Pearson correlation | 0.364 | 0.301 | 0.080 |
| p | 2.272 × 10−26 | 4.043 × 10−18 | 0.024 | |
| BSI interpersonal sensitivity | Pearson correlation | 0.355 | 0.398 | −0.026 |
| p | 5.627 × 10−25 | 1.259 × 10−31 | 0.471 | |
| BSI depression | Pearson correlation | 0.456 | 0.403 | 0.074 |
| p | 4.600 × 10−42 | 1.834 × 10−32 | 0.037 | |
| BSI anxiety | Pearson correlation | 0.404 | 0.384 | 0.039 |
| p | 1.355 × 10−32 | 2.087 × 10−29 | 0.271 | |
| BSI hostility | Pearson correlation | 0.334 | 0.343 | 0.007 |
| p | 4.033 × 10−22 | 2.014 × 10−23 | 0.852 | |
| BSI phobic anxiety | Pearson correlation | 0.302 | 0.276 | 0.040 |
| p | 2.952 × 10−18 | 2.199 × 10−15 | 0.256 | |
| BSI paranoid ideation | Pearson correlation | 0.363 | 0.395 | −0.013 |
| p | 3.710 × 10−26 | 5.287 × 10−31 | 0.707 | |
| BSI psychoticism | Pearson correlation | 0.368 | 0.346 | 0.040 |
| p | 6.039 × 10−27 | 8.015 × 10−24 | 0.266 | |
| CTNNA2 rs17019243 → RRS Rumination → BSI Score | |||||||
|---|---|---|---|---|---|---|---|
| GSI | Somatization | Obsessive- Compulsive | Depression | Anxiety | Hostility | Phobic Anxiety | Paranoid Ideation |
| 0.146 | 0.098 | 0.117 | 0.146 | 0.133 | 0.107 | 0.098 | 0.114 |
| (p = 0.020) | (p = 0.023) | (p = 0.020) | (p = 0.021) | (p = 0.020) | (p = 0.022) | (p = 0.022) | (p = 0.023) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eszlari, N.; Bagyura, Z.; Millinghoffer, A.; Nagy, T.; Juhasz, G.; Antal, P.; Merkely, B.; Bagdy, G. Catenin Alpha 2 May Be a Biomarker or Potential Drug Target in Psychiatric Disorders with Perseverative Negative Thinking. Pharmaceuticals 2021, 14, 850. https://doi.org/10.3390/ph14090850
Eszlari N, Bagyura Z, Millinghoffer A, Nagy T, Juhasz G, Antal P, Merkely B, Bagdy G. Catenin Alpha 2 May Be a Biomarker or Potential Drug Target in Psychiatric Disorders with Perseverative Negative Thinking. Pharmaceuticals. 2021; 14(9):850. https://doi.org/10.3390/ph14090850
Chicago/Turabian StyleEszlari, Nora, Zsolt Bagyura, Andras Millinghoffer, Tamas Nagy, Gabriella Juhasz, Peter Antal, Bela Merkely, and Gyorgy Bagdy. 2021. "Catenin Alpha 2 May Be a Biomarker or Potential Drug Target in Psychiatric Disorders with Perseverative Negative Thinking" Pharmaceuticals 14, no. 9: 850. https://doi.org/10.3390/ph14090850






