Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety
Abstract
1. Introduction
2. Materials and Methods
3. Results
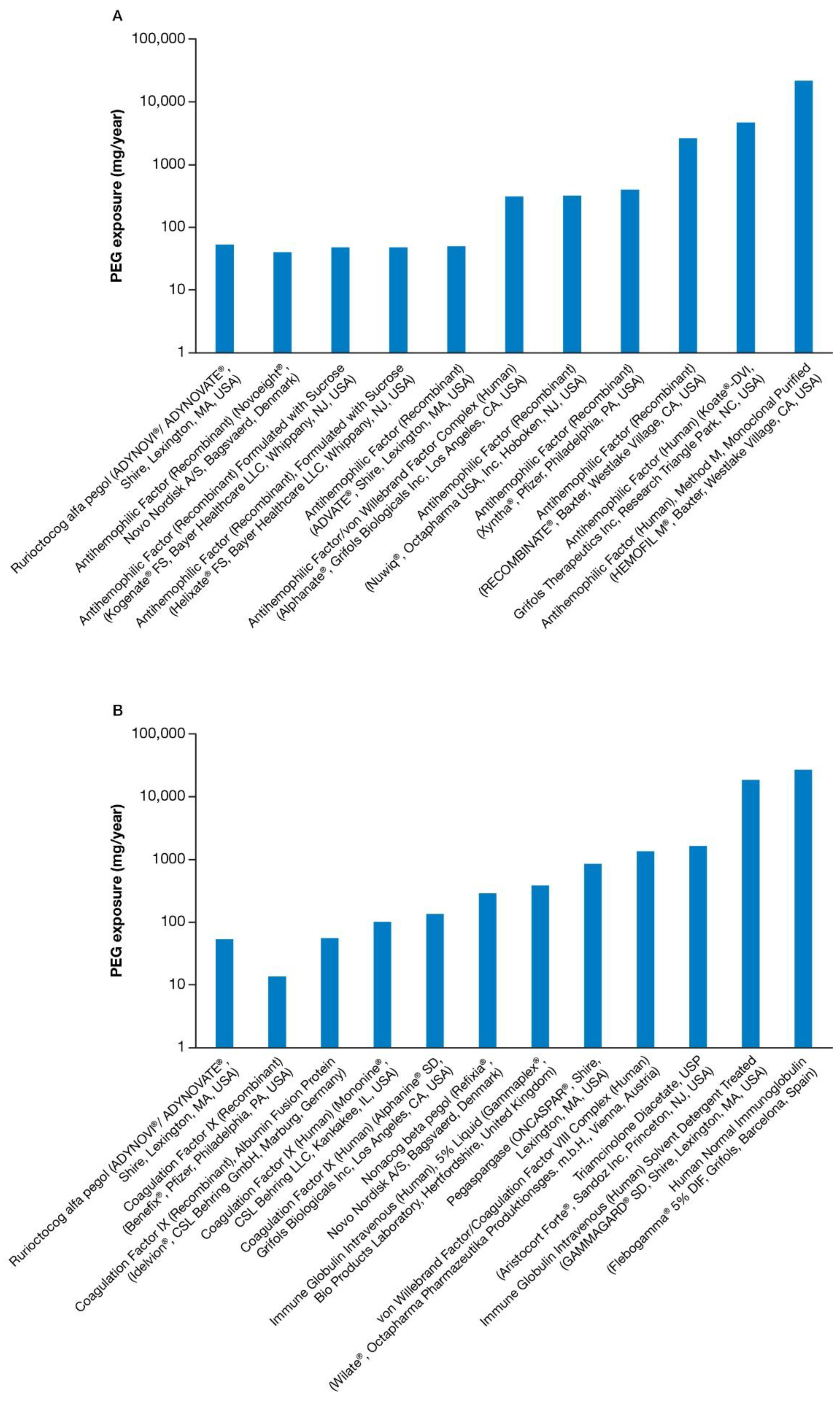
3.1. PEG Exposure
3.2. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swierczewska, M.; Lee, K.C.; Lee, S. What is the future of PEGylated therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.; Didier, E.; Harris, P.; Siegel, N.; Stadler, J.; Tilbury, L.; Smith, D. PEGylated proteins: Evaluation of their safety in the absence of definitive metabolism studies. Drug. Metab. Dispos. 2007, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ivens, I.A.; Baumann, A.; McDonald, T.A.; Humphries, T.J.; Michaels, L.A.; Mathew, P. PEGylated therapeutic proteins for haemophilia treatment: A review for haemophilia caregivers. Haemophilia 2013, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, C.F.; Simone, J.V.; Corrigan, J.J.; Seeler, R.A.; Edelstein, G.; Vanderheiden, J.; Schulman, I. Treatment of hemophilia with glycine-precipitated factor 8. N. Engl. J. Med. 1966, 275, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Rebinyn Package Insert. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004178/WC500232816.pdf (accessed on 28 November 2017).
- Grigoletto, A.; Maso, K.; Mero, A.; Rosato, A.; Schiavon, O.; Pasut, G. Drug and protein delivery by polymer conjugation. J. Drug Deliv. Sci. Technol. 2016, 32, 132–141. [Google Scholar] [CrossRef]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Stidl, R.; Fuchs, S.; Bossard, M.; Siekmann, J.; Turecek, P.L.; Putz, M. Safety of PEGylated recombinant human full-length coagulation factor VIII (BAX 855) in the overall context of PEG and PEG conjugates. Haemophilia 2016, 22, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Konkle, B.A.; Stasyshyn, O.; Chowdary, P.; Bevan, D.H.; Mant, T.; Shima, M.; Engl, W.; Dyck-Jones, J.; Fuerlinger, M.; Patrone, L.; et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood 2015, 126, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- FDA Regulatory Approval of Rebinyn: Summary Basis for Regulatory Action. Available online: https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM564338.pdf (accessed on 23 January 2018).
- Zhang, F.; Liu, M.R.; Wan, H.T. Discussion about several potential drawbacks of PEGylated therapeutic proteins. Biol. Pharm. Bull. 2014, 37, 335–339. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. CHMP Safety Working Party’s Response to the PDCO Regarding the Use of PEGylated Drug Products in the Pediatric Population. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/11/WC500135123.pdf (accessed on 2 January 2018).
- Collins, P.W.; Bjorkman, S.; Fischer, K.; Blanchette, V.; Oh, M.; Schroth, P.; Fritsch, S.; Casey, K.; Spotts, G.; Ewenstein, B.M. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: Influences of variance in pharmacokinetics and treatment regimens. J. Thromb. Haemost. 2010, 8, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ivens, I.A.; Achanzar, W.; Baumann, A.; Brandli-Baiocco, A.; Cavagnaro, J.; Dempster, M.; Depelchin, B.O.; Rovira, A.R.; Dill-Morton, L.; Lane, J.H.; et al. PEGylated Biopharmaceuticals: Current Experience and Considerations for Nonclinical Development. Toxicol. Pathol. 2015, 43, 959–983. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Nara, A.; Uemura, K. Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biol. Toxicol. 2012, 28, 125–131. [Google Scholar] [PubMed]
- Chen, R.; Duan, C.Y.; Chen, S.K.; Zhang, C.Y.; He, T.; Li, H.; Liu, Y.P.; Dai, R.Y. The suppressive role of p38 MAPK in cellular vacuole formation. J. Cell. Biochem. 2013, 114, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Henics, T.; Wheatley, D.N. Cytoplasmic vacuolation, adaptation and cell death: A view on new perspectives and features. Biol. Cell 1999, 91, 485–498. [Google Scholar] [PubMed]
- Bendele, A.; Seely, J.; Richey, C.; Sennello, G.; Shopp, G. Short communication: Renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol. Sci. 1998, 42, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Rudmann, D.G.; Alston, J.T.; Hanson, J.C.; Heidel, S. High molecular weight polyethylene glycol cellular distribution and PEG-associated cytoplasmic vacuolation is molecular weight dependent and does not require conjugation to proteins. Toxicol. Pathol. 2013, 41, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.B.; Mohan, P.; Narkewicz, M.R.; Molleston, J.P.; Nash, S.R.; Hu, S.; Wang, K.; Gries, J.M. Safety, efficacy and pharmacokinetics of peginterferon alpha2a (40 kd) in children with chronic hepatitis C. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 499–505. [Google Scholar] [CrossRef] [PubMed]
- CDC Pediatric Weight Chart. Available online: https://www.cdc.gov/growthcharts/clinical_charts.htm (accessed on 23 January 2018).
- Van Tellingen, O.; Beijnen, J.H.; Verweij, J.; Scherrenburg, E.J.; Nooijen, W.J.; Sparreboom, A. Rapid esterase-sensitive breakdown of polysorbate 80 and its impact on the plasma pharmacokinetics of docetaxel and metabolites in mice. Clin. Cancer Res. 1999, 5, 2918–2924. [Google Scholar] [PubMed]
- Zhang, R.; Wang, Y.; Tan, L.; Zhang, H.Y.; Yang, M. Analysis of polysorbate 80 and its related compounds by RP-HPLC with ELSD and MS detection. J. Chromatogr. Sci. 2012, 50, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.M.; Stephens, N.V.; Vincent, S.; Raghavan, N.; Sand, P.J. Determination of polysorbate 80 in parenteral formulations by high-performance liquid chromatography and evaporative light scattering detection. J. Chromatogr. A 2003, 1012, 81–86. [Google Scholar] [CrossRef]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia. 2013, 19, e1–e47. [Google Scholar] [CrossRef] [PubMed]
- ADYNOVATE Package Insert. Available online: http://www.adynovate.com/search_results.html?q=package+insert (accessed on 2 January 2018).
- Rasmussen, C.E.; Nowak, J.; Larsen, J.M.; Bottomley, A.; Rowles, A.; Offenberg, H. Evaluation of Nonacog Beta Pegol Long-term Safety in the Immune-deficient Rowett Nude Rat (Crl:NIH-Foxn1rnu). Toxicol. Pathol. 2016, 44, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Avramis, V.I.; Sencer, S.; Periclou, A.P.; Sather, H.; Bostrom, B.C.; Cohen, L.J.; Ettinger, A.G.; Ettinger, L.J.; Franklin, J.; Gaynon, P.S.; et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: A Children’s Cancer Group study. Blood 2002, 99, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Refixia EMA Authorization. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004178/WC500224408.pdf (accessed on 23 January 2018).
- Stafford, D.W. Extravascular FIX and coagulation. Thromb. J. 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, A.; Liu, Z.; Tan, S.; Chen, K.; Drager, D.; Liu, T.; Patarroyo-White, S.; Jiang, H.; Light, D.R. FcRn Rescues Recombinant Factor VIII Fc Fusion Protein from a VWF Independent FVIII Clearance Pathway in Mouse Hepatocytes. PLoS ONE 2015, 10, e0124930. [Google Scholar] [CrossRef] [PubMed]
- Orlova, N.A.; Kovnir, S.V.; Vorobiev, I.I.; Gabibov, A.G. Coagulation Factor IX for Hemophilia B Therapy. Acta Nat. 2012, 4, 62–73. [Google Scholar]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J. Pharm. Sci. 1994, 83, 601–606. [Google Scholar] [CrossRef] [PubMed]
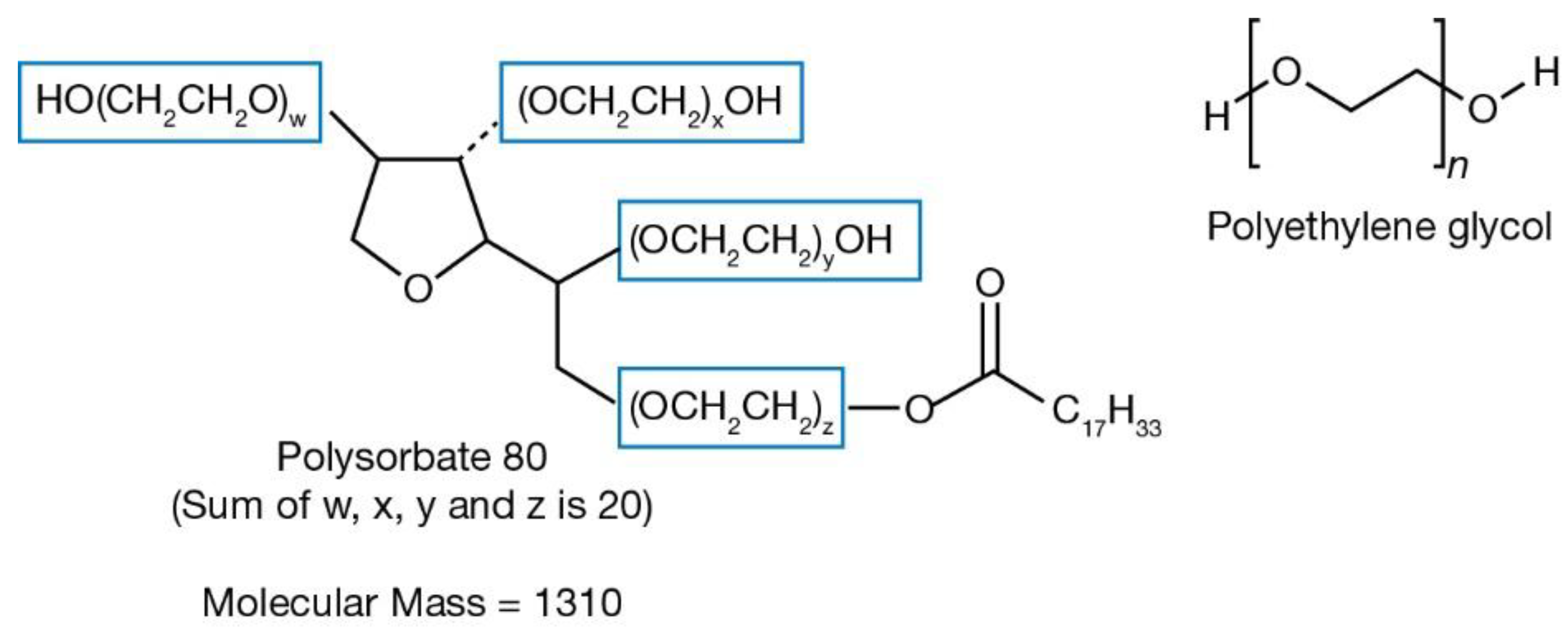
| Product (Brand Name, Manufacturer)/Year Approved | Dosage | Peg Conjugated | Peg as Excipient | Type of Peg | ||
|---|---|---|---|---|---|---|
| Per Dose (mg) | Per Year (mg) | Per Dose (mg) | Per Year (mg) | |||
| FVIII Replacement Therapies | ||||||
| Rurioctocog alfa pegol (ADYNOVI®/ADYNOVATE®, Shire, Lexington, MA, USA)/2015 | 50 IU/kg | <1 | 5 | <1 | 17 | 20 kDa PEG *, PS80 |
| Antihemophilic Factor (Recombinant) (ADVATE®, SHIRE, Lexington, MA, USA)/2003 | 25 IU/kg | 0 | 0 | <1 | 50 | PS80 |
| Antihemophilic Factor/von Willebrand Factor Complex (Human) (Alphanate®, Grifols Biologicals Inc., Los Angeles, CA, USA)/1978 | 25 IU/kg | 0 | 0 | <1 | 39 | PS80/PEG mixture |
| Antihemophilic Factor (Recombinant), Formulated with Sucrose (Helixate FS®, Bayer Healthcare LLC, Whippany, NJ, USA)/1993 | 25 IU/kg | 0 | 0 | <1 | 24 | PS80 |
| Antihemophilic Factor (Human), Method M, Monoclonal Purified (HEMOFIL M®, Baxter, Westlake Village, CA, USA)/1966 | 25 IU/kg | 0 | 0 | 18 | 2730 | PEG 3350 |
| Antihemophilic Factor (Human) (Koate®-DVI, Grifols Therapeutics Inc., Research Triangle Park, NC, USA)/1974 | 25 IU/kg | 0 | 0 | 8 | 1183 | PEG + PS80 |
| Antihemophilic Factor (Recombinant) Formulated with Sucrose (Kogenate® FS, Bayer Healthcare LLC, Whippany, NJ, USA)/1993 | 25 IU/kg | 0 | 0 | <1 | 24 | PS80 |
| Antihemophilic Factor (Recombinant) (NovoEight®, Novo Nordisk A/S, Bagsvaerd, Denmark)/2013 | 25 IU/kg | 0 | 0 | <1 | 40 | PS80 |
| Antihemophilic Factor (Recombinant) (Nuwiq®, Octapharma USA, INC, Hoboken, NJ, USA)/2015 | 25 IU/kg | 0 | 0 | 2 | 323 | P188 |
| Antihemophilic Factor (Recombinant) (RECOMBINATE®, Baxter, Westlake Village, CA, USA)/1992 | 25 IU/kg | 0 | 0 | 15 | 2378 | PEG 3350 + PS80 |
| Antihemophilic Factor (Recombinant) (Xyntha®, Pfizer, Philadelphia, PA, USA)/2008 | 25 IU/kg | 0 | 0 | 3 | 402 | PS80 |
| von Willebrand Factor/Coagulation Factor VIII Complex (Human) (Wilate®, Octapharma Pharmazeutika Produktionsges m.b.H., Vienna, Austria)/2009 | 25 IU/kg | 0 | 0 | 2 | 168 | PS80 |
| Factor IX (FIX) Replacement Therapies | ||||||
| Nonacog beta pegol (Refixia® †, Novo Nordisk A/S, Bagsvaerd, Denmark)/2017 | 40 IU/kg | 1 | 56 | <1 | 5 | 40 kDa PEG *, PS80 |
| Coagulation Factor IX (Human) (Alphanine® SD, Grifols Biologicals Inc., Los Angeles, CA, USA)/1998 | 25 IU/kg | 0 | 0 | <1 | 17 | PS80 |
| Coagulation Factor IX (Recombinant) (Benefix®, Pfizer, Philadelphia, PA, USA)/1997 | 25 IU/kg | 0 | 0 | <1 | 13 | PS80 |
| Coagulation Factor IX (Recombinant), Albumin Fusion Protein (Idelvion®, CSL Behring GmbH, Marburg, Germany)/2016 | 25 IU/kg | 0 | 0 | <1 | 5 | PS80 |
| Coagulation Factor IX (Human) (Mononine®, CSL Behring LLC, Kankakee, IL, USA)/1992 | 25 IU/kg | 0 | 0 | <1 | 13 | PS80 |
| Other Pediatric-indicated Therapies | ||||||
| Pegaspargase (ONCASPAR®, Shire, Lexington, MA, USA)/1994 | 2500 IU/m2 | 11 | 274 | 0 | 0 | 5 kDa PEG * |
| Triamcinolone Diacetate, USP (Aristocort Forte®, Sandoz Inc., Princeton, NJ, USA)/1961 | 40 mg/dose | 0 | 0 | 31 | 1627 | PEG + PS80 |
| Human Normal Immunoglobulin (Flebogamma® 5% DIF, Grifols, Barcelona, Spain)/2006 | 300 mg/kg | 0 | 0 | 180 | 2674 | PEG |
| Immune Globulin Intravenous (Human) Solvent Detergent Treated (GAMMAGARD® SD, Shire, Lexington, MA, USA)/1986 | 300 g/kg | 0 | 0 | 124 | 1840 | PEG + PS80 |
| Immune Globulin Intravenous (Human), 5% Liquid (Gammaplex®, Bio Products Laboratory, Hertfordshire, UK)/2009 | 300 mg/kg | 0 | 0 | 2 | 29 | PS80 |
| Drug | Renal Adverse Events | Liver Adverse Events | CNS Adverse Events |
|---|---|---|---|
| Rurioctocog alfa pegol (ADYNOVI®/ADYNOVATE®, Shire, Lexington, MA, USA) | NA | NA | NA |
| Antihemophilic Factor (Recombinant) (ADVATE®, Shire, Lexington, MA, USA) | NA | NA | NA |
| Antihemophilic Factor/von Willebrand Factor Complex (Human) (Alphanate®, Grifols Biologicals Inc., Los Angeles, CA, USA) | NA | NA | NA |
| Antihemophilic Factor (Recombinant), Formulated with Sucrose (Helixate FS®, Bayer Healthcare LLC, Whippany, NJ, USA) | NA | NA | NA |
| Antihemophilic Factor (Human), Method M, Monoclonal Purified (HEMOFIL M®, Baxter, Westlake Village, CA, USA) | NA | NA | NA |
| Antihemophilic Factor (Human) (Koate®-DVI, Grifols Therapeutics Inc., Research Triangle Park, NC, USA) | NA | NA | NA |
| Antihemophilic Factor (Recombinant) Formulated with Sucrose (Kogenate® FS, Bayer Healthcare LLC, Whippany, NJ, USA) | NA | NA | NA |
| Antihemophilic Factor (Recombinant) (NovoEight®, Novo Nordisk A/S, Bagsvaerd, Denmark) | NA | Increased hepatic enzymes | NA |
| Antihemophilic Factor (Recombinant) (Nuwiq®, Octapharma USA, Inc., Hoboken, NJ, USA) | NA | NA | NA |
| Antihemophilic Factor (Recombinant) (RECOMBINATE®, Baxter, Westlake Village, CA, USA) | NA | NA | NA |
| Antihemophilic Factor (Recombinant) (Xyntha®, Pfizer, Philadelphia, PA, USA) | NA | NA | NA |
| Coagulation Factor IX (Human) (AlphaNine® SD, Grifols Biologicals Inc., Los Angeles, CA, USA) | NA | NA | NA |
| Coagulation Factor IX (Recombinant), Albumin Fusion Protein (Idelvion®, CSL Behring GMBH, Marburg, Germany) | NA | NA | Dizziness |
| Nonacog beta pegol (Refixia®/Rebinyn®, Novo Nordisk A/S, Bagsvaerd, Denmark) | NA | NA | NA |
| von Willebrand Factor/Coagulation Factor VIII Complex (Human) (Wilate®, Octapharma Pharmazeutika Produktionsges m.b.H., Vienna, Austria) | NA | NA | NA |
| Coagulation Factor IX (Recombinant) (Benefix®, Pfizer, Philadelphia, PA, USA) | Rare: Renal infarct | NA | NA |
| Coagulation Factor IX (Human) (Mononine®, CSL Behring LLC, Kankakee, IL, USA) | Nephrotic syndrome in hemophilia patients with a history of hypersensitivity reactions | NA | NA |
| Immune Globulin Intravenous (Human) Solvent Detergent Treated (Gammagard® SD, Shire, Lexington, MA, USA) | Common among IG products: Acute renal dysfunction/failure, osmotic nephropathy | Common amongst IG products: hepatic dysfunction | Common among IG products: Coma, loss of consciousness, seizures, tremor, aseptic meningitis syndrome |
| Immune Globulin Intravenous (Human), 5% Liquid (Gammaplex®, Bio Products Laboratory, Hertfordshire, UK) | Common among IG products: Acute renal dysfunction/failure, osmotic nephropathy | Common among IG products: hepatic dysfunction, abdominal pain | Migraine, aseptic meningitis Common among IG products: Coma, loss of consciousness, seizures, tremor, aseptic meningitis syndrome |
| Human Normal Immunoglobulin (Flebogamma® 5% DIF, Grifols, Barcelona, Spain) | Common among IG products: Acute renal dysfunction/failure, osmotic nephropathy | Common among IG products: Hepatic dysfunction, abdominal pain | Common among IG products: Coma, loss of consciousness, seizures, tremor, aseptic meningitis syndrome |
| Triamcinolone Diacetate, USP (Aristocort Forte®, Sandoz Inc., Princeton, NJ, USA) | Common among corticosteroids: elevation of blood pressure, salt and water retention, and increased excretion of potassium and calcium, renal failure | Bowel/bladder dysfunction, elevation in serum liver enzyme levels, hepatomegaly Common among corticosteroids: hepatic failure | Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychic disorders, vertigo. Arachnoiditis, meningitis, paraparesis/paraplegia, and sensory disturbances Common neurological AEs among corticosteroids: Spinal cord infarction, paraplegia, quadriplegia, cortical blindness, seizure, neurological deterioration, and stroke |
| Pegaspargase (ONCASPAR®, Shire, Lexington, MA, USA) | Increased BUN, increased creatinine, urinary frequency, hematuria due to thrombocytopenia, severe hemorrhagic cystitis, renal dysfunction, and renal failure. | Jaundice, ascites, and hypoalbuminemia, hepatomegaly, fatty changes in the liver and liver failure. Hepatotoxicity and abnormal liver function, including elevations of AST, ALT, ALP, bilirubin, and depression of SA, and plasma fibrinogen | CNS thrombosis/hemorrhage |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stidl, R.; Denne, M.; Goldstine, J.; Kadish, B.; Korakas, K.I.; Turecek, P.L. Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety. Pharmaceuticals 2018, 11, 75. https://doi.org/10.3390/ph11030075
Stidl R, Denne M, Goldstine J, Kadish B, Korakas KI, Turecek PL. Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety. Pharmaceuticals. 2018; 11(3):75. https://doi.org/10.3390/ph11030075
Chicago/Turabian StyleStidl, Reinhard, Michael Denne, Jimena Goldstine, Bill Kadish, Katherine I. Korakas, and Peter L. Turecek. 2018. "Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety" Pharmaceuticals 11, no. 3: 75. https://doi.org/10.3390/ph11030075
APA StyleStidl, R., Denne, M., Goldstine, J., Kadish, B., Korakas, K. I., & Turecek, P. L. (2018). Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety. Pharmaceuticals, 11(3), 75. https://doi.org/10.3390/ph11030075





