Amperometric Sensor for Detection of Chloride Ions
Abstract
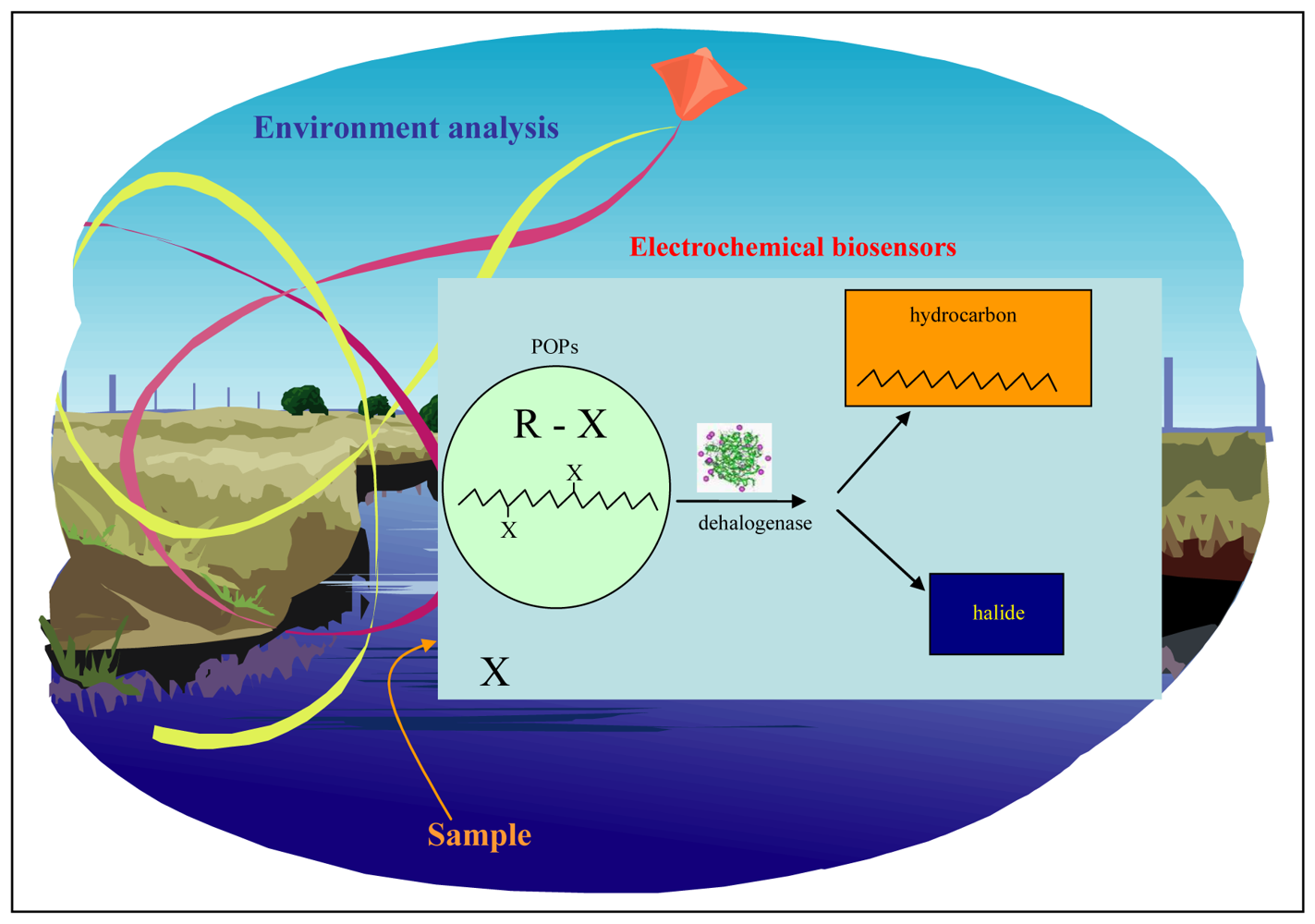
:1. Introduction
2. Material and Methods
2.1 Chemicals, material and pH measurements
2.2 Voltammetric measurements
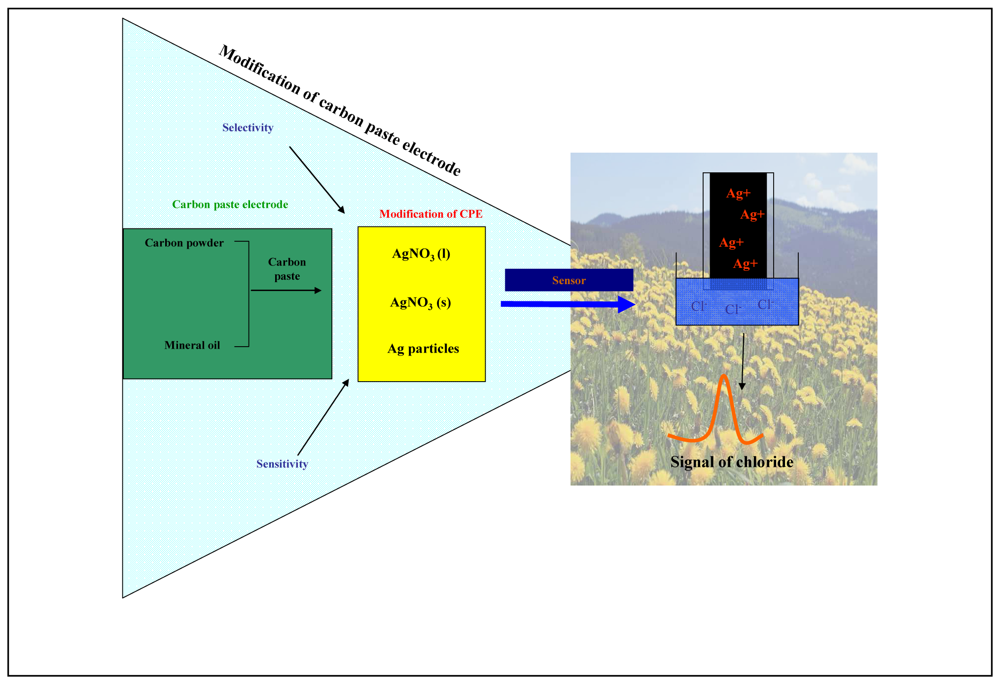
2.3 Preparation of carbon paste electrode and its modification
2.4 Amperometric measurements
2.5 Descriptive statistics
3. Results and Discussion
3.1 Carbon paste electrode modified by solid AgNO3
3.2 Carbon paste electrode modified by solution of AgNO3
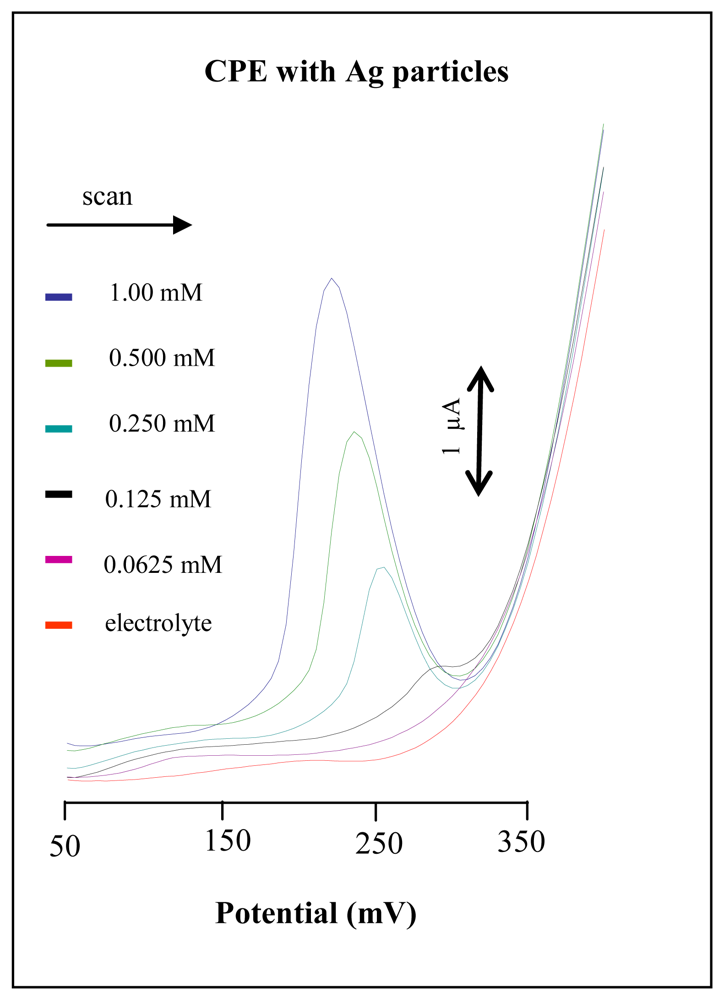
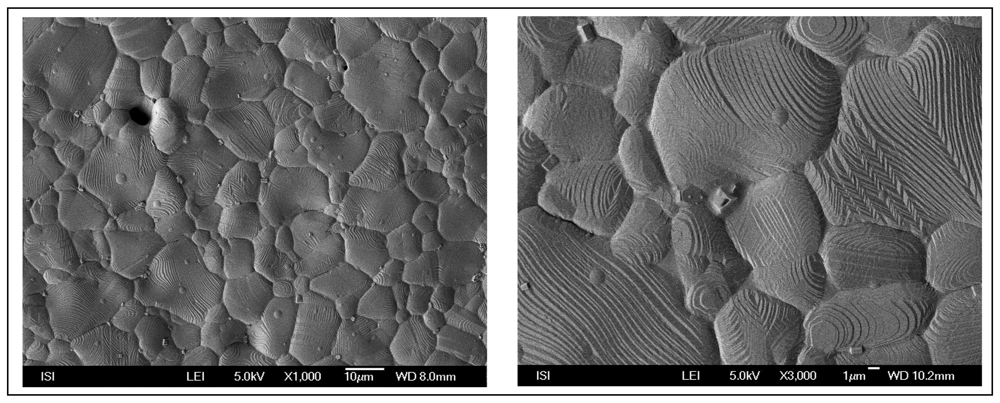
3.3 Carbon paste electrode modified by silver microparticles
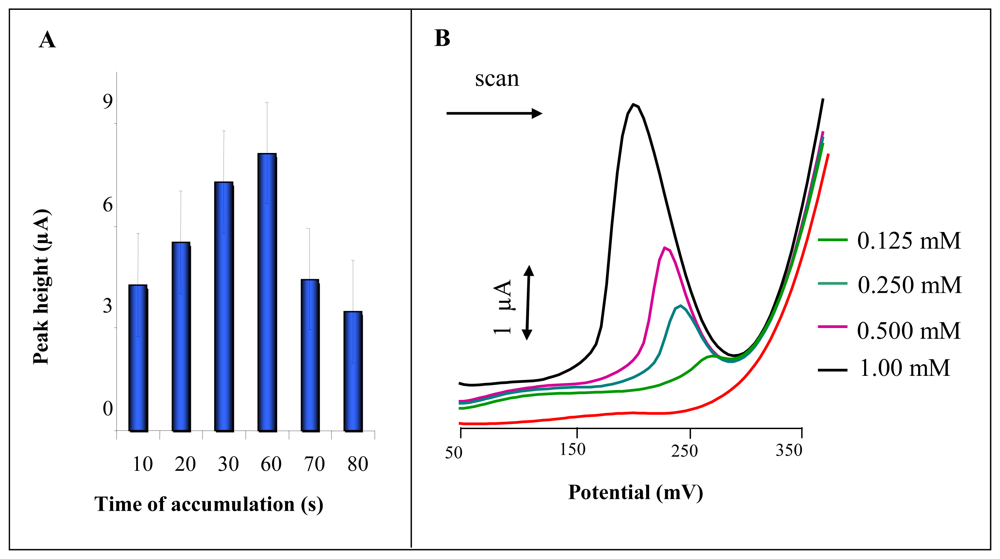
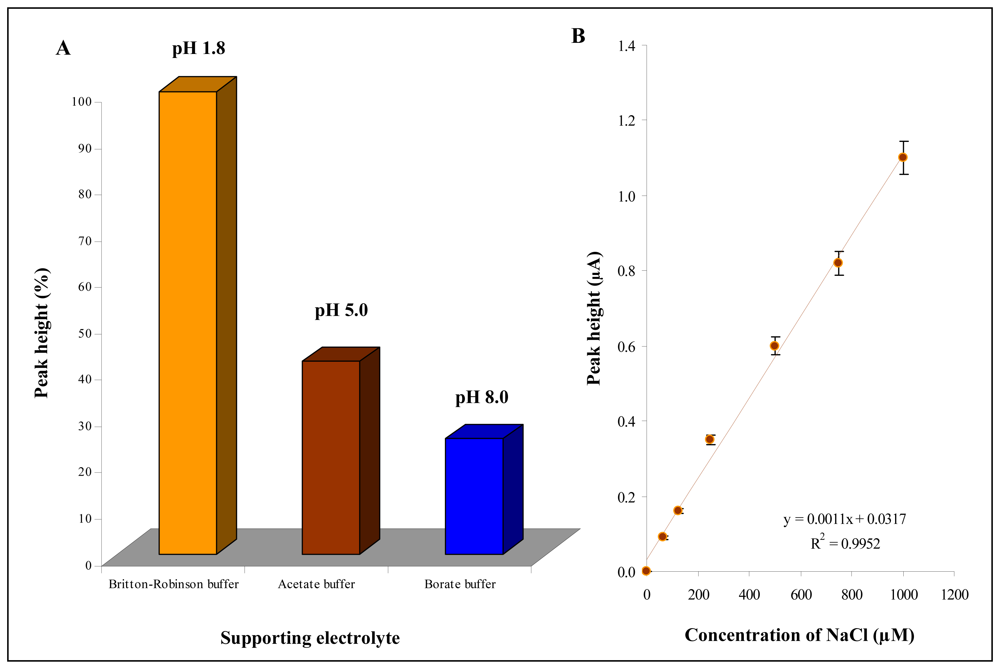
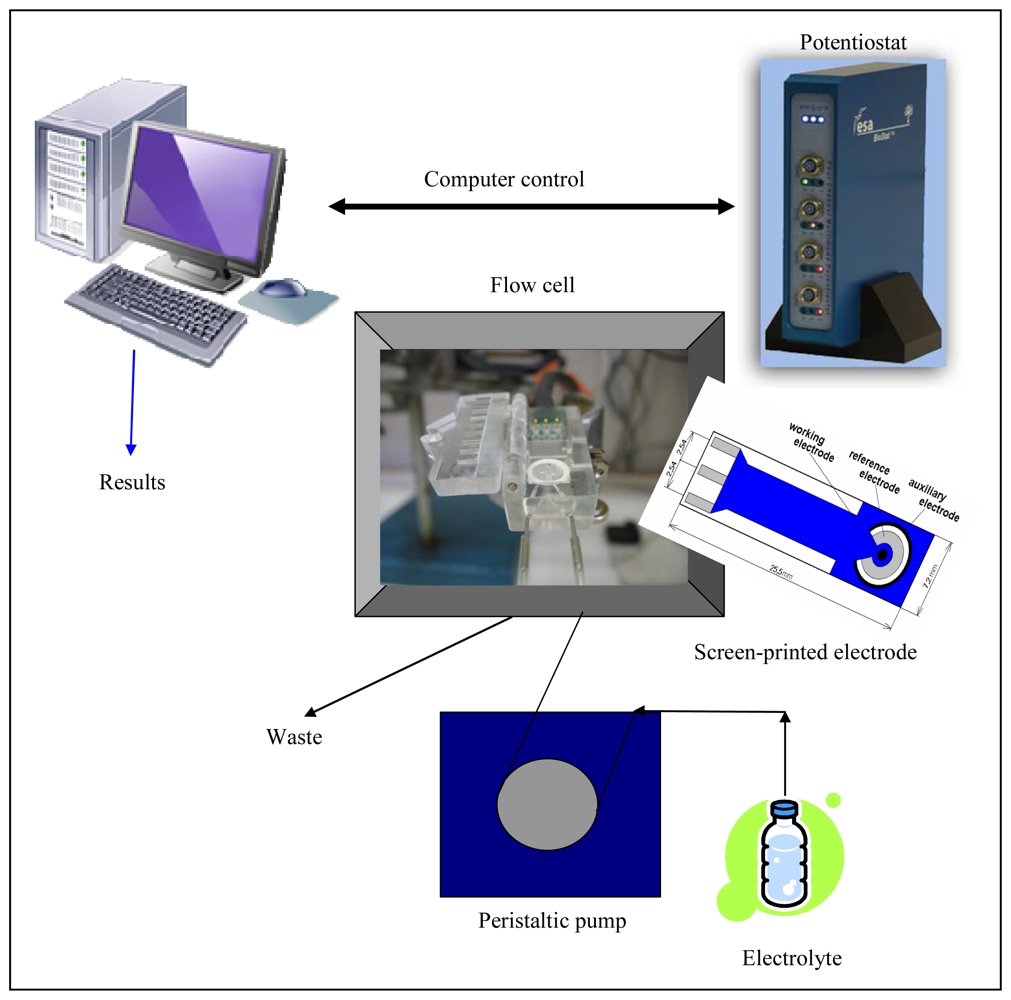
3.4 Miniaturization of measuring device
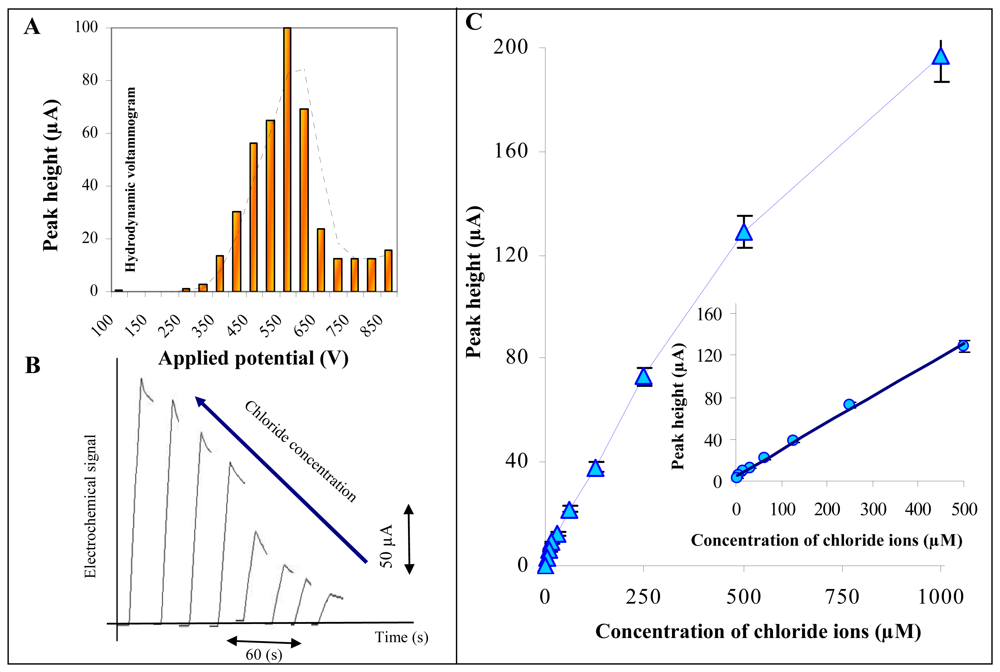
Influence of applied potential and chloride concentrations
4. Conclusions
Acknowledgments
- †Dedicated to the UNESCO International Year of Planet Earth
References
- Jiang, Q.S.; Mak, D.; Devidas, S.; Schwiebert, E.M.; Bragin, A.; Zhang, Y.L.; Skach, W.R.; Guggino, W.B.; Foskett, J.K.; Engelhardt, J.F. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J. Cell Biol. 1998, 143, 645–657. [Google Scholar]
- Huber, C.; Werner, T.; Krause, C.; Klimant, I.; Wolfbeis, O.S. Energy transfer-based lifetime sensing of chloride using a luminescent transition metal complex. Anal. Chim. Acta 1998, 364, 143–151. [Google Scholar]
- Montemor, M.F.; Alves, J.H.; Simoes, A.M.; Fernandes, J.C.S.; Lourenco, Z.; Costa, A.J.S.; Appleton, A.J.; Ferreira, M.G.S. Multiprobe chloride sensor for in situ monitoring of reinforced concrete structures. Cem. Concr. Compos. 2006, 28, 233–236. [Google Scholar]
- Huber, C.; Klimant, I.; Krause, C.; Werner, T.; Mayr, T.; Wolfbeis, O.S. Optical sensor for seawater salinity. Fresenius J. Anal. Chem. 2000, 368, 196–202. [Google Scholar]
- Martin, A.; Narayanaswamy, R. Studies on quenching of fluorescence of reagents in aqueous solution leading to an optical chloride-ion sensor. Sens. Actuator B-Chem. 1997, 39, 330–333. [Google Scholar]
- Babu, J.N.; Bhalla, V.; Kumar, M.; Mahajan, R.K.; Puri, R.K. A chloride selective sensor based on a calix[4]arene possessing a urea moiety. Tetrahedron Lett. 2008, 49, 2772–2775. [Google Scholar]
- Badr, I.H.A.; Diaz, M.; Hawthorne, M.F.; Bachas, L.G. Mercuracarborand “anti-crown ether”- based chloride sensitive liquid/polymeric membrane electrodes. Anal. Chem. 1999, 71, 1371–1377. [Google Scholar]
- Ratjen, F.; Doring, G. Cystic fibrosis. Lancet 2003, 361, 681–689. [Google Scholar]
- Junsomboon, J.; Jakmunee, J. Determination of chloride in admixtures and aggregates for cement by a simple flow injection potentiometric system. Talanta 2008, 76, 365–368. [Google Scholar]
- Hahn, F. Novel valve for automatic calibration of a chloride sensor for river monitoring. Biosyst. Eng. 2005, 92, 275–284. [Google Scholar]
- Sebkova, S. Determination of chlorides on composite silver electrodes. Chem. Listy 2003, 97, 201–205. [Google Scholar]
- Wu, R.H.; Shao, X.G. Application of near-infrared spectra in the determination of water soluble chloride ion in plant samples. Spectrosc. Spectr. Anal. 2006, 26, 617–619. [Google Scholar]
- Philippi, M.; dos Santos, H.S.; Martins, A.O.; Azevedo, C.M.N.; Pires, M. Alternative spectrophotometric method for standardization of chlorite aqueous solutions. Anal. Chim. Acta 2007, 585, 361–365. [Google Scholar]
- Cao, H.; Dong, H.W. Rapid and sensitive determination of trace chloride ion in drinks using resonance light scattering technique. J. Autom. Methods Manag. Chem. 2008, Article Number 745636. 5. [Google Scholar]
- Kumar, K.G.; John, K.S.; Indira, C.J. A chloride ion-selective potentiometric sensor based on a polymeric schiff base complex. Indian J. Chem. Technol. 2006, 13, 13–16. [Google Scholar]
- Shishkanova, T.V.; Sykora, D.; Sessler, J.L.; Kral, V. Potentiometric response and mechanism of anionic recognition of heterocalixarene-based ion selective electrodes. Anal. Chim. Acta 2007, 587, 247–253. [Google Scholar]
- Mesquita, R.B.R.; Fernandes, S.M.V.; Rangel, A. Turbidimetric determination of chloride in different types of water using a single sequential injection analysis system. J. Environ. Monit. 2002, 4, 458–461. [Google Scholar]
- Pimenta, A.M.; Araujo, A.N.; Conceicao, M.; Montenegro, B.S.M.; Pasquini, C.; Rohwedder, J.J.R.; Raimundo, I.M. Chloride-selective membrane electrodes and optodes based on an indium(III) porphyrin for the determination of chloride in a sequential injection analysis system. J. Pharm. Biomed. Anal. 2004, 36, 49–55. [Google Scholar]
- Bonifacio, V.G.; Figueiredo-Filho, L.C.; Marcolino, L.H.; Fatibello-Filho, O. An improved flow system for chloride determination in natural waters exploiting solid-phase reactor and long pathlength spectrophotometry. Talanta 2007, 72, 663–667. [Google Scholar]
- Krizkova, S.; Ryant, P.; Krystofova, O.; Adam, V.; Galiova, M.; Beklova, M.; Babula, P.; Kaiser, J.; Novotny, K.; Novotny, J.; Liska, M.; Malina, R.; Zehnalek, J.; Hubalek, J.; Havel, L.; Kizek, R. Multi-instrumental analysis of tissues of sunflower plants treated with silver(I) ions - Plants as bioindicators of environmental pollution. Sensors 2008, 8, 445–463. [Google Scholar]
- Adam, V.; Zitka, O.; Dolezal, P.; Zeman, L.; Horna, A.; Hubalek, J.; Sileny, J.; Krizkova, S.; Trnkova, L.; Kizek, R. Lactoferrin isolation using monolithic column coupled with spectrometric or micro-amperometric detector. Sensors 2008, 8, 464–487. [Google Scholar]
- Adam, V.; Mikelova, R.; Hubalek, J.; Hanustiak, P.; Beklova, M.; Hodek, P.; Horna, A.; Trnkova, L.; Stiborova, M.; Zeman, L.; Kizek, R. Utilizing of square wave voltammetry to detect flavonoids in the presence of human urine. Sensors 2007, 7, 2402–2418. [Google Scholar]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; Zehnalek, J.; Kizek, R. Spectrometric and voltammetric analysis of urease - nickel nanoelectrode as an electrochemical sensor. Sensors 2007, 7, 1238–1255. [Google Scholar]
- Zitka, O.; Huska, D.; Krizkova, S.; Adam, V.; Chavis, G.J.; Trnkova, L.; Horna, A.; Hubalek, J.; Kizek, R. An investigation of glutathione-platinum(II) interactions by means of the flow injection analysis using glassy carbon electrode. Sensors 2007, 7, 1256–1270. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Adam, V.; Havel, L.; Kramer, K.J.; Babula, P.; Kizek, R. A fluorimetric sensor for detection of one living cell. Sensors 2007, 7, 222–238. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 6, 1483–1497. [Google Scholar]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New hydrodynamic electrochemical arrangement for cadmium ions detection using thick-film chemical sensor electrodes. Sensors 2006, 6, 1498–1512. [Google Scholar]
- Trnkova, L.; Jelen, F.; Petrlova, J.; Adam, V.; Potesil, D.; Kizek, R. Elimination voltammetry with linear scan as a new detection method for DNA sensors. Sensors 2005, 5, 448–464. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy-metal ion biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Mikelova, R.; Prokop, Z.; Stejskal, K.; Adam, V.; Beklova, M.; Trnkova, L.; Kulichova, B.; Horna, A.; Chaloupkova, R.; Damborsky, J.; Kizek, R. Enzymatic reaction coupled with flow-injection analysis with charged aerosol, coulometric, or amperometric detection for estimation of contamination of the environment by pesticides. Chromatographia 2008, 67, S47–S53. [Google Scholar]
- Huska, D.; Krizkova, S.; Adam, V.; Hubalek, J.; Trnkova, L.; Prusa, R.; Havel, L.; Kizek, R. Paramagnetic beads coupled with electrochemical detection as a tool to investigate transcriptome. Tumor Biol. 2007, 28, 124–124. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Zitka, O.; Hubalek, J.; Prusa, R.; Adam, V.; Wang, J.; Beklova, M.; Sures, B.; Kizek, R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B-Chem. 2007, 127, 112–119. [Google Scholar]
- Adam, V.; Hanustiak, P.; Krizkova, S.; Beklova, M.; Zehnalek, J.; Trnkova, L.; Horna, A.; Sures, B.; Kizek, R. Palladium biosensor. Electroanalysis 2007, 19, 1909–1914. [Google Scholar]
- Kukacka, J.; Krizkova, S.; Zitka, O.; Prusa, R.; Adam, V.; Sures, B.; Beklova, M.; Kizek, R. Study of nucleic acids interactions with platinum based cytostatics using biosensor. Faseb J. 2007, 21, A262–A262. [Google Scholar]
- Huska, D.; Zitka, O.; Adam, V.; Beklova, M.; Krizkova, S.; Zeman, L.; Horna, A.; Havel, L.; Zehnalek, J.; Kizek, R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci. 2007, 52, 37–43. [Google Scholar]
- Krizkova, S.; Adam, V.; Petrlova, J.; Zitka, O.; Stejskal, K.; Zehnalek, J.; Sures, B.; Trnkova, L.; Beklova, M.; Kizek, R. A suggestion of electrochemical biosensor for study of platinum(II)-DNA interactions. Electroanalysis 2007, 19, 331–338. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. Determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Petrek, J.; Adam, V.; Potesil, D.; Havel, L.; Mikelova, R.; Trnkova, L.; Kizek, R. Electrochemical study of S-nitrosoglutathione and nitric oxide by carbon fibre NO sensor and cyclic voltammetry - possible way of monitoring of nitric oxide. Electrochim. Acta 2006, 51, 5087–5094. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Potesil, D.; Mikelova, R.; Adam, V.; Kizek, R.; Prusa, R. Change of the protein p53 electrochemical signal according to its structural form - Quick and sensitive distinguishing of native, denatured, and aggregated form of the “guardian of the genome”. Protein J. 2006, 25, 23–32. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Prusa, R.; Potesil, D.; Masarik, M.; Adam, V.; Kizek, R.; Jelen, F. Fast and sensitive electrochemical detection of native, denatured, and aggregated forms of tumor suppressor protein p53. Mol. Biol. Cell 2004, 15, 249A–249A. [Google Scholar]
- Palecek, E.; Kizek, R.; Havran, L.; Billova, S.; Fojta, M. Electrochemical enzyme-linked immunoassay in a DNA hybridization sensor. Anal. Chim. Acta 2002, 469, 73–83. [Google Scholar]
- Kizek, R.; Havran, L.; Fojta, M.; Palecek, E. Determination of nanogram quantities of osmium-labeled single stranded DNA by differential pulse stripping voltammetry. Bioelectrochemistry 2002, 55, 119–121. [Google Scholar]
- Kizek, R.; Trnkova, L.; Sevcikova, S.; Smarda, J.; Jelen, F. Silver electrode as a sensor for determination of zinc in cell cultivation medium. Anal. Biochem. 2002, 301, 8–13. [Google Scholar]
- deSilva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: review. Sens. Actuator B- Chem. 1999, 54, 3–15. [Google Scholar]
- Wang, J.; Musameh, M.; Lin, Y.H. Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J. Am. Chem. Soc. 2003, 125, 2408–2409. [Google Scholar]
- Wang, J. From DNA biosensors to gene chips. Nucleic Acids Res. 2000, 28, 3011–3016. [Google Scholar]
- Wang, J.; Musameh, M. Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal. Chem. 2003, 75, 2075–2079. [Google Scholar]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar]
- Palecek, E.; Fojta, M.; Tomschik, M.; Wang, J. Electrochemical biosensors for DNA hybridization and DNA damage. Biosens. Bioelectron. 1998, 13, 621–628. [Google Scholar]
- Wang, J.; Palecek, E.; Nielsen, P.E.; Rivas, G.; Cai, X.H.; Shiraishi, H.; Dontha, N.; Luo, D.B.; Farias, P.A.M. Peptide nucleic acid probes for sequence-specific DNA biosensors. J. Am. Chem. Soc. 1996, 118, 7667–7670. [Google Scholar]
- Raiteri, R.; Grattarola, M.; Butt, H.J.; Skladal, P. Micromechanical cantilever-based biosensors. Sens. Actuator B-Chem. 2001, 79, 115–126. [Google Scholar]
- Wang, Y.; Xu, H.; Zhang, J.M.; Li, G. Electrochemical sensors for clinic analysis. Sensors 2008, 8, 2043–2081. [Google Scholar]
- O'Toole, M.; Diamond, D. Absorbance based light emitting diode optical sensors and sensing devices. Sensors 2008, 8, 2453–2479. [Google Scholar]
- Michikawa, Y.; Suga, T.; Ohtsuka, Y.; Matsumoto, I.; Ishikawa, A.; Ishikawa, K.; Iwakawa, M.; Imai, T. Visible genotype sensor array. Sensors 2008, 8, 2722–2735. [Google Scholar]
- Belluzo, M.S.; Ribone, M.E.; Lagier, C.M. Assembling amperometric biosensors for clinical diagnostics. Sensors 2008, 8, 1366–1399. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Voros, J.; Reimhult, E. Electrochemical biosensors - Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar]
- Bacci, M.; Cucci, C.; Mencaglia, A.A.; Mignani, A.G. Innovative sensors for environmental monitoring in museums. Sensors 2008, 8, 1984–2005. [Google Scholar]
- Rahman, M.A.; Kumar, P.; Park, D.S.; Shim, Y.B. Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8, 118–141. [Google Scholar]
- Bosch, M.E.; Sanchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Recent development in optical fiber biosensors. Sensors 2007, 7, 797–859. [Google Scholar]
- Jaffrezic-Renault, N.; Martelet, C.; Chevolot, Y.; Cloarec, J.P. Biosensors and bio-bar code assays based on biofunctionalized magnetic microbeads. Sensors 2007, 7, 589–614. [Google Scholar]
- Schneider, H.J.; Kato, K.; Strongin, R.M. Chemomechanical polymers as sensors and actuators for biological and medicinal applications. Sensors 2007, 7, 1578–1611. [Google Scholar]
- Bai, H.; Shi, G.Q. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar]
- Kocherginsky, N.M.; Wang, Z. Polyaniline membrane based potentiometric sensor for ascorbic acid, other redox active species and chloride. J. Electroanal. Chem. 2007, 611, 162–168. [Google Scholar]
- Schazmann, B.; Alhashimy, N.; Diamond, D. Chloride selective calix[4]arene optical sensor combining urea functionality with pyrene excimer transduction. J. Am. Chem. Soc. 2006, 128, 8607–8614. [Google Scholar]
- Sundaram, R.; Hariprasad, K.S. Synthesis of chloride ion-selective potentiometric sensor based on coordination polymer complex. Indian J. Chem. Technol. 2007, 14, 451–458. [Google Scholar]
- Xu, C.; Qin, Y.; Bakker, E. Optical chloride sensor based on [9]mercuracarborand-3 with massively expanded measuring range. Talanta 2004, 63, 180–184. [Google Scholar]
- Zhang, W.; Rozniecka, E.; Malinowska, E.; Parzuchowski, P.; Meyerhoff, M.E. Optical chloride sensor based on dimer-monomer equilibrium of indium(III) octaethylporphyrin in polymeric film. Anal. Chem. 2002, 74, 4548–4557. [Google Scholar]
- Krizkova, S.; Hrdinova, V.; Adam, V.; Burgess, E.P.J.; Kramer, K.J.; Masarik, M.; Kizek, R. Chip-based CE for avidin determination in transgenic tobacco and its comparison with square-wave voltammetry and standard gel electrophoresis. Chromatographia 2008, 67, S75–S81. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Supalkova, V.; Masarik, M.; Adam, V.; Havel, L.; Kramer, K.J.; Kizek, R. The determination of avidin in genetically modified maize by voltammetric techniques. Plant Soil Environ. 2007, 53, 345–349. [Google Scholar]
- Kizek, R.; Masarik, M.; Kramer, K.J.; Potesil, D.; Bailey, M.; Howard, J.A.; Klejdus, B.; Mikelova, R.; Adam, V.; Trnkova, L.; Jelen, F. An analysis of avidin, biotin and their interaction at attomole levels by voltammetric and chromatographic techniques. Anal. Bioanal. Chem. 2005, 381, 1167–1178. [Google Scholar]
- Masarik, M.; Kizek, R.; Kramer, K.J.; Billova, S.; Brazdova, M.; Vacek, J.; Bailey, M.; Jelen, F.; Howard, J.A. Application of avidin-biotin technology and adsorptive transfer stripping square-wave voltammetry for detection of DNA hybridization and avidin in transgenic avidin maize. Anal. Chem. 2003, 75, 2663–2669. [Google Scholar]
- White, N.M.; Turner, J.D. Thick-film sensors: Past, present and future. Meas. Sci. Technol. 1997, 8, 1–20. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of Detection. Anal. Chem. 1983, 55, A712–A724. [Google Scholar]
- Sebkova, S.; Navratil, T.; Kopanica, M. Silver composite electrode for voltammetric determination of halogenides. Anal. Lett. 2004, 37, 603–628. [Google Scholar]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar]
- Fidalgo-Used, N.; Blanco-Gonzalez, E.; Sanz-Medel, A. Sample handling strategies for the determination of persistent trace organic contaminants from biota samples. Anal. Chim. Acta 2007, 590, 1–16. [Google Scholar]
- Godduhn, A.; Duffy, L.K. Multi-generation health risks of persistent organic pollution in the far north: use of the precautionary approach in the Stockholm Convention. Environ. Sci. Policy 2003, 6, 341–353. [Google Scholar]
- Horak, J. Dioxins as a source of hazard for the environment and health. Chem. Listy 2002, 96, 863–868. [Google Scholar]
- Lohmann, R.; Breivik, K.; Dachs, J.; Muir, D. Global fate of POPs: Current and future research directions. Environ. Pollut. 2007, 150, 150–165. [Google Scholar]
- Muir, D.; Sverko, E. Analytical methods for PCBs and organochlorine pesticides in environmental monitoring and surveillance: a critical appraisal. Anal. Bioanal. Chem. 2006, 386, 769–789. [Google Scholar]
- Muir, D.C.G.; Howard, P.H. Are there other persistent organic pollutants? A challenge for environmental chemists. Environ. Sci. Technol. 2006, 40, 7157–7166. [Google Scholar]
- Oakley, A.J.; Klvana, M.; Otyepka, M.; Nagata, Y.; Wilce, M.C.J.; Damborsky, J. Crystal structure of haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26 at 0.95 angstrom resolution: Dynamics of catalytic residues. Biochemistry 2004, 43, 870–878. [Google Scholar]
- Chaloupkova, R.; Sykorova, J.; Prokop, Z.; Jesenska, A.; Monincovaa, M.; Pavlova, M.; Tsuda, M.; Nagata, Y.; Damborsky, J. Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by engineering of its entrance tunnel. J. Biol. Chem. 2003, 278, 52622–52628. [Google Scholar]
- Streltsov, V.A.; Prokop, Z.; Damborsky, J.; Nagata, Y.; Oakley, A.; Wilce, M.C.J. Haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26: X-ray crystallographic studies of dehalogenation of brominated substrates (vol 42, pg 10104, 2003). Biochemistry 2003, 42, 12719–12720. [Google Scholar]
- Prokop, Z.; Monincova, M.; Chaloupkova, R.; Klvana, M.; Nagata, Y.; Janssen, D.B.; Damborsky, J. Catalytic mechanism of the haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. J. Biol. Chem. 2003, 278, 45094–45100. [Google Scholar]
- Bosma, T.; Damborsky, J.; Stucki, G.; Janssen, D.B. Biodegradation of 1,2,3-trichloropropane through directed evolution and heterologous expression of a haloalkane dehalogenase gene. Appl. Environ. Microbiol. 2002, 68, 3582–3587. [Google Scholar]
- Nagata, Y.; Mori, K.; Takagi, M.; Murzin, A.G.; Damborsky, J. Identification of protein fold and catalytic residues of gamma-hexachlorocyclohexane dehydrochlorinase LinA. Proteins 2001, 45, 471–477. [Google Scholar]
- Damborsky, J.; Rorije, E.; Jesenska, A.; Nagata, Y.; Klopman, G.; Peijnenburg, W. Structure-specificity relationships for haloalkane dehalogenases. Environ. Toxicol. Chem. 2001, 20, 2681–2689. [Google Scholar]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Trnkova, L.; Adam, V.; Hubalek, J.; Babula, P.; Kizek, R. Amperometric Sensor for Detection of Chloride Ions. Sensors 2008, 8, 5619-5636. https://doi.org/10.3390/s8095619
Trnkova L, Adam V, Hubalek J, Babula P, Kizek R. Amperometric Sensor for Detection of Chloride Ions. Sensors. 2008; 8(9):5619-5636. https://doi.org/10.3390/s8095619
Chicago/Turabian StyleTrnkova, Libuse, Vojtech Adam, Jaromir Hubalek, Petr Babula, and Rene Kizek. 2008. "Amperometric Sensor for Detection of Chloride Ions" Sensors 8, no. 9: 5619-5636. https://doi.org/10.3390/s8095619
APA StyleTrnkova, L., Adam, V., Hubalek, J., Babula, P., & Kizek, R. (2008). Amperometric Sensor for Detection of Chloride Ions. Sensors, 8(9), 5619-5636. https://doi.org/10.3390/s8095619



