DNA Optical Detection Based on Porous Silicon Technology: from Biosensors to Biochips
Abstract
:1. Introduction
2. Experimental Section
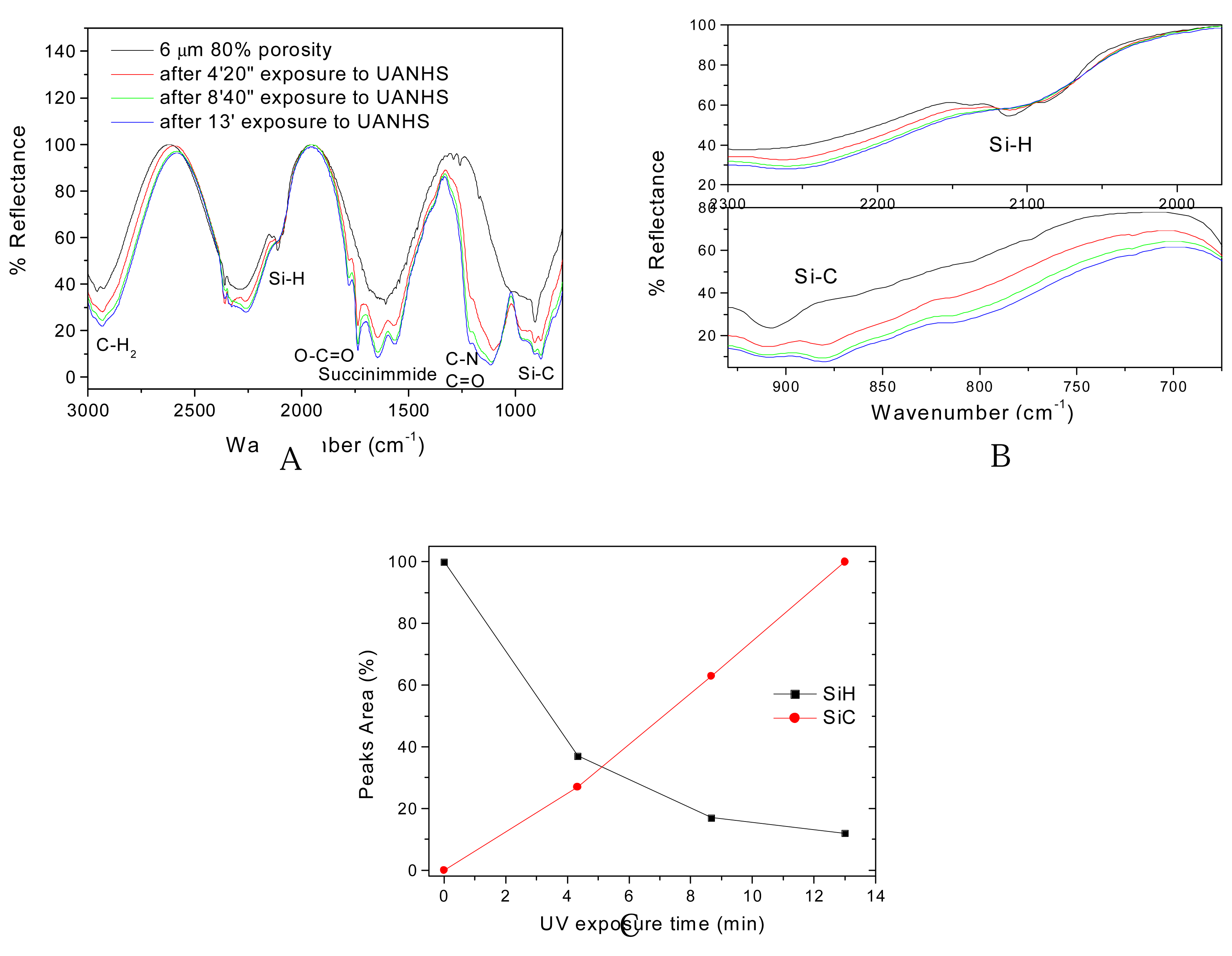
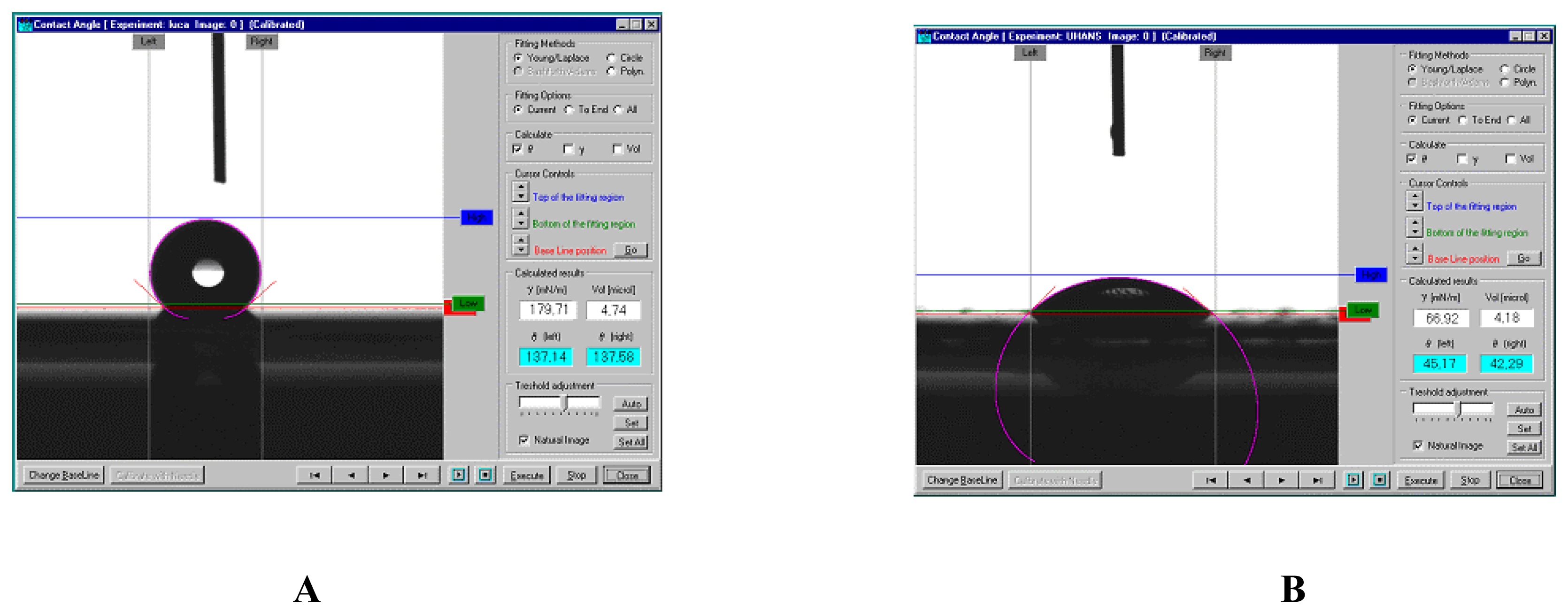
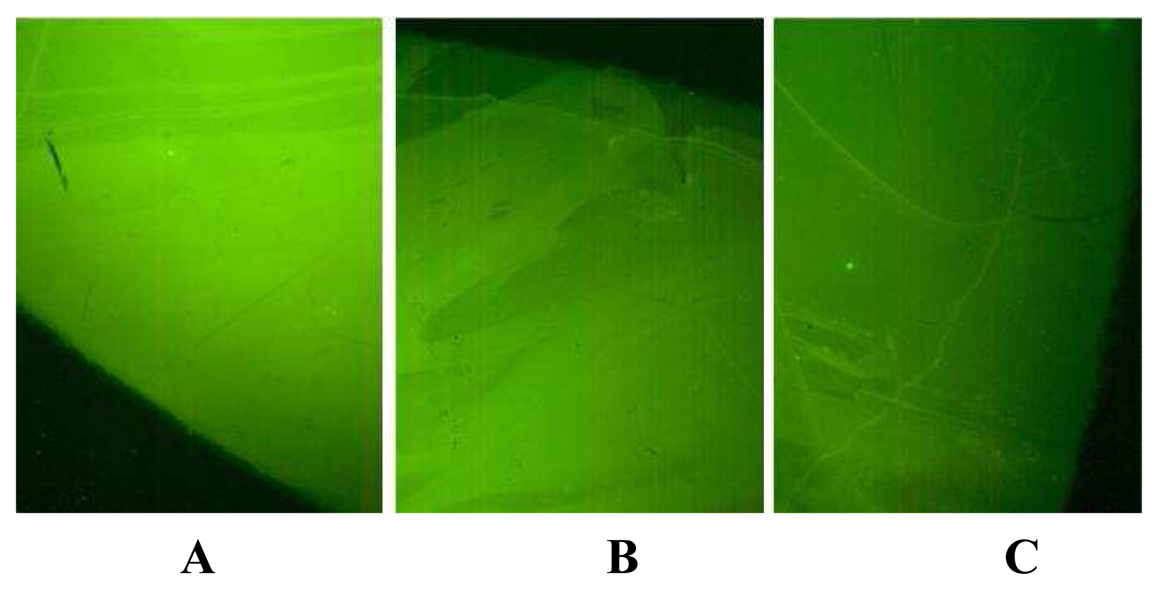
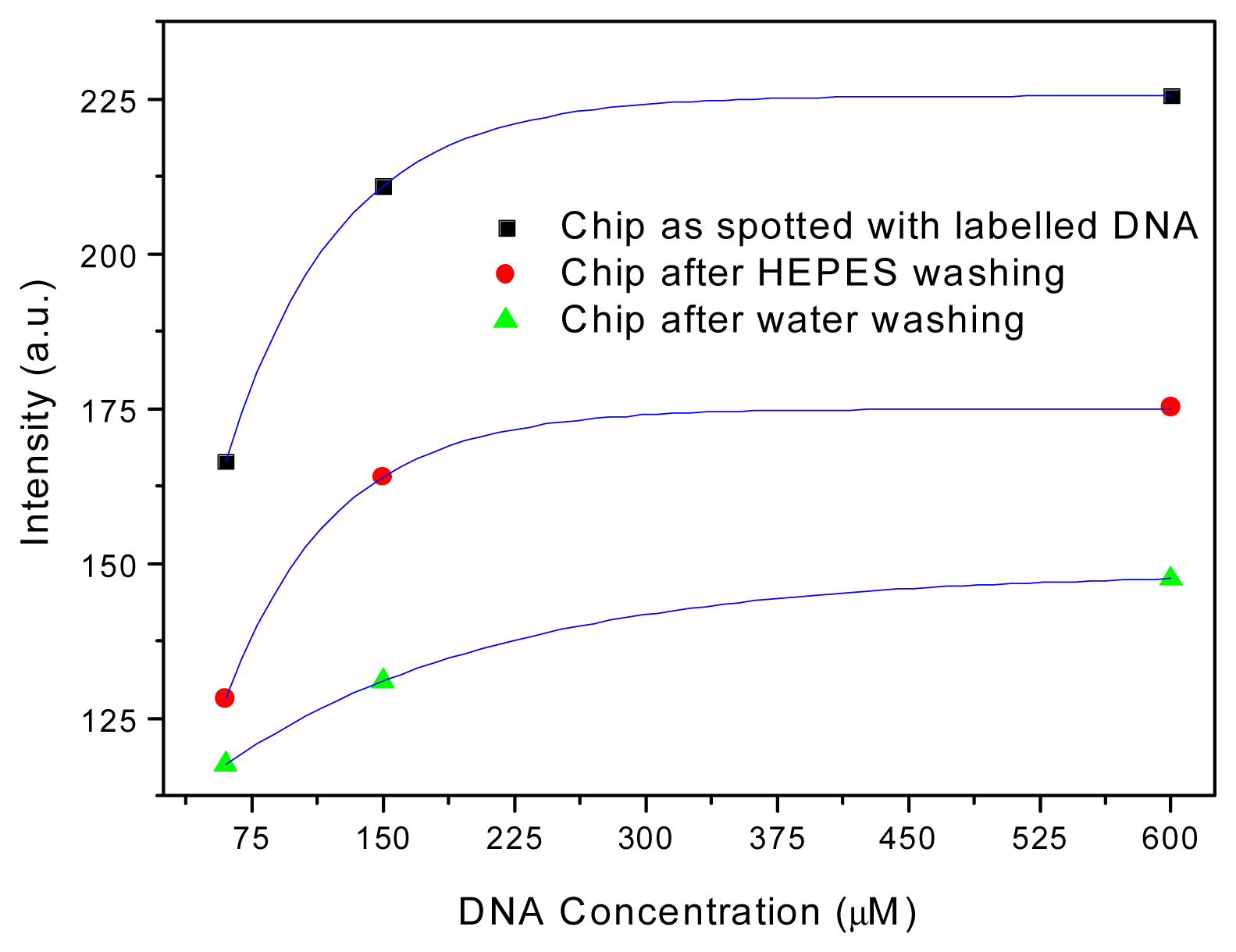
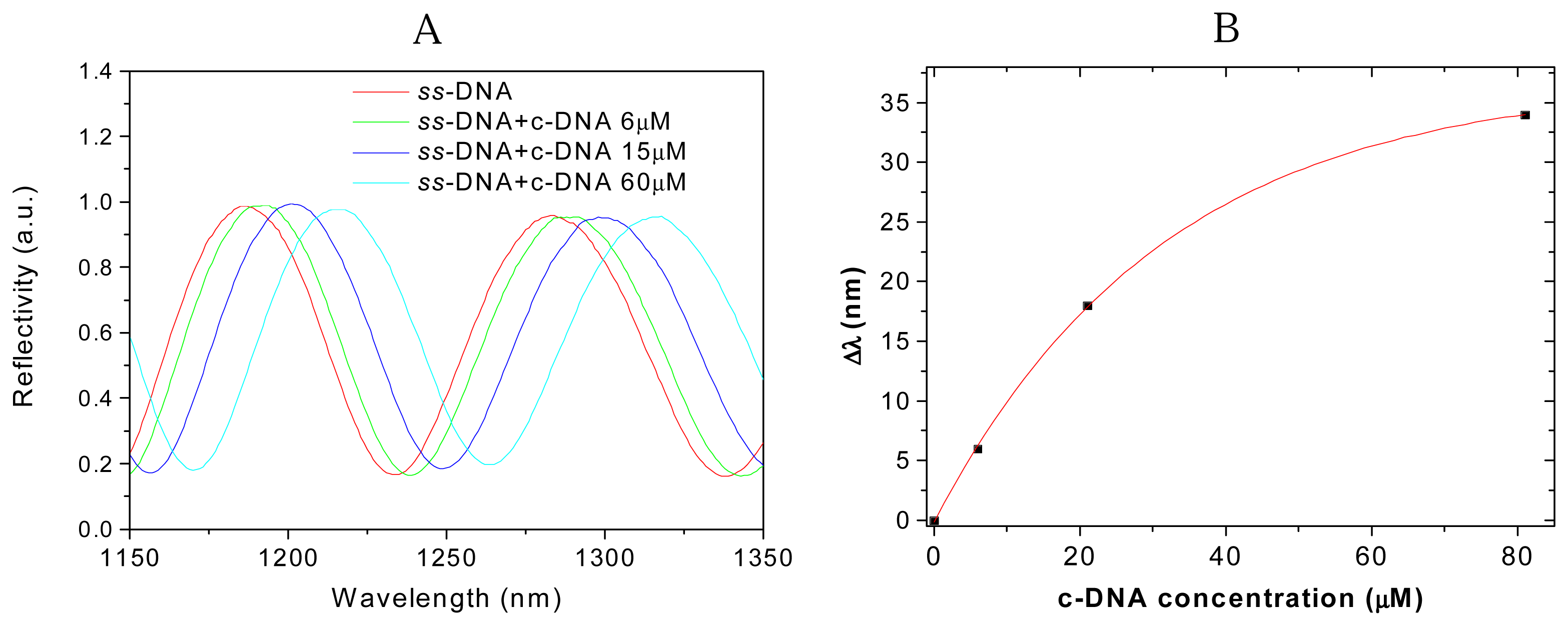
3. Results and Discussion
Acknowledgments
References and Notes
- Optical Biosensors; Ligler, F.S.; Rowe Taitt, C.A. (Eds.) Elsevier: Amsterdam, The Netherlands, 2004.
- Salitermkan, S. S. Fundamentals of BioMEMS and Medical Microdevices; Wiley-Interscience, SPIE PRESS: Bellingham, Washington USA, 2006. [Google Scholar]
- Lin, V. S. Y.; Motesharei, K.; Dancil, K. P. S.; Sailor, M. J.; Ghadiri, M. R. A porous silicon-based optical interferometric biosensor. Science 1997, 278, 840–843. [Google Scholar]
- De Stefano, L.; Moretti, L.; Rendina, I.; Rossi, A. M. Time-resolved sensing of chemical species in porous silicon optical microcavity. Sensor and Actuators B 2004, 100, 168–172. [Google Scholar]
- Dancil, K. -P. S.; Greiner, D. P.; Sailor, M. J. A Porous Silicon Optical Biosensor: Detection of Reversible Binding of IgG to a Protein A-Modified Surface. J. Am. Chem. Soc. 1999, 121, 7925–7930. [Google Scholar]
- Snow, P. A.; Squire, E. K.; Russel, P. St. J.; Canham, L. T. Vapor sensing using the optical properties of porous silicon Bragg mirrors. J. Appl. Phys. 1999, 86, 1781–1784. [Google Scholar]
- Lorenzo, E.; Oton, C. J.; Capuj, N. E.; Ghulinyan, M.; Navarro-Urrios, D.; Gaburro, Z.; Pavesi, L. Porous silicon-based rugate filters. Appl. Opt. 2005, 44, 5415–5421. [Google Scholar]
- Ouyang, H.; Chrtistophersen, M.; Viard, R.; Miller, B. L.; Fauchet, P. M. Macroporous silicon microcavities for macromolecule detection. Adv. Funct. Mater. 2005, 15, 1851–1859. [Google Scholar]
- De Stefano, L.; Rotiroti, L.; Rea, I.; Rendina, I.; Moretti, L.; Di Francia, G.; Massera, E.; Lamberti, A.; Arcari, P.; Sangez, C. Porous silicon-based optical biochips. Journal of Optics A: Pure and Applied Optics 2006, 8, S540–S544. [Google Scholar]
- Wongmanerod, C.; Zangooie, S.; Arwin, H. Determination of pore size distribution and surface area of thin porous silicon layers by spectroscopic ellipsometry. Applied Surface Science 2001, 172, 117–125. [Google Scholar]
- Yin, H.B.; Brown, T.; Gref, R.; Wilkinson, J.S.; Melvin, T. Chemical modification and micropatterning of Si(100) with oligonucleotides. Microelectronic Engineering 2004, 73-74, 830–836. [Google Scholar]
| Current Density (mA/cm2) | Porosity (%) | Etch Rate (nm/s) | Thickness (μm) | Etch Time (s) |
|---|---|---|---|---|
| 50 | 60 | 193 | 2.0 | 10.4 |
| 4.0 | 20.7 | |||
| 6.0 | 31.1 | |||
| 125 | 70 | 247 | 2.0 | 8.1 |
| 4.0 | 16.2 | |||
| 6.0 | 24.3 | |||
| 150 | 80 | 137 | 2.0 | 14.6 |
| 4.0 | 29.2 | |||
| 6.0 | 43.8 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
De Stefano, L.; Arcari, P.; Lamberti, A.; Sanges, C.; Rotiroti, L.; Rea, I.; Rendina, I. DNA Optical Detection Based on Porous Silicon Technology: from Biosensors to Biochips. Sensors 2007, 7, 214-221. https://doi.org/10.3390/s7020214
De Stefano L, Arcari P, Lamberti A, Sanges C, Rotiroti L, Rea I, Rendina I. DNA Optical Detection Based on Porous Silicon Technology: from Biosensors to Biochips. Sensors. 2007; 7(2):214-221. https://doi.org/10.3390/s7020214
Chicago/Turabian StyleDe Stefano, Luca, Paolo Arcari, Annalisa Lamberti, Carmen Sanges, Lucia Rotiroti, Ilaria Rea, and Ivo Rendina. 2007. "DNA Optical Detection Based on Porous Silicon Technology: from Biosensors to Biochips" Sensors 7, no. 2: 214-221. https://doi.org/10.3390/s7020214




