Optical Slot-Waveguide Based Biochemical Sensors
Abstract
:1. Introduction
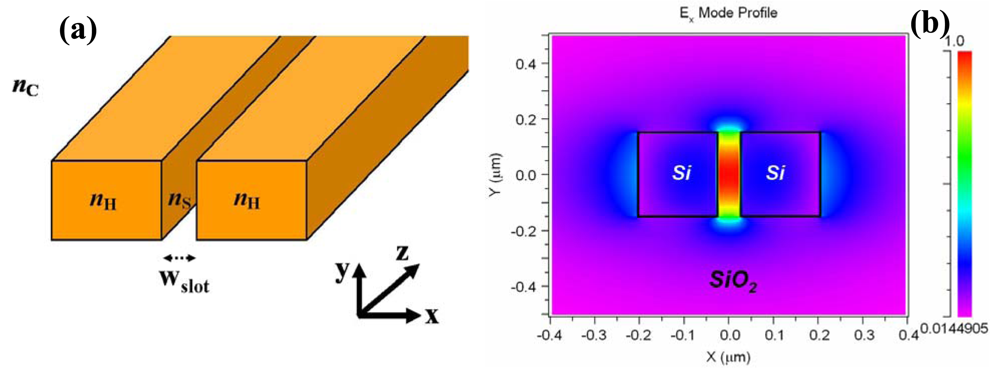
2. Slot-Waveguide Based Refractometric Sensors
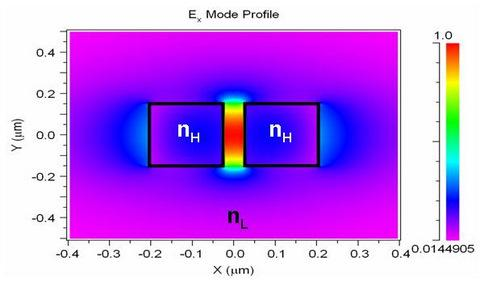
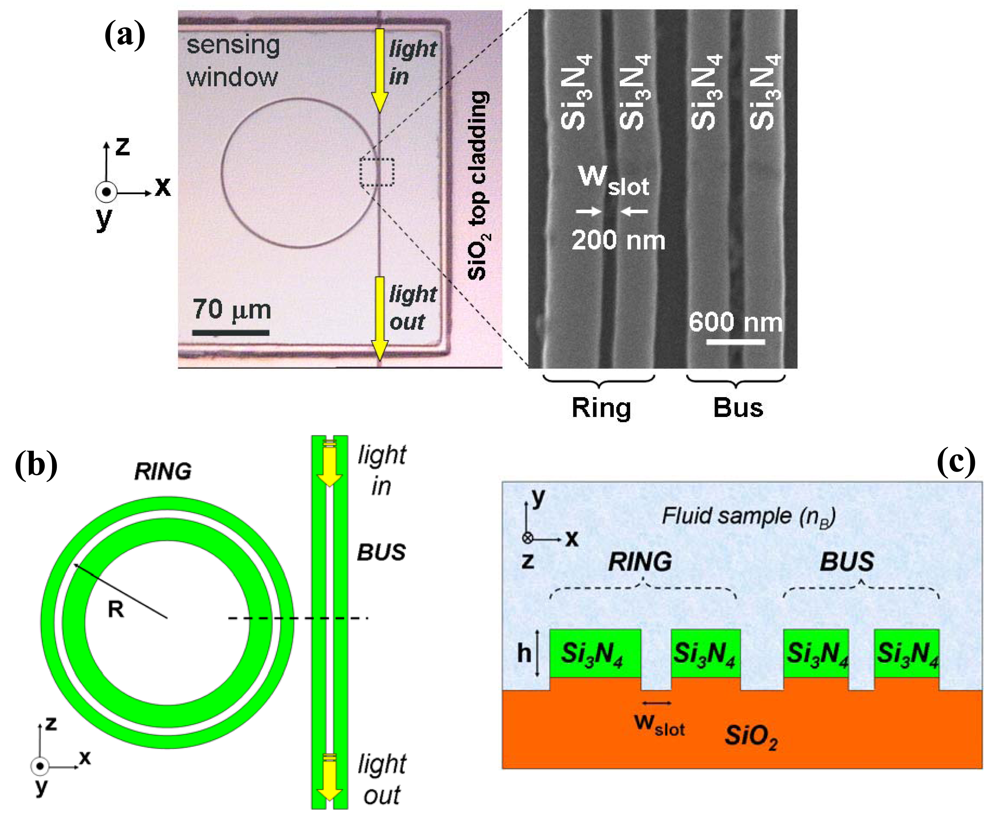
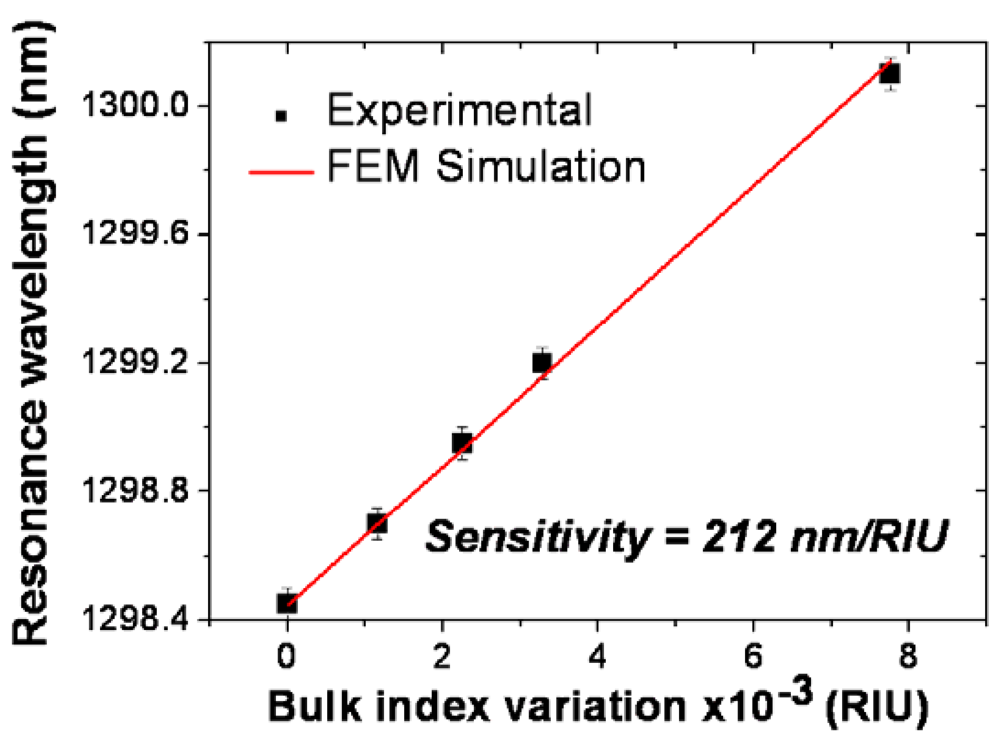
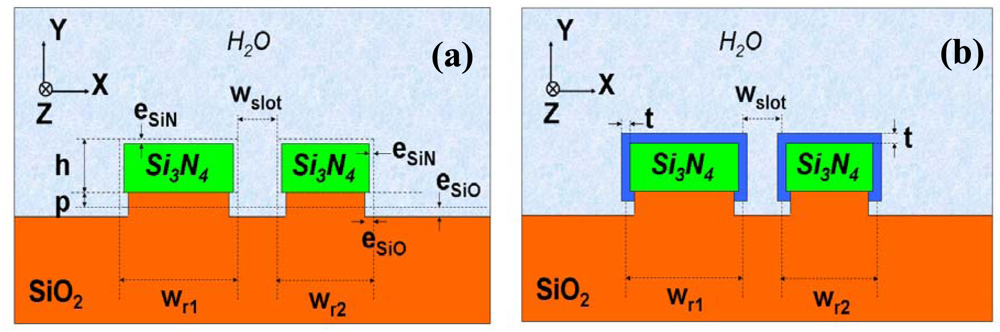
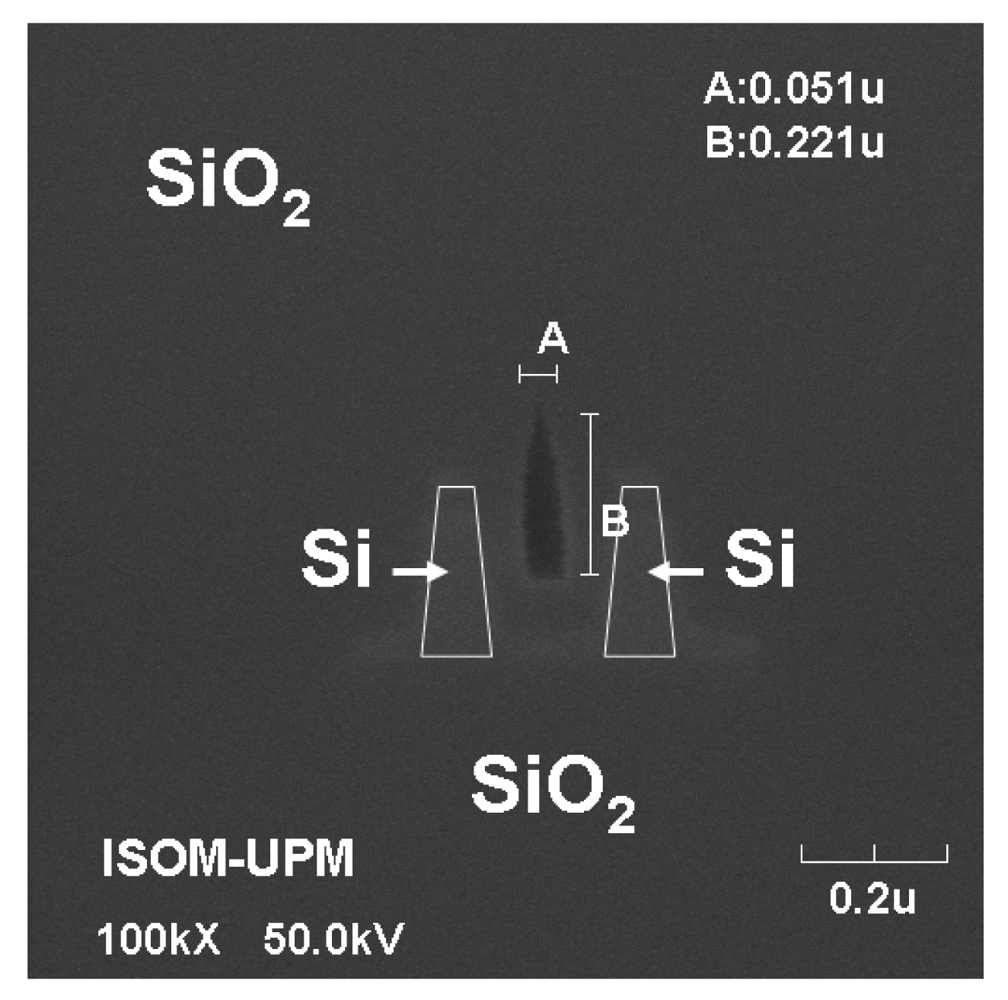
2.1. Silicon nitride slot-waveguide microring resonator based sensors
2.2. Silicon slot-waveguide microring resonator based sensors
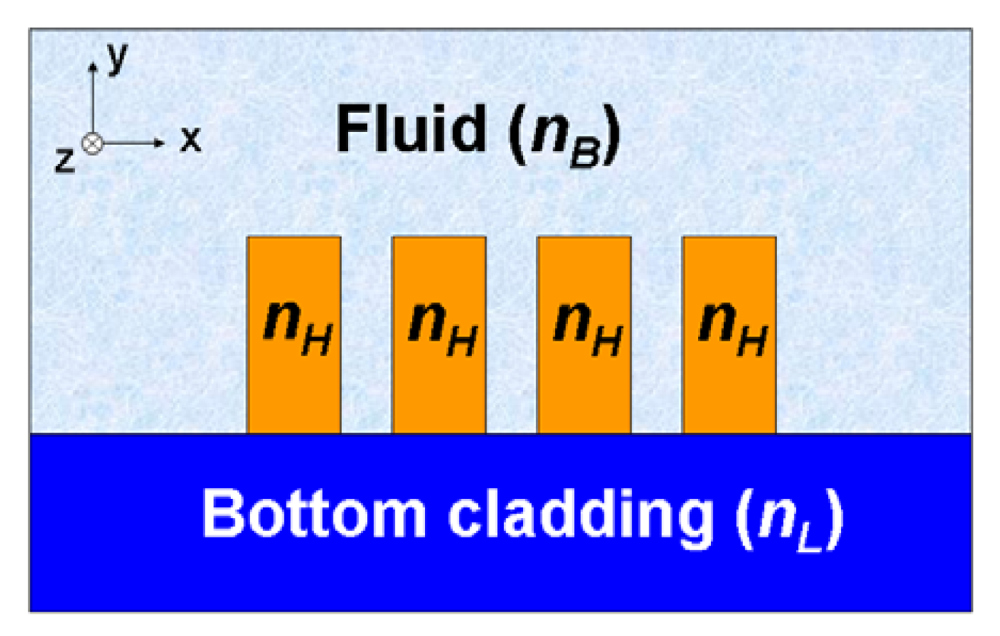
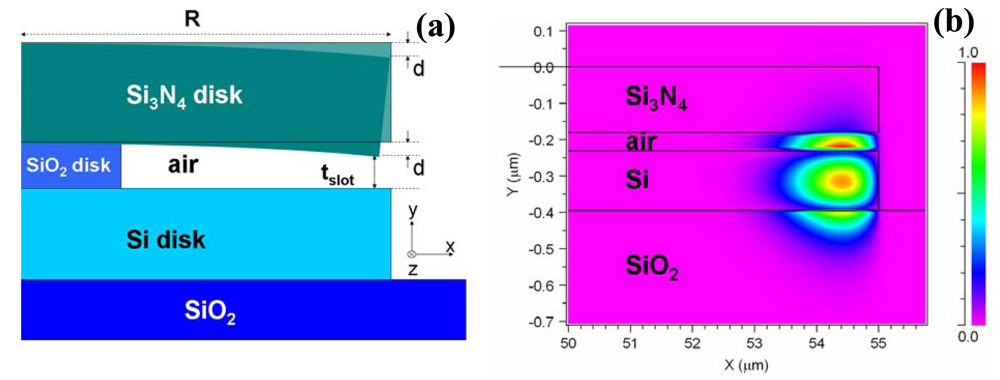
2.3. Other slot-waveguide based RI sensor configurations
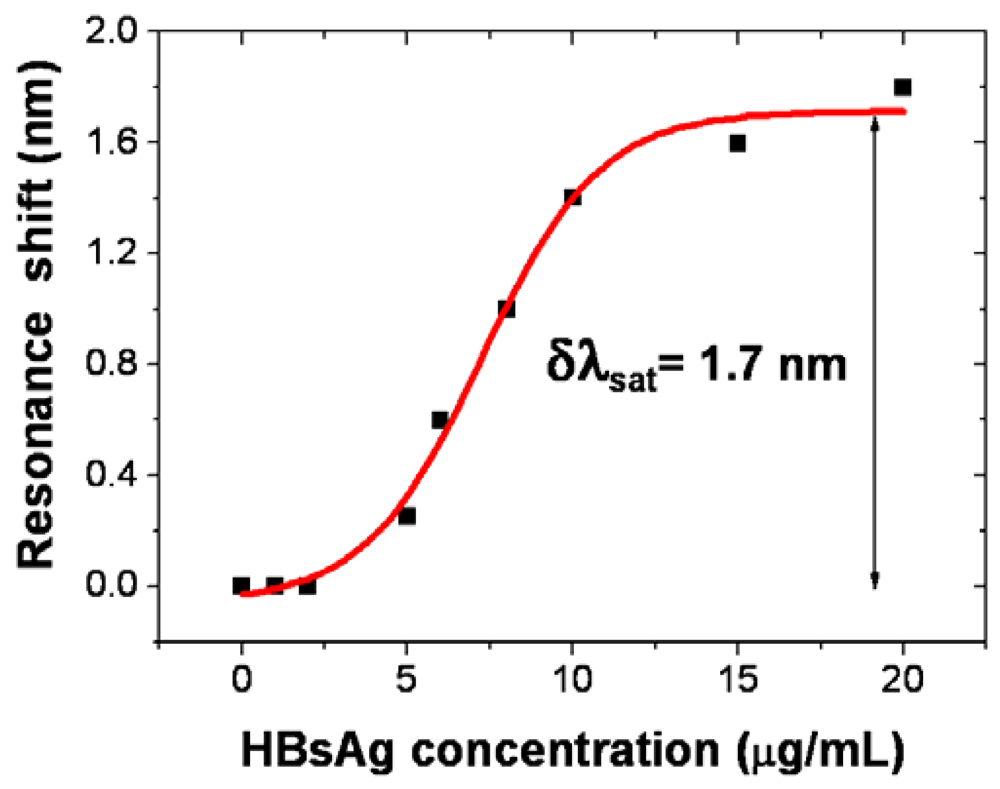
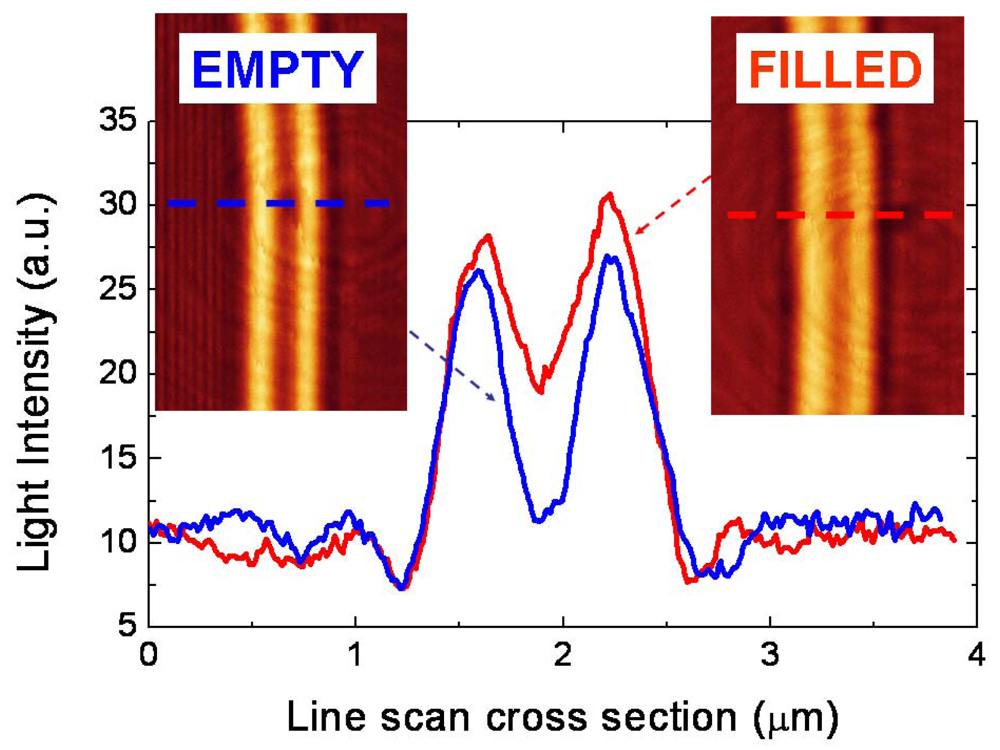
3. Labeling-Based Optical Slot-Waveguide Sensors
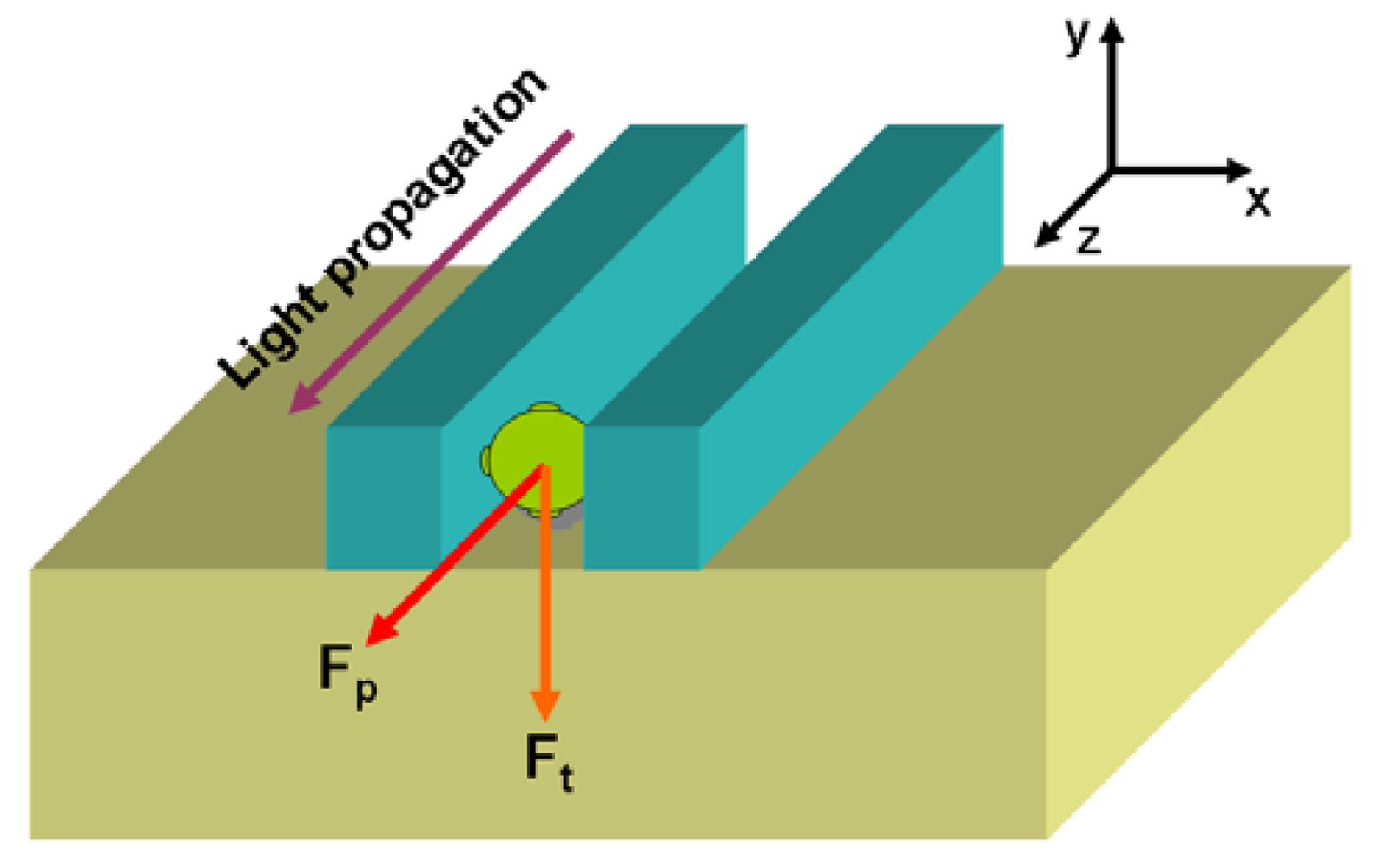
4. Opto-Mechanical Slot-Waveguide Based Sensors
5. Conclusions
Acknowledgments
References and Notes
- Brecht, A.; Gauglitz, G. Optical probes and transducers. Biosens. Bioelectron. 1995, 10, 923–936. [Google Scholar]
- Zinoviev, K.; Carrascosa, L.G.; del Río, J.S.; Sepúlveda, B.; Domínguez, C.; Lechuga, L.M. Silicon photonic biosensors for lab-on-a-chip applications. Adv. Opt. Tech. 2008, 383927, 1–6. [Google Scholar]
- Saito, Y.; Kanaya, T.; Nomura, A.; Kano, T. Experimental trial of a hollow-core waveguide used as an absorption cell for concentration measurement of NH3 gas with a CO2 laser. Opt. Lett. 1993, 18, 2150–2152. [Google Scholar]
- Almeida, V.R.; Xu, Q.; Barrios, C.A.; Lipson, M. Guiding and confining light in void nanostructure. Opt. Lett. 2004, 29, 1209–1211. [Google Scholar]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: a review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar]
- Luff, B.J.; Harris, R.D.; Wilkinson, J.S.; Wilson, R.; Schiffrin, D.J. Integrated-optical directional coupler biosensor. Opt. Lett. 1996, 21, 618–620. [Google Scholar]
- Heideman, R.G.; Kooyman, R.P.H.; Greve, J. Performance of a highly sensitive optical waveguide Mach-Zehnder interferometer immunosensor. Sens. Actuat. B 1993, 10, 209–217. [Google Scholar]
- Krioukov, E.; Klunder, D.J.; Driessen, A.; Greve, J.; Otto, C. Sensor based on an integrated optical microcavity. Opt. Lett. 2002, 27, 512–514. [Google Scholar]
- Yalçin, A.; Popat, K.C.; Aldridge, J.C.; Desai, T.A.; Hryniewicz, J.; Chbouki, N.; Little, B.E.; King, O.; Van, V.; Chu, S.; Gill, D.; Anthes-Washburn, M.; Ünlü, M.S.; Goldberg, B.B. Optical sensing of biomolecules using microring resonators. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 148–155. [Google Scholar]
- De Vos, K.; Bartolozzi, I.; Schacht, E.; Bienstman, P.; Baets, R. Silicon-on-Insulator microring resonator for sensitive and label-free biosensing. Opt. Express 2007, 15, 7610–7615. [Google Scholar]
- Armani, A.M.; Kulkarni, E.P.; Fraser, S.E.; Flagan, R.C.; Vahala, K.J. Label-free, single-molecule detection with optical microcavities. Science 2007, 317, 783–786. [Google Scholar]
- Barrios, C.A.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Sohlström, H.; Holgado, M.; Casquel, R. Slot-waveguide biochemical sensor. Opt. Lett. 2007, 32, 3080–3082. [Google Scholar]
- Barrios, C.A.; Bañuls, M.J.; Gonzalez-Pedro, V.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Maquieira, A.; Sohlström, H.; Holgado, M.; Casquel, R. Label-free optical biosensing with slot-waveguides. Opt. Lett. 2008, 33, 708–710. [Google Scholar]
- Robinson, J.T.; Chen, L.; Lipson, M. On-chip gas detection in silicon optical microcavities. Opt. Express 2008, 16, 4296–4301. [Google Scholar]
- Claes, T.; Girones, J.; De Vos, K.; Bienstman, P.; Baets, R. Label-free biosensing with silicon-on-insulator slotted racetrack resonator. Proceedings Symposium IEEE/LEOS Benelux Chapter, Twente, The Netherlands; 2008; pp. 23–26. [Google Scholar]
- Passaro, V.M.N.; Dell'Olio, F.; Ciminelli, C.; Armenise, M.N. Efficient chemical sensing by coupled slot SOI waveguides. Sensors 2009, 9, 1012–1032. [Google Scholar]
- Barrios, C.A.; Sánchez, B.; Gylfason, K.B.; Griol, A.; Sohlström, H.; Holgado, M.; Casquel, R. Demonstration of slot-waveguide structures on silicon nitride / silicon oxide platform. Opt. Express 2007, 15, 6846–6856. [Google Scholar]
- Barrios, C.A. Analysis and modeling of a silicon nitride slot-waveguide ring resonator biochemical sensor. Proceedings of SPIE, San Diego, CA, USA; 2009; pp. 154–196. [Google Scholar]
- Barrios, C.A.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Sohlström, H.; Holgado, M.; Casquel, R. Slot-waveguide biochemical sensor: erratum. Opt. Lett. 2008, 33, 2554–2555. [Google Scholar]
- Barrios, C.A.; González-Pedro, V.; Banuls, M.J.; Maquieira, A. Private communication.
- Barrios, C.A.; Holgado, M.; Guarneros, O.; Gylfason, K.B.; Sánchez, B.; Casquel, R.; Sohlström, H. Reconfiguration of microring resonators by liquid adhesion. Appl. Phys. Lett. 2008, 93, 203114. [Google Scholar]
- Gylfason, K.B.; Carlborg, C.F.; Kaźmierczak, A.; Dortu, F.; Sohlström, H.; Vivien, L.; Ronan, G.; Barrios, C.A.; van der Wijngaart, W.; Stemme, G. A packaged optical slot-waveguide ring resonator sensor array for multiplex assays in labs-on-chip. In Proc. MicroTAS.; Jeju, Korea, 2009. [Google Scholar]
- Dell'Olio, F.; Passaro, V.M.N. Optical sensing by optimized silicon slot waveguides. Opt. Express 2007, 15, 4977–4993. [Google Scholar]
- Tu, X.; Xu, X.; Chen, S.; Yu, J.; Wang, Q. Simulation demonstration and experimental fabrication of a multiple-slot waveguide. IEEE Photon. Technol. Lett. 2008, 20, 333–335. [Google Scholar]
- Xu, Q.; Almeida, V.R.; Panepucci, R.R.; Lipson, M. Experimental demonstration of guiding and confining light in nanometer-size low-refractive-index material. Opt. Lett. 2004, 29, 1626–1628. [Google Scholar]
- Yang, S.-H.; Cooper, M.L.; Bandaru, P.R.; Mookherjea, S. Giant birefringence in multi-slotted silicon nanophotonic waveguides. Opt. Express 2008, 16, 8306–8316. [Google Scholar]
- Vivien, L.; Marris-Morini, D.; Griol, A.; Gylfason, K.B.; Hill, D.; Alvarez, J.; Sohlström, H.; Hurtado, J.; Bouville, D.; Cassan, E. Vertical multiple-slot waveguide ring resonators in silicon nitride. Opt. Express 2008, 16, 17237–17242. [Google Scholar]
- Barrios, C.A.; Xu, Q.; Shakya, J.; Manolatou, C.; Lipson, M. Compact Silicon Slot-Waveguide Disk Resonator. Proceedings of Conference on Lasers and Electro-optics (CLEO), Long Beach, CA, USA; 2006. [Google Scholar] [CrossRef]
- Schmidt, B.; Barrios, C.A.; Lipson, M. Si3N4-SiO2-Si slot-waveguide disk resonators in a silicon photonic platform. Group IV Semiconductor Nanostructures. In Material Research Society (MRS) Fall Meeting; Boston, MA, December 2006; paper L2.8. [Google Scholar]
- Srivastava, R.; Bao, C.; Gómez-Reino, C. Planar-surface-waveguide evanescent-wave chemicals sensors. Sens. Actuat. A 1996, 51, 165–171. [Google Scholar]
- Bernini, R.; Cennamo, N.; Minardo, A.; Zeni, L. Planar waveguides for fluorescence-based biosensing: optimization and analysis. IEEE Sensors J. 2006, 6, 1218–1226. [Google Scholar]
- Ng, L.N.; Luf, B.J.; Zervas, M.N.; Wilkinson, J.S. Forces on a Rayleigh particle in the cover region of a planar waveguide. J. Lightwave Technol. 2000, 18, 388–400. [Google Scholar]
- Yang, A.H.J.; Moore, S.D.; Schmidt, B.S.; Klug, M.; Lipson, M.; Erickson, D. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature 2009, 457, 71–75. [Google Scholar]
- Zinoviev, K.; Domínguez, C.; Plaza, J.A.; Cadalso Busto, V.J.; Lechuga, L.M. A novel optical waveguide microcantilever sensor for the detection of nanomechanical forces. J. Lightw. Technol. 2006, 24, 2133–2138. [Google Scholar]
- Barrios, C.A. Ultrasensitive nanomechanical photonic sensor based on horizontal slot-waveguide resonator. IEEE Phot. Tech. Lett. 2006, 18, 2419–2421. [Google Scholar]
- Barrios, C.A. Ultrasensitive nanomechanical photonic sensor. Proceeding of SPIE, San Diego, CA, USA; 2007; 6593, pp. 65930X-1–65930X-5. [Google Scholar]
- Almeida, V.R.; Panepucci, R.R. NOEMs devices based on slot-waveguides. Silicon Slot-Waveguide as NOEMS Photonic Platform. Proceedings of the Frontiers in Optics, Rochester, New York, NY, USA; 2006. [Google Scholar]

© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barrios, C.A. Optical Slot-Waveguide Based Biochemical Sensors. Sensors 2009, 9, 4751-4765. https://doi.org/10.3390/s90604751
Barrios CA. Optical Slot-Waveguide Based Biochemical Sensors. Sensors. 2009; 9(6):4751-4765. https://doi.org/10.3390/s90604751
Chicago/Turabian StyleBarrios, Carlos Angulo. 2009. "Optical Slot-Waveguide Based Biochemical Sensors" Sensors 9, no. 6: 4751-4765. https://doi.org/10.3390/s90604751