Synthesis and Evaluation of the Antidepressant-like Properties of HBK-10, a Novel 2-Methoxyphenylpiperazine Derivative Targeting the 5-HT1A and D2 Receptors
Abstract
:1. Introduction
2. Results
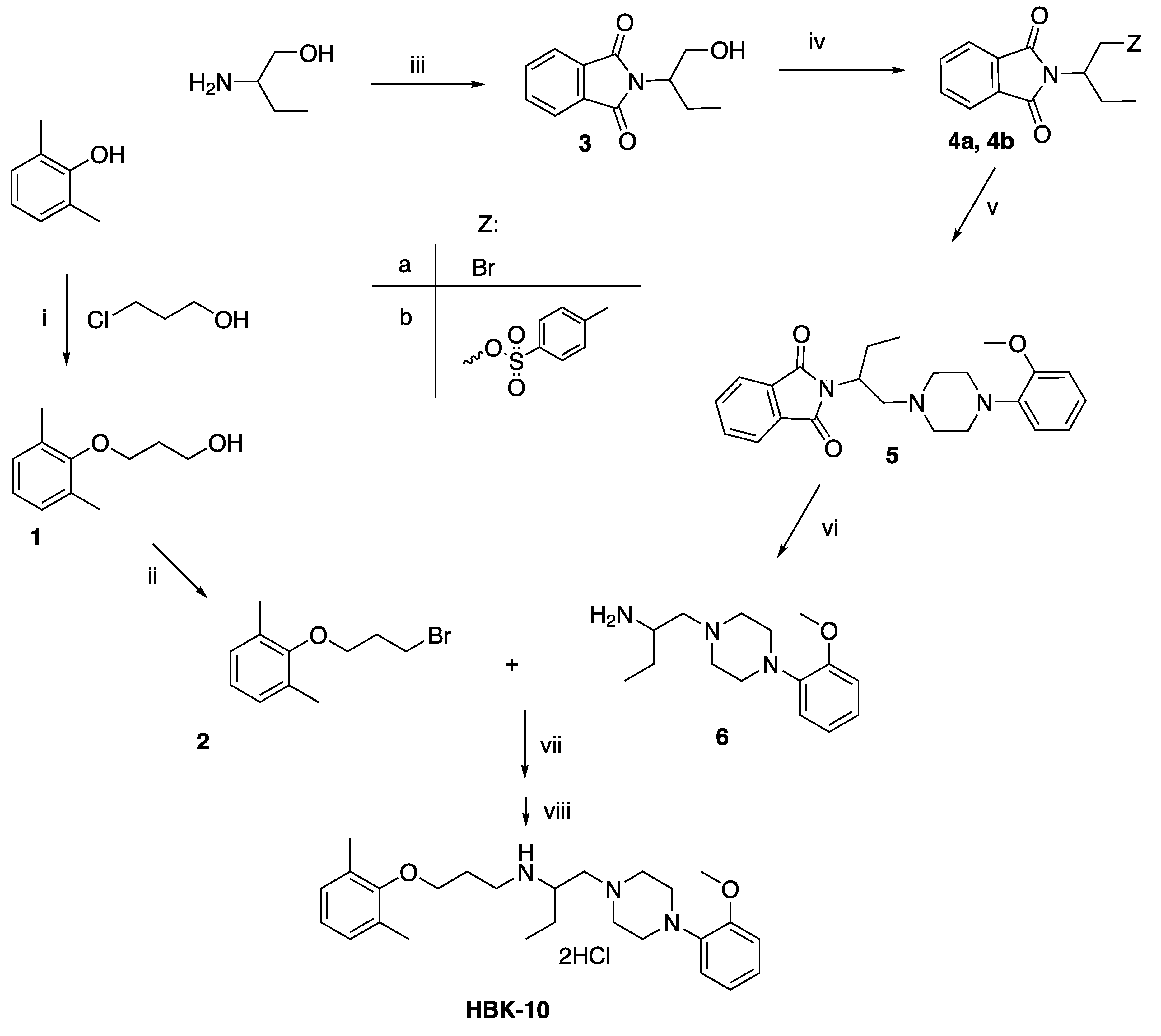
2.1. Chemistry
2.2. In Vitro Cytotoxicity and Metabolic Stability
2.3. In Vitro Pharmacology
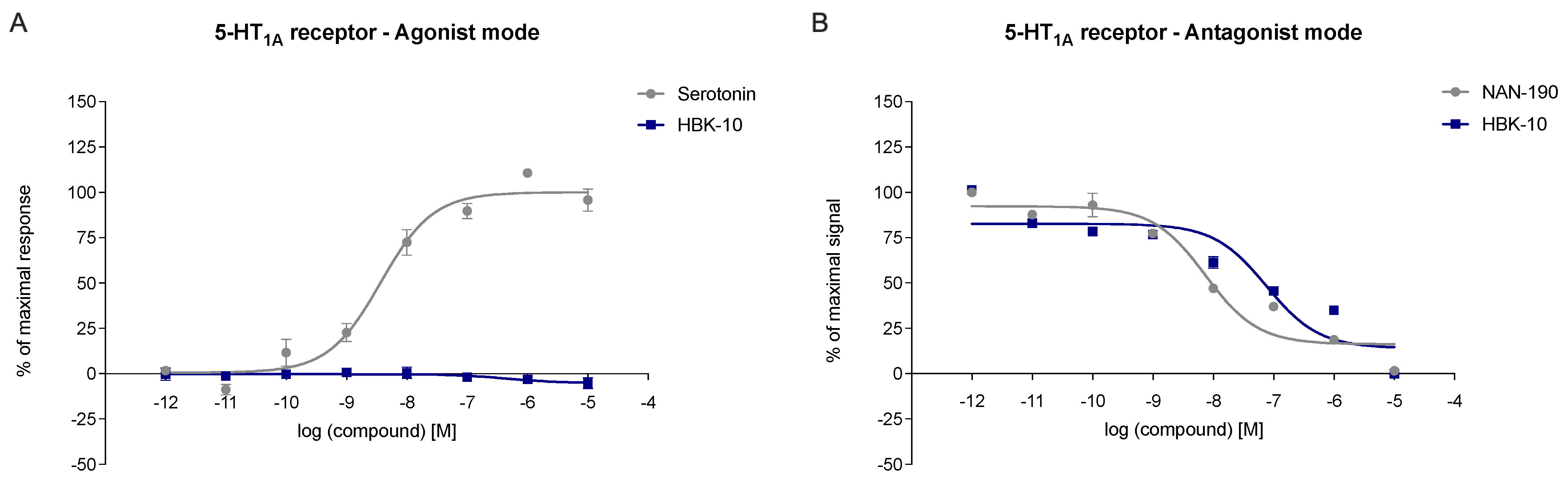
2.3.1. HBK-10 Showed High Affinity for Serotonergic 5-HT1A and Dopaminergic D2 Receptors
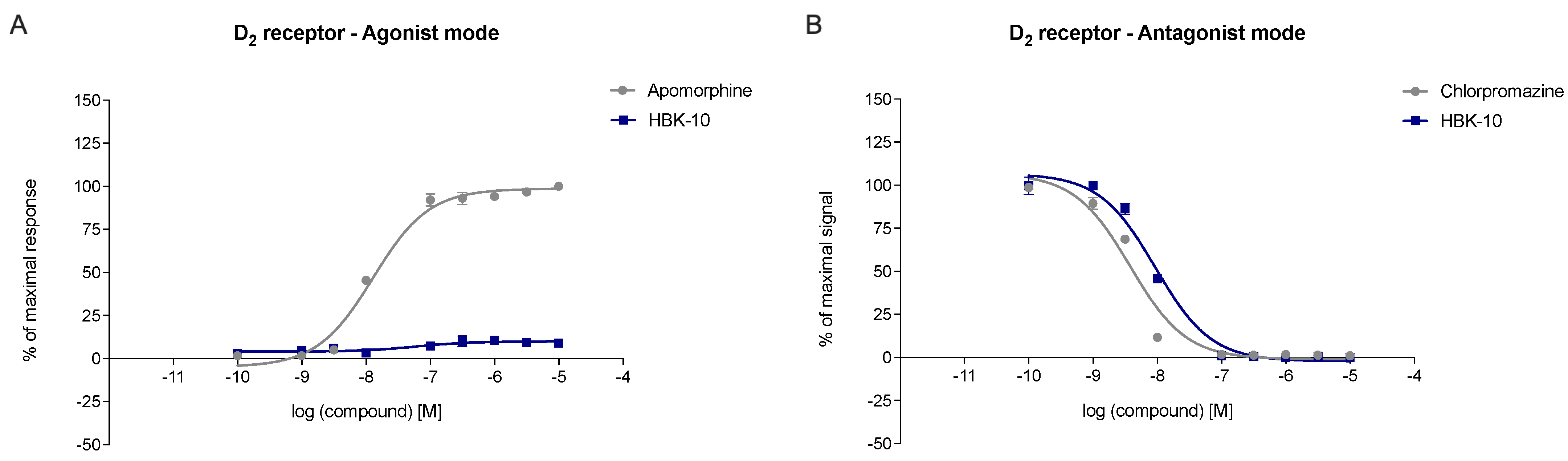
2.3.2. HBK-10 Antagonized 5-HT1A and D2 Receptors
2.4. In Vivo Pharmacology
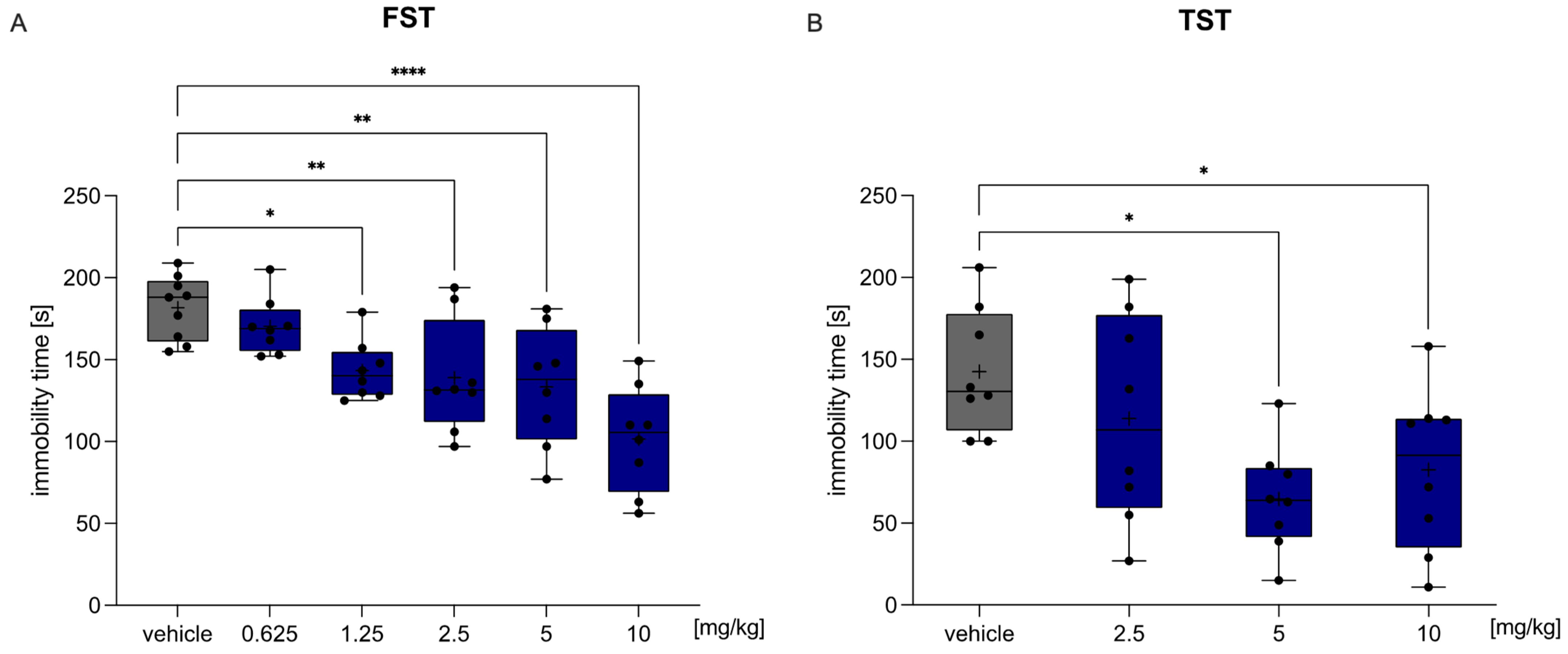
2.4.1. HBK-10 Showed Antidepressant-like Activity in Mice after Acute Administration
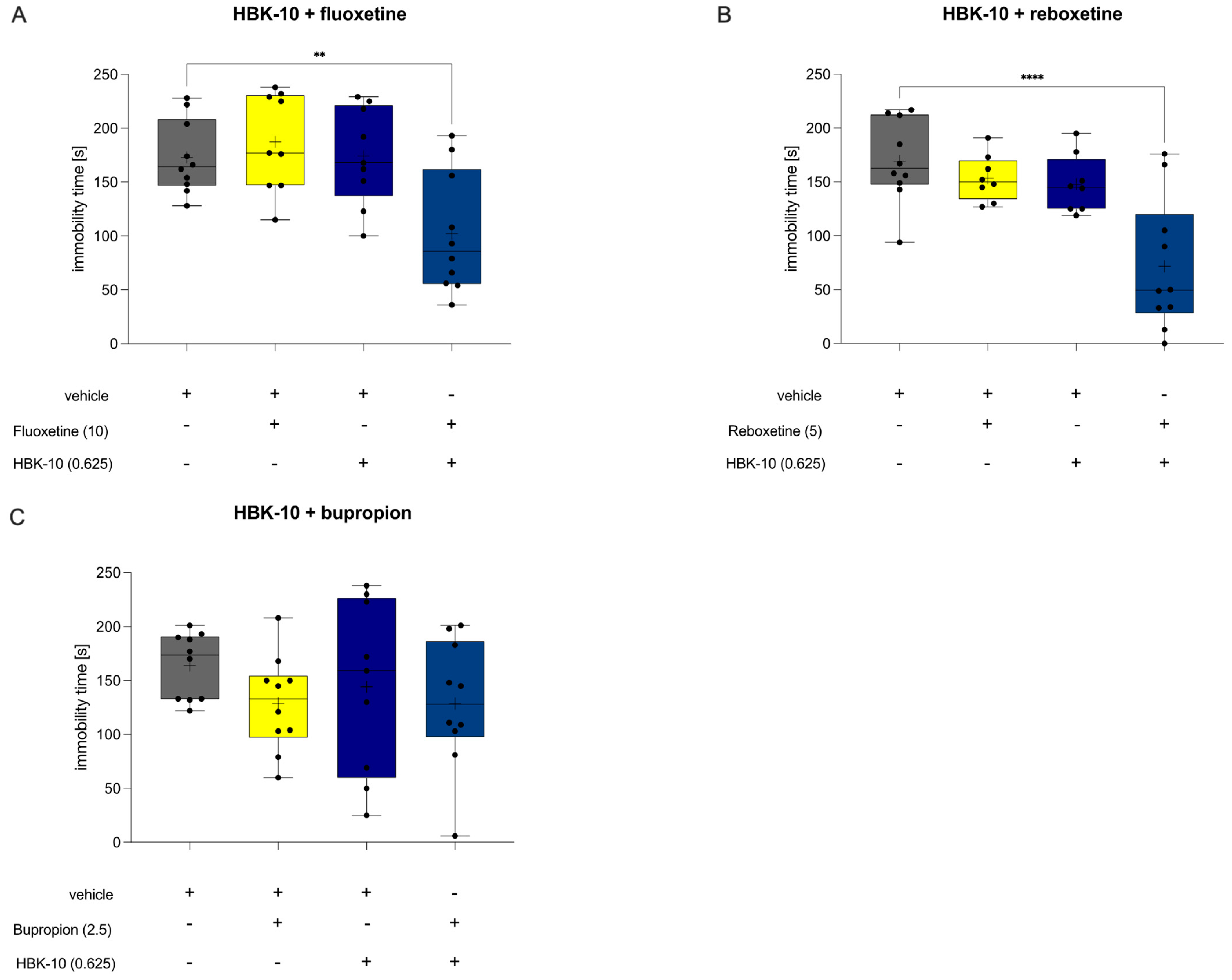
2.4.2. The Combined Administration of Sub-Effective Doses of HBK-10 and Fluoxetine Showed Antidepressant-like Effect
2.4.3. The Combined Administration of Sub-Effective Doses of HBK-10 and Reboxetine Showed Antidepressant-like Effect
2.4.4. The Combined Administration of Sub-Effective Doses of HBK-10 and Bupropion Did Not Show Antidepressant-like Effect
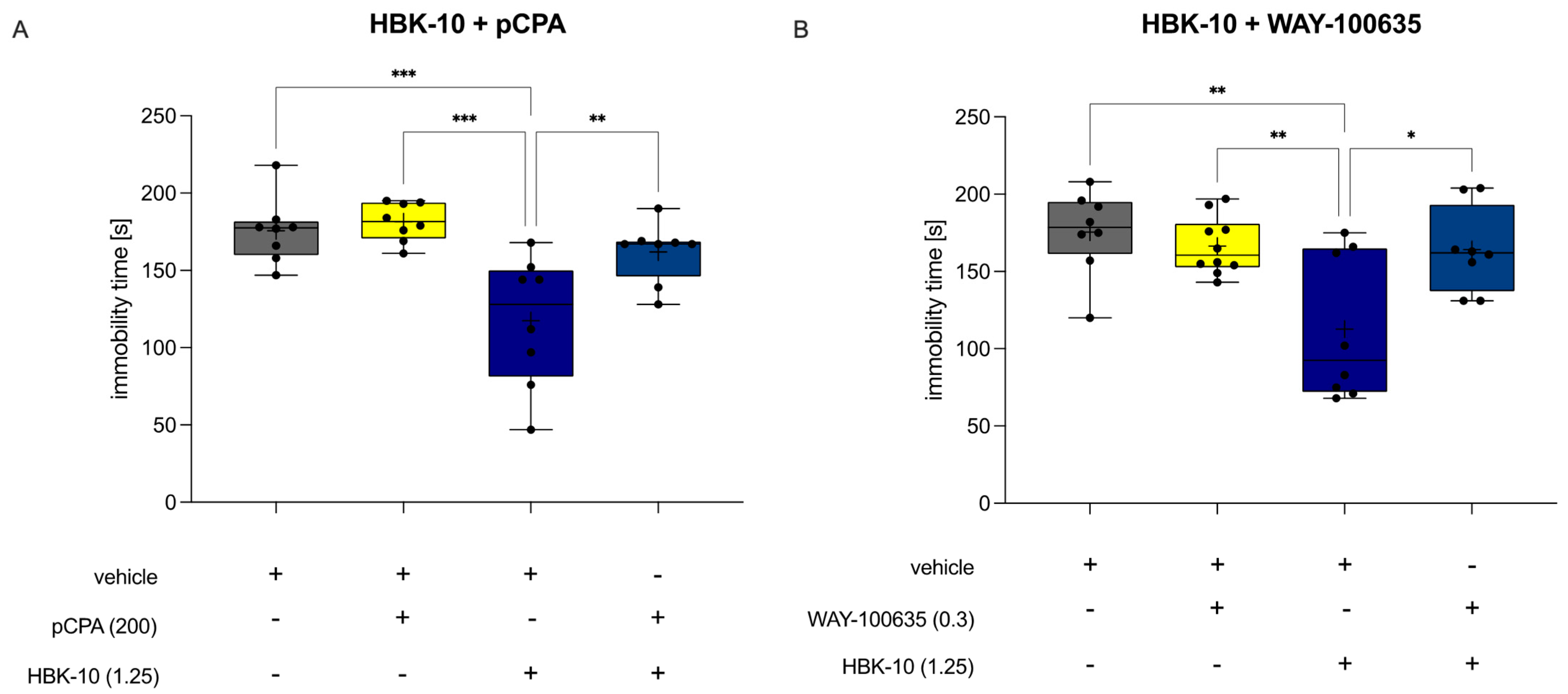
2.4.5. HBK-10 Did Not Show Antidepressant-like Activity after Pretreatment with pCPA
2.4.6. HBK-10 Did Not Show Antidepressant-like Activity after Pretreatment with WAY-100635
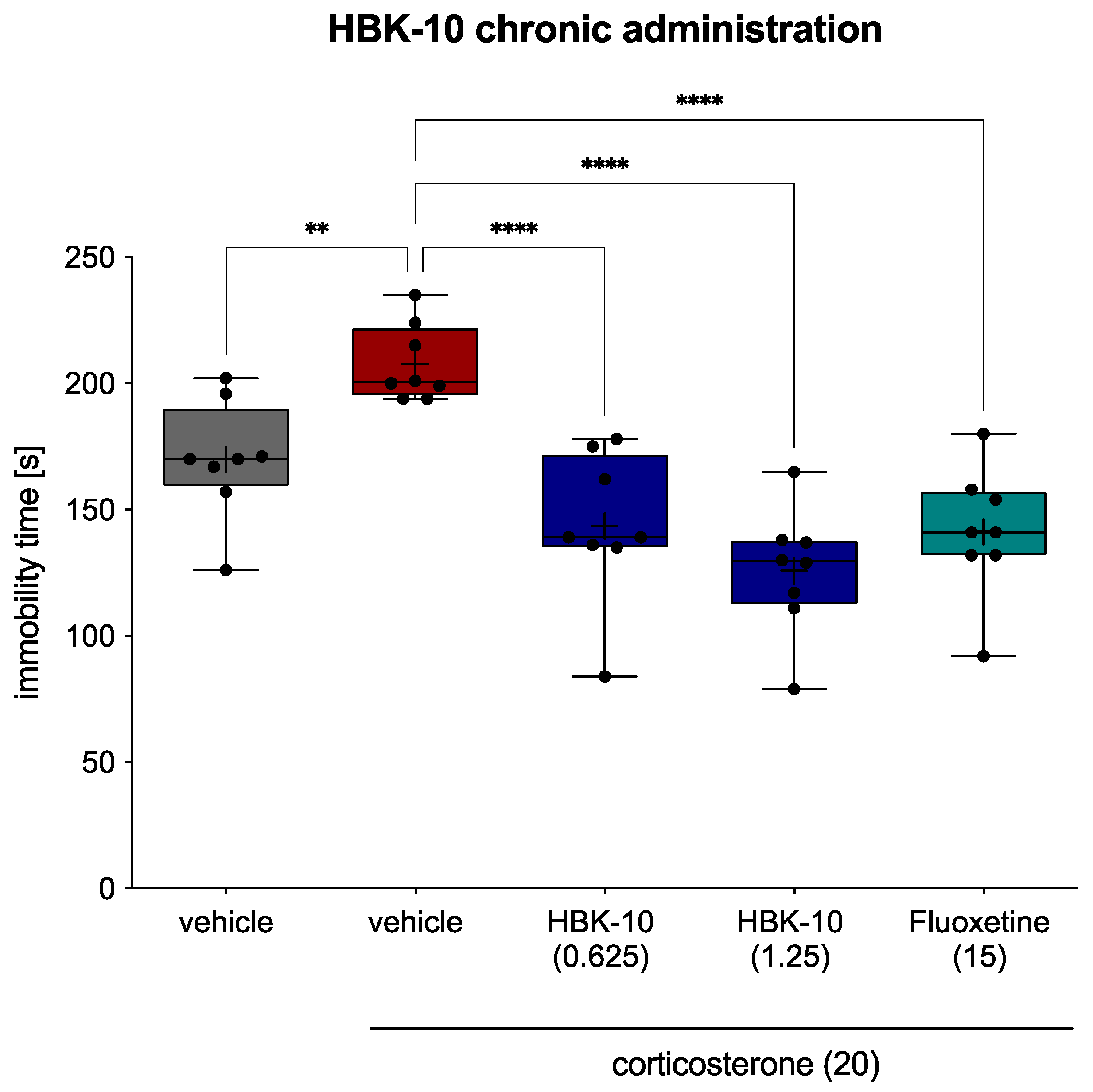
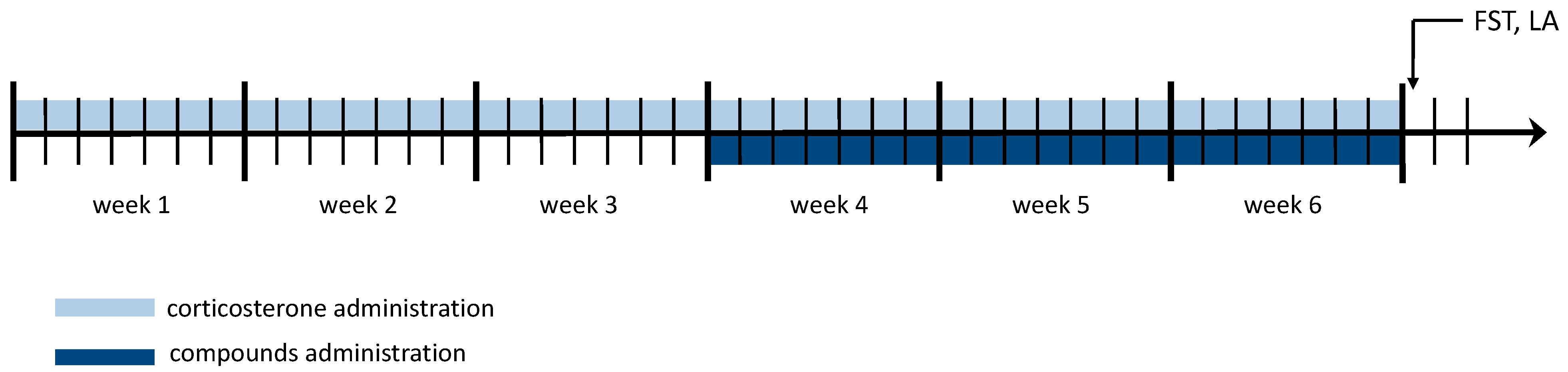
2.4.7. HBK-10 Showed Antidepressant-like Activity in the Forced Swim Test in the Corticosterone-Treated Mice after a Chronic Administration
2.4.8. HBK-10 Did Not Alter the Locomotor Activity in the Corticosterone-Treated Mice after a Chronic Administration
2.4.9. HBK-10 Decreases Locomotor Activity in Mice at Higher Doses
2.4.10. HBK-10 Influenced Motor Coordination in Mice at Highest Dose Tested
3. Discussion
4. Materials and Methods
4.1. Chemistry
Synthesis
4.2. In Vitro Studies
4.2.1. Cytotoxicity
4.2.2. Metabolic Stability
4.2.3. Radioligand Binding Assays
4.2.4. Functional Assays for 5-HT1A Receptor
4.2.5. Functional Assays for D2 Receptor
4.3. In Vivo Studies
4.3.1. Animals
4.3.2. Drugs
4.3.3. Forced Swim Test
Serotonin Synthesis Blockade
The Blockade of 5-HT1A Receptors
4.3.4. Tail Suspension Test
4.3.5. Spontaneous Locomotor Activity in Mice
4.3.6. Rotarod Test
4.3.7. Chimney Test
4.3.8. Corticosterone-Induced Mouse Model of Depression
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ettman, C.K.; Abdalla, S.M.; Cohen, G.H.; Sampson, L.; Vivier, P.M.; Galea, S. Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2019686. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Stassen, H.H.; Angst, J. Delayed onset of action of antidepressants: Fact or fiction? CNS Drugs 1998, 9, 177–184. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Antidepressant adherence: Are patients taking their medications? Innov. Clin. Neurosci. 2012, 9, 41–46. [Google Scholar]
- Lader, M. Limitations of current medical treatments for depression: Disturbed circadian rhythms as a possible therapeutic target. Eur. Neuropsychopharmacol. 2007, 17, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Angst, J.; Angst, F.; Stassen, H.H. Suicide risk in patients with major depressive disorder. J. Clin. Psychiatry 1999, 60, 57–62. [Google Scholar] [PubMed]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Sałaciak, K.; Pytka, K. Biased agonism in drug discovery: Is there a future for biased 5-HT 1A receptor agonists in the treatment of neuropsychiatric diseases? Pharmacol. Ther. 2021, 227, 107872. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-HT1A receptor function in major depressive disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bogaert, M.; Oosting, R.; Toth, M.; Groenink, L.; van Oorschot, R.; Olivier, B. Effects of genetic background and null mutation of 5-HT1A receptors on basal and stress-induced body temperature: Modulation by serotonergic and GABAA-ergic drugs. Eur. J. Pharmacol. 2006, 550, 84–90. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Parsey, R.V. Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120004. [Google Scholar] [CrossRef] [Green Version]
- Albert, P.R.; Vahid-Ansari, F.; Luckhart, C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: Pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front. Behav. Neurosci. 2014, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Richardson-Jones, J.W.; Craige, C.P.; Nguyen, T.H.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Guiard, B.P.; Beck, S.G.; Hen, R.; et al. Serotonin-1A Autoreceptors Are Necessary and Sufficient for the Normal Formation of Circuits Underlying Innate Anxiety. J. Neurosci. 2011, 31, 6008–6018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marona, H.; Filipek, B.; Kubacka, M.; Nowak, G. Pochodne 1-(Aroksyalkilo)-4-2(Metoksyfenylo)piperazyny i ich Zastosowania. Available online: https://uprp.gov.pl/sites/default/files/bup/2010/02/bup02_2010.pdf (accessed on 10 April 2021).
- Kubacka, M.; Mogilski, S.; Bednarski, M.; Nowiński, L.; Dudek, M.; Zmudzka, E.; Siwek, A.; Waszkielewicz, A.M.; Marona, H.; Satała, G.; et al. Antidepressant-like activity of aroxyalkyl derivatives of 2-methoxyphenylpiperazine and evidence for the involvement of serotonin receptor subtypes in their mechanism of action. Pharmacol. Biochem. Behav. 2016, 141, 28–41. [Google Scholar] [CrossRef]
- Marona, H.; Kubacka, M.; Filipek, B.; Siwek, A.; Dybała, M.; Szneler, E.; Pociecha, T.; Gunia, A.; Waszkielewicz, A.M. Synthesis, alpha-adrenoceptors affinity and alpha 1-adrenoceptor antagonistic properties of some 1,4-substituted piperazine derivatives. Pharmazie 2011, 66, 733–739. [Google Scholar] [PubMed]
- Marona, H.; Antkiewicz-Michaluk, L. Synthesis and anticonvulsant activity of 1,2-aminoalkanol derivatives. Acta Pol. Pharm. 1998, 55, 487–498. [Google Scholar]
- Waszkielewicz, A.M.; Pytka, K.; Rapacz, A.; Wełna, E.; Jarzyna, M.; Satała, G.; Bojarski, A.; Sapa, J.; Zmudzki, P.; Filipek, B.; et al. Synthesis and evaluation of antidepressant-like activity of some 4-substituted 1-(2-methoxyphenyl) piperazine derivatives. Chem. Biol. Drug Des. 2014, 85, 326–335. [Google Scholar] [CrossRef]
- Pytka, K.; Partyka, A.; Jastrzębska-Więsek, M.; Siwek, A.; Głuch-Lutwin, M.; Mordyl, B.; Kazek, G.; Rapacz, A.; Olczyk, A.; Gałuszka, A.; et al. Antidepressant- and Anxiolytic-Like Effects of New Dual 5-HT1A and 5-HT7 Antagonists in Animal Models. PLoS ONE 2015, 10, e0142499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pytka, K.; Gawlik, K.; Pawlica-Gosiewska, D.; Witalis, J.; Waszkielewicz, A. HBK-14 and HBK-15 with antidepressant-like and/or memory-enhancing properties increase serotonin levels in the hippocampus after chronic treatment in mice. Metab. Brain Dis. 2017, 32, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pytka, K.; Głuch-Lutwin, M.; Kotańska, M.; Waszkielewicz, A.; Kij, A.; Walczak, M. Single Administration of HBK-15—A Triple 5-HT1A, 5-HT7, and 5-HT3 Receptor Antagonist—Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Mol. Neurobiol. 2018, 55, 3931–3945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pytka, K.; Głuch-Lutwin, M.; Kotańska, M.; Żmudzka, E.; Jakubczyk, M.; Waszkielewicz, A.; Janiszewska, P.; Walczak, M. HBK-15 protects mice from stress-induced behavioral disturbances and changes in corticosterone, BDNF, and NGF levels. Behav. Brain Res. 2017, 333, 54–66. [Google Scholar] [CrossRef]
- Orjales, A.; Alonso-Cires, L.; Labeaga, L.; Corcóstegui, R. New (2-Methoxyphenyl)piperazine Derivatives as 5-HT1A Receptor Ligands with Reduced α1-Adrenergic Activity. Synthesis and Structure-Affinity Relationships. J. Med. Chem. 1995, 38, 1273–1277. [Google Scholar] [CrossRef]
- Marona, H. Synteza niektórych pochodnych N-alkiloftalimidów. Acta Pol. Pharm. 1987, 44, 286–291. [Google Scholar]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Kucwaj-Brysz, K.; Wesołowska, A.; Kieć-Kononowicz, K.; Handzlik, J. MF-8, a novel promising arylpiperazine-hydantoin based 5-HT(7) receptor antagonist: In vitro drug-likeness studies and in vivo pharmacological evaluation. Bioorg. Med. Chem. Lett. 2018, 28, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Solanki, A. Comparative in-vitro Intrinsic Clearance of Imipramine in Multiple Species Liver Microsomes: Human, Rat, Mouse and Dog. J. Drug Metab. Toxicol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Lee, C.; Kook, Y.J.; Oh, S.J.; Kang, J.S.; Kim, H.-J.; Han, G. Improving potency and metabolic stability by introducing an alkenyl linker to pyridine-based histone deacetylase inhibitors for orally available RUNX3 modulators. Eur. J. Med. Chem. 2017, 126, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011, 14, 8.10A.1–8.10A.14. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, M.; Miyazaki, S.; Onodera, K. Effects of theophylline in p-chlorophenylalanine-treated mice in a light/dark test. Methods Find. Exp. Clin. Pharmacol. 1996, 18, 513–520. [Google Scholar] [CrossRef]
- Fletcher, A.; Forster, E.A.; Bill, D.J.; Brown, G.; Cliffe, I.A.; Hartley, J.E.; Jones, D.E.; McLenachan, A.; Stanhope, K.J.; Critchley, D.J.; et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996, 73, 337–353. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef]
- Bogdanova, O.V.; Kanekar, S.; D’Anci, K.E.; Renshaw, P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013, 118, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Strömberg, C. Interactions of antidepressants and ethanol on spontaneous locomotor activity and rotarod performance in NMRI and C57BL/6 mice. J. Psychopharmacol. 1988, 2, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Szkutnik-Fiedler, D.; Kus, K.; Balcerkiewicz, M.; Grzesḱowiak, E.; Nowakowska, E.; Burda, K.; Ratajczak, P.; Sadowski, C. Concomitant use of tramadol and venlafaxine-Evaluation of antidepressant-like activity and other behavioral effects in rats. Pharmacol. Rep. 2012, 64, 1350–1358. [Google Scholar] [CrossRef]
- Watzman, N.; Barry, H., 3rd. Drug effects on motor coordination. Psychopharmacologia 1968, 12, 414–423. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Gammans, R.E.; Mayol, R.F.; LaBudde, J.A. Metabolism and disposition of buspirone. Am. J. Med. 1986, 80, 41–51. [Google Scholar] [CrossRef]
- Słoczyńska, K.; Gunia-Krzyżak, A.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Żelaszczyk, D.; Popiół, J.; Pękala, E. Metabolic stability and its role in the discovery of new chemical entities. Acta Pharm. 2019, 69, 345–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pytka, K.; Głuch-Lutwin, M.; Żmudzka, E.; Sałaciak, K.; Siwek, A.; Niemczyk, K.; Walczak, M.; Smolik, M.; Olczyk, A.; Gałuszka, A.; et al. HBK-17, a 5-HT1A Receptor Ligand With Anxiolytic-Like Activity, Preferentially Activates ß-Arrestin Signaling. Front. Pharmacol. 2018, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Brossard, G.; Hautbois, C.; Roux, S. Models of Affective Illness: Forced Swimming and Tail Suspension Tests in Rodents. Curr. Protoc. Pharmacol. 2000, 10, 1–9. [Google Scholar] [CrossRef]
- Pytka, K.; Rapacz, A.; Zygmunt, M.; Olczyk, A.; Waszkielewicz, A.; Sapa, J.; Filipek, B. Antidepressant-like activity of a new piperazine derivative of xanthone in the forced swim test in mice: The involvement of serotonergic system. Pharmacol. Rep. 2015, 67, 160–165. [Google Scholar] [CrossRef]
- Manosso, L.M.; Neis, V.B.; Moretti, M.; Daufenbach, J.F.; Freitas, A.E.; Colla, A.R.; Rodrigues, A.L.S. Antidepressant-like effect of α-tocopherol in a mouse model of depressive-like behavior induced by TNF-α. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska, A.; Satała, G.; Kołaczkowski, M.; Bucki, A.; Głuch-Lutwin, M.; Świerczek, A.; Pociecha, K.; Partyka, A.; Jastrzębska-Więsek, M.; Lubelska, A.; et al. Novel anilide and benzylamide derivatives of arylpiperazinylalkanoic acids as 5-HT1A/5-HT7 receptor antagonists and phosphodiesterase 4/7 inhibitors with procognitive and antidepressant activity. Eur. J. Med. Chem. 2020, 201, 112437. [Google Scholar] [CrossRef]
- Czopek, A.; Byrtus, H.; Kołaczkowski, M.; Pawłowski, M.; Dybała, M.; Nowak, G.; Tatarczyńska, E.; Wesołowska, A.; Chojnacka-Wójcik, E. Synthesis and pharmacological evaluation of new 5-(cyclo)alkyl-5-phenyl- and 5-spiroimidazolidine-2,4-dione derivatives. Novel 5-HT1A receptor agonist with potential antidepressant and anxiolytic activity. Eur. J. Med. Chem. 2010, 45, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Oficialdegui, A.M.; Martinez, J.; Pérez, S.; Heras, B.; Irurzun, M.; Palop, J.A.; Tordera, R.; Lasheras, B.; Del Río, J.; Monge, A. Design, synthesis and biological evaluation of new 3-[(4-aryl)piperazin- 1-yl]-1-arylpropane derivatives as potential antidepressants with a dual mode of action: Serotonin reuptake inhibition and 5-HT(1A) receptor antagonism. Farmaco 2000, 55, 345–353. [Google Scholar] [CrossRef]
- Gu, Z.S.; Xiao, Y.; Zhang, Q.W.; Li, J.Q. Synthesis and antidepressant activity of a series of arylalkanol and aralkyl piperazine derivatives targeting SSRI/5-HT1A/5-HT7. Bioorg. Med. Chem. Lett. 2017, 27, 5420–5423. [Google Scholar] [CrossRef]
- Gu, Z.S.; Zhou, A.N.; Xiao, Y.; Zhang, Q.W.; Li, J.Q. Synthesis and antidepressant-like activity of novel aralkyl piperazine derivatives targeting SSRI/5-HT1A/5-HT7. Eur. J. Med. Chem. 2018, 144, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Kazek, G.; Siwek, A.; Mordyl, B.; Głuch-Lutwin, M.; Rapacz, A.; Olczyk, A.; Gałuszka, A.; Waszkielewicz, A.; Marona, H.; et al. HBK-7—A new xanthone derivative and a 5-HT1A receptor antagonist with antidepressant-like properties. Pharmacol. Biochem. Behav. 2016, 146–147, 35–43. [Google Scholar] [CrossRef]
- Sałaciak, K.; Głuch-Lutwin, M.; Siwek, A.; Szafarz, M.; Kazek, G.; Bednarski, M.; Nowiński, L.; Mitchell, E.; Jastrzębska-Więsek, M.; Partyka, A.; et al. The antidepressant-like activity of chiral xanthone derivatives may be mediated by 5-HT1A receptor and β-arrestin signalling. J. Psychopharmacol. 2020, 34, 1431–1442. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Roberts, R.C.; Conley, R.C.; Tamminga, C.A.; Pandey, G.N. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J. Neurochem. 2001, 77, 916–928. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.P.; Rosa, J.M.; Colla, A.R.S.; Lieberknecht, V.; Oliveira, Á.; Rodrigues, A.L.S. Creatine, Similar to Ketamine, Counteracts Depressive-Like Behavior Induced by Corticosterone via PI3K/Akt/mTOR Pathway. Mol. Neurobiol. 2016, 53, 6818–6834. [Google Scholar] [CrossRef]
- Pytka, K.; Podkowa, K.; Rapacz, A.; Podkowa, A.; Zmudzka, E.; Olczyk, A.; Sapa, J.; Filipek, B. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol. Rep. 2016, 68, 263–274. [Google Scholar] [CrossRef]
- Sterner, E.Y.; Kalynchuk, L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: Relevance to depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 777–790. [Google Scholar] [CrossRef]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef]
- Jacobsen, J.P.R.; Mørk, A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006, 1110, 221–225. [Google Scholar] [CrossRef]
- Fairchild, G.; Leitch, M.M.; Ingram, C.D. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology 2003, 45, 925–934. [Google Scholar] [CrossRef]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.P.; et al. Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galeotti, N.; Ghelardini, C.; Bartolini, A. Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology 2001, 40, 75–84. [Google Scholar] [CrossRef]
- Pytka, K.; Socała, K.; Rapacz, A.; Nieoczym, D.; Pieróg, M.; Gryboś, A.; Siwek, A.; Waszkielwicz, A.; Wlaź, P. HBK-14 and HBK-15, triple 5-HT1A, 5-HT7 and 5-HT3 antagonists with potent antidepressant- and anxiolytic-like properties, increase seizure threshold in various seizure tests in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Głuch-Lutwin, M.; Knutelska, J.; Jakubczyk, M.; Waszkielewicz, A.; Kotańska, M. HBK-14 and HBK-15 Do Not Influence Blood Pressure, Lipid Profile, Glucose Level, or Liver Enzymes Activity after Chronic Treatment in Rats. PLoS ONE 2016, 11, e0165495. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Lustyk, K.; Żmudzka, E.; Kotańska, M.; Siwek, A.; Zygmunt, M.; Dziedziczak, A.; Śniecikowska, J.; Olczyk, A.; Gałuszka, A.; et al. Chemically Homogenous Compounds with Antagonistic Properties at All α1-Adrenoceptor Subtypes but not β1-Adrenoceptor Attenuate Adrenaline-Induced Arrhythmia in Rats. Front. Pharmacol. 2016, 7, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obach, R.S. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999, 27, 1350–1359. [Google Scholar] [PubMed]
- Pytka, K.; Walczak, M.; Kij, A.; Rapacz, A.; Siwek, A.; Kazek, G.; Olczyk, A.; Gałuszka, A.; Waszkielewicz, A.; Marona, H.; et al. The antidepressant-like activity of 6-methoxy-2-[4-(2-methoxyphenyl)piperazin-1-yl]-9H-xanthen-9-one involves serotonergic 5-HT1A and 5-HT2A/C receptors activation. Eur. J. Pharmacol. 2015, 764, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Zmudzka, E.; Lustyk, K.; Rapacz, A.; Olczyk, A.; Galuszka, A.; Waszkielewicz, A.; Marona, H.; Sapa, J.; Barbara, F. The antidepressant- and anxiolytic-like activities of new xanthone derivative with piperazine moiety in behavioral tests in mice. Indian J. Pharmacol. 2016, 48, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Boissier, P.J.-R.; Tardy, J.; Diverres, J.-C. A new simple method to explore the tranquilizing action: The chimney test. Med. Exp. 1960, 3, 81–84. [Google Scholar] [CrossRef]
| HBK-10 Concentration | Absorbance (OD570 nm) |
Viability (% ± SEM) |
|---|---|---|
| Blank | 0.516 | 100 |
| 1 | 0.531 | 102 ± 3.3 |
| 5 | 0.569 | 109 ± 0.9 |
| 25 | 0.463 | 99 ± 2.4 |
| Treatment | Protein Concentration | % Remaining after 30 min | t1/2 min | Intrinsic Clearance (Clint) |
|---|---|---|---|---|
| HBK-10 | 0.8 | 36 | 25 | 34.6 |
| Buspirone | 0.5 | 0.29 a | - | - |
| Aripiprazole | 1.0 | - | 217 b | 12.5 b |
| Imipramine | 0.5 | - | 11.0 c | 125.5 c |
| Treatment | Serotonergic pKi [nM] | Dopaminergic pKi [nM] | ||||
|---|---|---|---|---|---|---|
| 5-HT1A a,c | 5-HT2A a,d | SERT b,e | 5-HT6 a,f | 5-HT7 a,g | D2 a,h | |
| HBK-10 | 8.29 ± 0.10 | 6.72 ± 0.31 | 5.43 ± 0.32 | 5.43 ± 0.22 | 5.20 ± 0.20 | 7.30 ± 0.19 |
| Serotonin | 8.62 ± 0.13 | - | - | - | - | - |
| Mianserin | - | 8.92 ± 0.06 | - | - | - | - |
| Risperidone | - | 9.70 ± 0.19 | - | - | - | - |
| Escitalopram | - | - | 8.68 ± 0.03 | - | - | - |
| Clozapine | - | - | - | 8.40 ± 0.13 | 7.74 ± 0.16 | - |
| Haloperidol | - | - | - | - | - | 8.96 ± 0.07 |
| Agonist Mode | Antagonist Mode | |||||
|---|---|---|---|---|---|---|
| Treatment | Emax % | pEC50 ± SEM | Emax % | pIC50 ± SEM | Kb | R2 |
| nM | Kb | |||||
| Serotonin | 100% | 8.08 ± 0.36 | - | - | - | - |
| NAN-190 | - | - | 2% | 7.72 ± 0.114 | 6.4 | 0.919 |
| HBK-10 | 2% | n.c. | 0% | 6.72 ± 0.093 | 64 | 0.831 |
| Agonist Mode | Antagonist Mode | |||||
|---|---|---|---|---|---|---|
| Treatment | Emax % | pEC50 ± SEM | Emax % | pIC50 ± SEM | Kb nM | R2 Kb |
| Apomorphine | 100% | 7.89 ± 0.36 | 1% | n.c. | n.c. | n.c. |
| Chlorpromazine | 5% | n.c. | 1% | 8.53 | 0.88 | 0.956 |
| HBK-10 | 9% | n.c. | 0% | 8.03 | 2.76 | 0.985 |
| Treatment | Corticosterone Injections | Dose (mg/kg) | Number of Crossings ± SEM |
|---|---|---|---|
| vehicle | - | - | 515.9 ± 41.18 |
| vehicle | + | - | 402.9 ± 43.30 |
| HBK-10 | + | 0.625 | 431.1 ± 47.92 |
| HBK-10 | + | 1.25 | 453.9 ± 41.27 |
| Fluoxetine | + | 15 | 462.5 ± 41.81 |
| Treatment | Dose (mg/kg) | Number of Crossings ± SEM | ED50 (mg/kg) |
|---|---|---|---|
| vehicle | - | 355.1 ± 31.38 | 32.3 (19.2–54.3) |
| HBK-10 | 10 | 355.1 ± 49.92 | |
| 20 | 329.8 ± 34.52 | ||
| 40 | 155.4 ± 29.59 *** | ||
| 60 | 22.17 ± 2.03 **** |
| Rotarod Test | Chimney Test | |||||
|---|---|---|---|---|---|---|
| Treatment | Dose (mg/kg) | % of Animals That Fell from Rotating Rod | TD50 (mg/kg) | Dose (mg/kg) | % of Animals That Did Not Climb out the Chimney | TD50 (mg/kg) |
| HBK-10 | 44.3 (37.8–51.8) | 10 | 20 | 21.7 (13.0–36.4) | ||
| 40 | 29 | 20 | 40 | |||
| 45 | 63 | 30 | 64 | |||
| 60 | 86 | 40 | 87 | |||
| 60 | 90 | |||||
| Receptor | Radioligand/Final Concentration | Blank (Nonspecific) | Buffer | Incubation Conditions |
|---|---|---|---|---|
| 5-HT1A | [3H]8-OH-DPAT 1 nM | 10 µM serotonin | 50 mM Tris-HCl pH 7.4 10 mM MgSO4, 0.5 mM EDTA, 0.1% ascorbic acid | 60 min, 27 °C |
| 5-HT2A | [3H]-ketanserin 0.5 nM | 10 µM mianserin | 50 mM Tris-HCl pH 7.4 4 mM CaCl2, 0.1% ascorbic acid | 60 min, 27 °C |
| 5-HT6 | [3H]-LSD 2 nM | 10 µM methiothepin | 50 mM Tris–HCl pH 7.4 0.5 mM EDTA, 4 mM MgCl2 | 60 min, 37 °C |
| 5-HT7 | [3H]-5-CT 0.6 nM | 10 µM serotonin | 50 mM Tris–HCl pH 7.4 4 mM MgCl2, 10 µM pargyline, 0.1% ascorbic acid | 60 min, 37 °C |
| SERT | [3H]-citalopram 1 nM | 1 µM imipramine | 50 mM Tris-HCl pH 7.7 150 mM NaCl, 5 mM KCl | 60 min, 23 °C |
| D2 | [3H]-methylspiperon 0.4 nM | 10 µM (+)-butaclamol | 50 mM HEPES, pH 7.4, 50 mM NaCl, 5 mM MgCl2, 0.5 mM EDTA | 60 min, 37 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sałaciak, K.; Malikowska-Racia, N.; Lustyk, K.; Siwek, A.; Głuch-Lutwin, M.; Kazek, G.; Popiół, J.; Sapa, J.; Marona, H.; Żelaszczyk, D.; et al. Synthesis and Evaluation of the Antidepressant-like Properties of HBK-10, a Novel 2-Methoxyphenylpiperazine Derivative Targeting the 5-HT1A and D2 Receptors. Pharmaceuticals 2021, 14, 744. https://doi.org/10.3390/ph14080744
Sałaciak K, Malikowska-Racia N, Lustyk K, Siwek A, Głuch-Lutwin M, Kazek G, Popiół J, Sapa J, Marona H, Żelaszczyk D, et al. Synthesis and Evaluation of the Antidepressant-like Properties of HBK-10, a Novel 2-Methoxyphenylpiperazine Derivative Targeting the 5-HT1A and D2 Receptors. Pharmaceuticals. 2021; 14(8):744. https://doi.org/10.3390/ph14080744
Chicago/Turabian StyleSałaciak, Kinga, Natalia Malikowska-Racia, Klaudia Lustyk, Agata Siwek, Monika Głuch-Lutwin, Grzegorz Kazek, Justyna Popiół, Jacek Sapa, Henryk Marona, Dorota Żelaszczyk, and et al. 2021. "Synthesis and Evaluation of the Antidepressant-like Properties of HBK-10, a Novel 2-Methoxyphenylpiperazine Derivative Targeting the 5-HT1A and D2 Receptors" Pharmaceuticals 14, no. 8: 744. https://doi.org/10.3390/ph14080744