Carbon-based Composite Electrodes: Preparation, Characterization and Application in Electroanalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface characterization
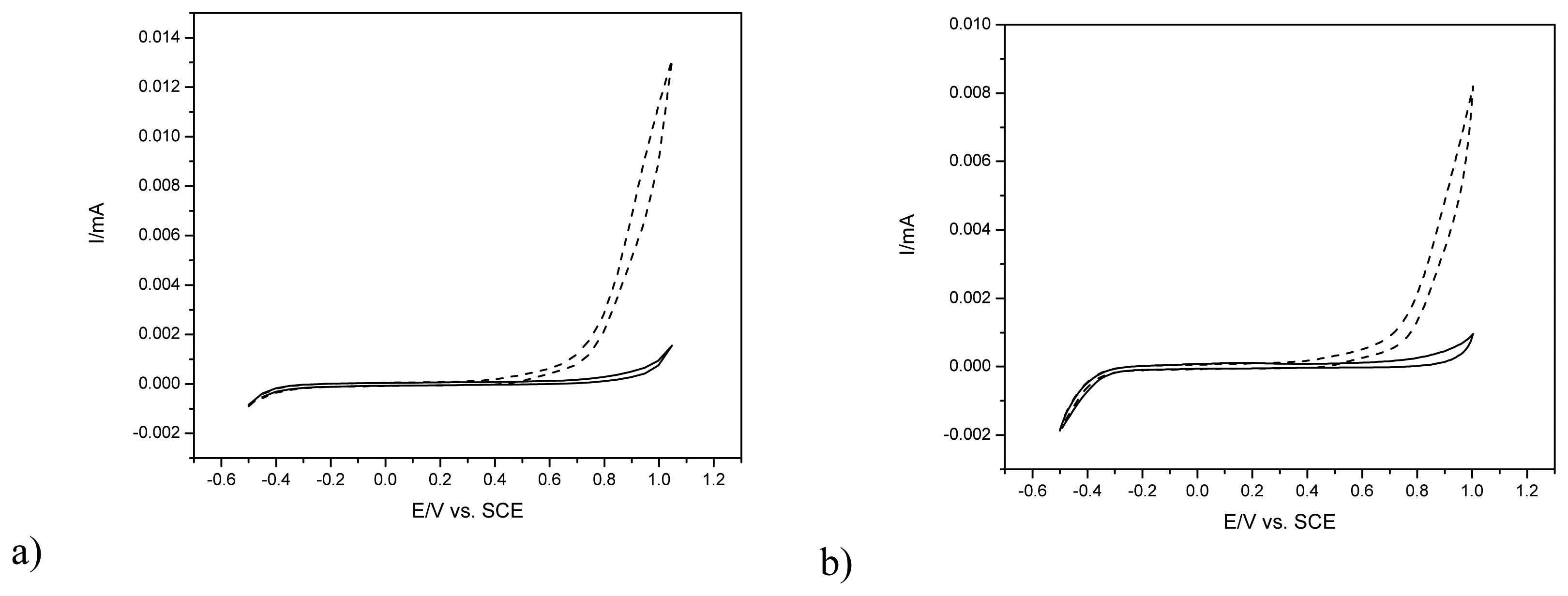
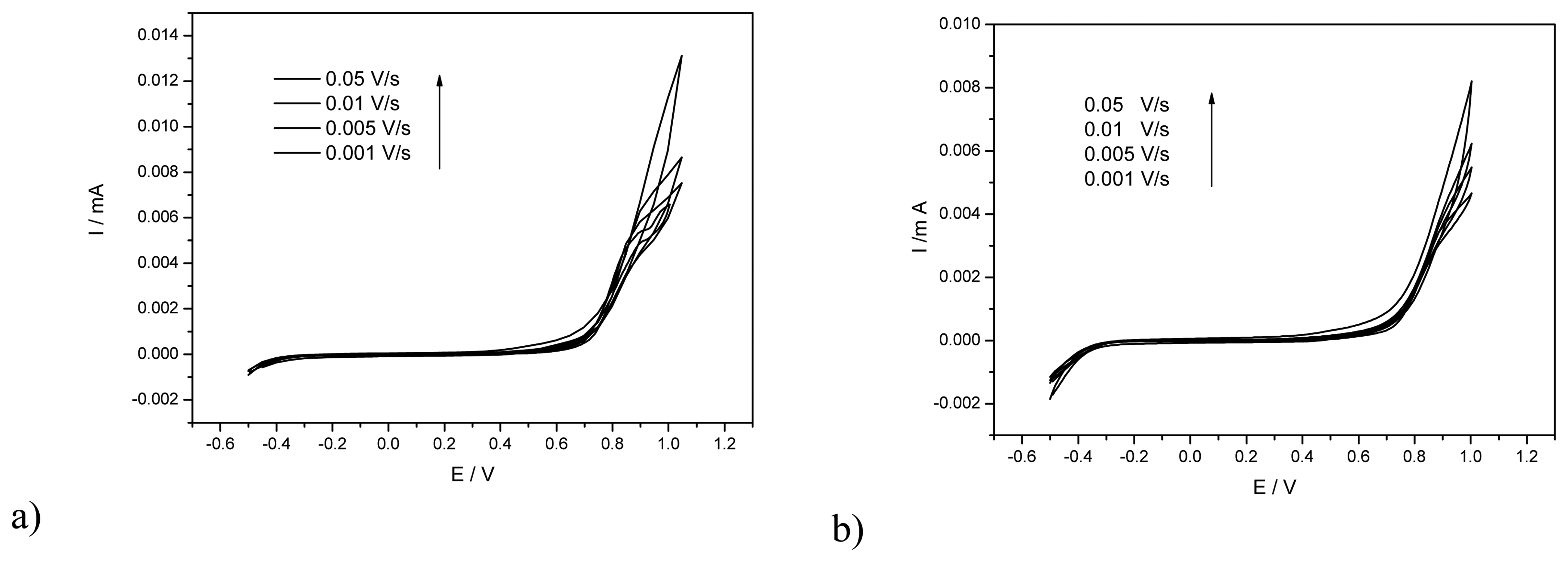
2.2. Electrochemical behavior
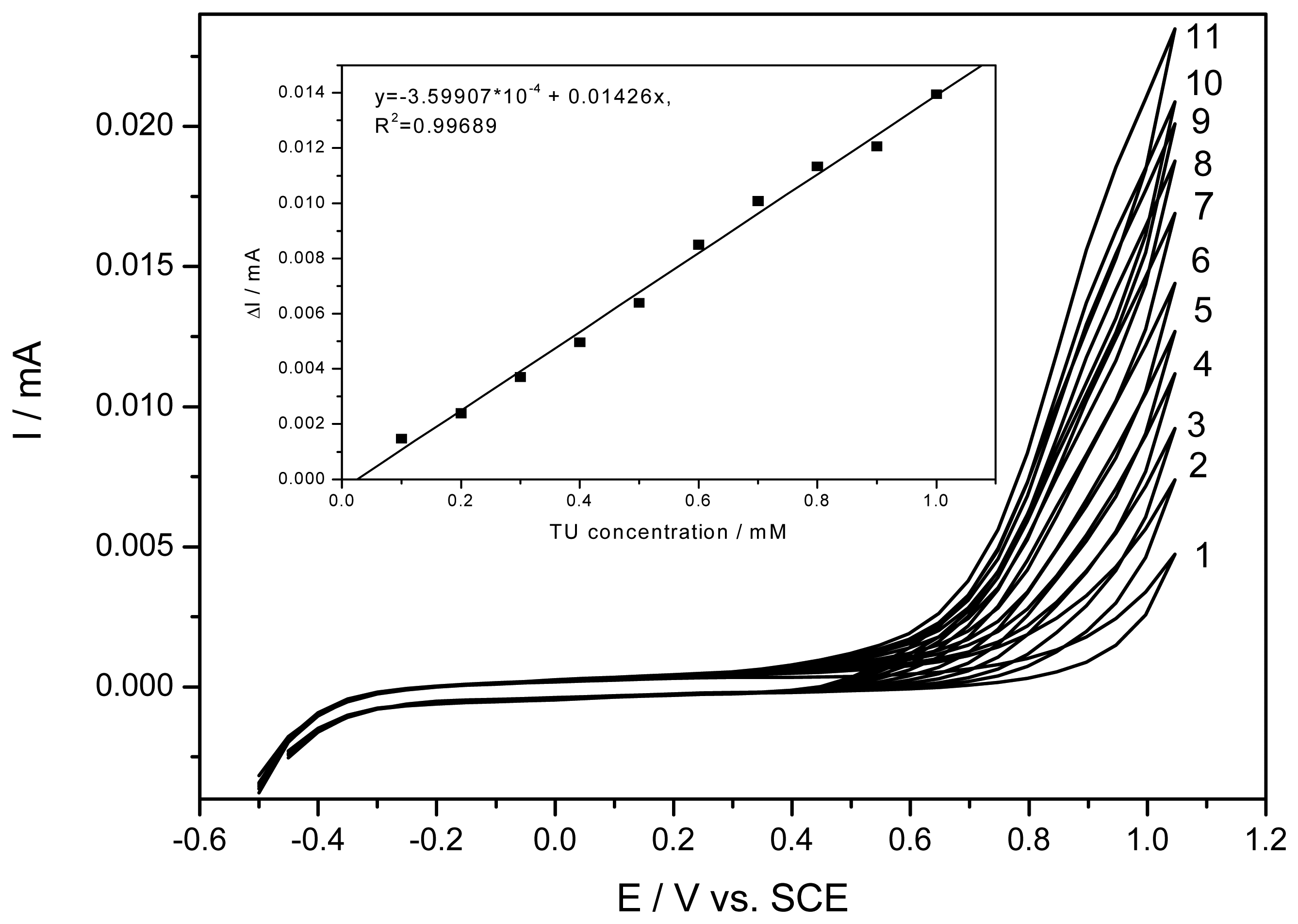
2.3. Electrochemical detection in solution of low ionic strength
3. Experimental Section
4. Conclusions
Acknowledgments
References and Notes
- Tu, Y.; Lin, Y.; Yantasee, W.; Ren, Z. Carbon Nanotubes Based Nanoelectrode Arrays: Fabrication, Evaluattion, and Application in Voltammetric Analysis. Electroanalysis 2005, 17, 79–84. [Google Scholar]
- Wang, J. Analytical Electrochemistry; VCH Publishers: New York, NY, 2000; Chapter 4; pp. 100–139. [Google Scholar]
- Rassaei, L.; Sillanpaa, M.; Bonne, M.J.; Marken, F. Carbon Nanofiber-Polystyrene Composite Eectrodes for Electroanalytical Processes. Electroanalysis 2007, 19(14), 1461–1466. [Google Scholar]
- Zhu, L.; Tian, C.; Zhai, J.; Yang, R. Sol-gel Derived Carbon Nanotubes Ceramic Composite Electrodes for Electrochemical Sensing. Sens. Actuators B. 2007, 125, 254–261. [Google Scholar]
- Pumera, M.; Merkoci, A.; Alegret, S. Carbon Nanotube-epoxy Composites for Electrochemical Sensing. Sens. Actuators B. 2006, 113, 617–622. [Google Scholar]
- Ballarin, B.; Cordero-Rando, M.M.; Blanco, E.; Hidalgo-Hidalgo De Cisneros, J. L.; Seeber, R.; Tonelli, D. New Rigid Conducting Composites for Electrochemical Sensors. Collect. Czech. Chem. Commun. 2003, 68, 1420–1436. [Google Scholar]
- Ramirez-Garcia, S.; Alegret, S.; Cespedes, F.; Forster, R.J. Carbon Composite Electrodes: Surface and Electrochemical Properties. Analyst 2002, 127, 1512–1519. [Google Scholar]
- Gabay, T.; Ben-David, M.; Kalifa, I.; Sorkin, R.; Abrahams, Z.R.; Ben-Jacob, E.; Hanein, Y. Electro-chemical and Biological Properties of Carbon Nanotubes based Multi-electrode Arrays. Nanotechnology 2007, 18, 035201:1–035201:6. [Google Scholar]
- Manso, J.; Mena, M.L.; Yanez-Sedeno, P.; Pingarron, J. Electrochemical Biosensors Based on Colloidal Gold-nanotube Composite Electrodes. J. Electroanal. Chem. 2007, 603(1), 1–7. [Google Scholar]
- Sun, D.; Zhu, L.; Zhu, G. Glassy Carbon Ceramic Composite Electrodes. Anal. Chim. Acta 2006, 564, 243–247. [Google Scholar]
- Mendes, R.K.; Cervini, P.; Cavalheiro, E. T. G. The Use of a Graphite-Castor Oil Polyurethane Composite Electrode for the Determination of Hidroquinone in Photographic Developers. Talanta. 2006, 68, 708–712. [Google Scholar]
- O'Hare, D.; Macpherson, J.V.; Wilson, A. On the Microelectrode Behaviour of Graphite-Epoxy Composite Electrodes. Electrochem. Commun. 2002, 4, 245–250. [Google Scholar]
- Marken, F.; Gerrard, M. L.; Mellor, I. M.; Mortimer, R. J.; Madden, C. E.; Fletcher, S.; Holt, K.; Foord, J. S.; Dahm, F. Voltammetry at Carbon Nanofiber Electrodes. Electrochem. Commun. 2001, 3, 177–180. [Google Scholar]
- Manea, F.; Radovan, C.; Schoonman, J. Amperometric Determination of Thiourea in Alkaline Media on a Copper Oxides-Copper Electrode. J. Appl. Electrochem. 2006, 36, 1075–1081. [Google Scholar]
- Spataru, N.; Spataru, T.; Fujishima, A. Voltammetric Determination of Thiourea at Conductive Diamond. Electroanalysis 2005, 17(9), 800–805. [Google Scholar]
- Stulik, K.; Amatore, C.; Holub, K.; Marecek, V.; Kutner, W. Microelectrodes. Definitions, Characterization, and Applications. Pure Appl. Chem. 2000, 72(8), 1483–1492. [Google Scholar]
- Simm, A.O.; Banks, C. E.; Ward-Jones, S.; Davies, T. J.; Lawrence, N. S.; Jones, T. G. J.; Jiang, L.; Compton, R.G. Boron-doped Diamond Microdisc Arrays: Electrochemical Characterisation and Their Use as a Substrate for The Production of Microelectrode Arrays of Diverse Metals (Ag, Au, Cu) via Electrodeposition. Analyst 2005, 130, 1303–1311. [Google Scholar]
- Davies, T.J.; Compton, R.G. The Cyclic and Linear Sweep Voltammetry of Regular and Random Arrays of Microdisc Electrodes: Theory. J. Electroanal. Chem. 2005, 585, 63–82. [Google Scholar]
- Cięciwa, A.; Wuthrich, R.; Comninellis, C. Electrochemical Characterization of Mechanically Implanted Boron-doped Diamond Electrodes. Electrochem. Commun. 2006, 8, 375–382. [Google Scholar]
- Soh, K.L.; Kang, W.P.; Davidson, J.L.; Basu, S.; Wong, Y.M.; Cliffel, D.E.; Bonds, A.B.; Swain, G.M. Diamond-derived Microelectrodes Array for Electrochemical Analysis. Diam. Relat. Mater. 2004, 13, 2009–2015. [Google Scholar]
- Feeney, R.; Kounaves, S. P. Microfabricated Ultramicroelectrode Arrays: Developments, Advances, and Applications in Environmental Analysis. Electroanalysis 2000, 12(9), 677–684. [Google Scholar]
- Fletcher, S.; Horne, M.D. Random Assemblies of Microelectrodes (RAM electrodes) for Electrochemical Studies. Electrochem. Commun. 1999, 1, 502–512. [Google Scholar]
- Lawrence, N.S.; Pagels, M.; Meredith, A.; Jones, T. G. J.; Hall, C.E.; Pickles, C. S. J.; Godfried, H. P.; Banks, C. E.; Compton, R. G.; Jiang, L. Electroanalytical Applications of Boron-doped Diamond Microelectrode Arrays. Talanta 2006, 69, 829–834. [Google Scholar]
- Lee, H.J.; Beriet, C.; Ferrigno, R.; Girault, H. Cyclic Voltammetry at Regular Microdisc Electrode Array. J. Electroanal. Chem. 2001, 502, 138–145. [Google Scholar]


| Technique used | Concentration range(mM) | Electrode sensitivity (mA·mM-1) | Correlation coefficient (R2) | LOD (mM) | RSD (%) |
|---|---|---|---|---|---|
| CV | 0.1-1 | 0.01426 | 0.9992 | 0.02 | 2.5 |
| CA | 0.5-4 | 0.00348 | 0.9832 | 0.2 | 3.2 |
| Carbon -based composite type | Electrical resistance*(Ω) |
|---|---|
| 20EG-Epoxy | 7.3 |
| 20EG-PS | 5.8 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Corb, I.; Manea, F.; Radovan, C.; Pop, A.; Burtica, G.; Malchev, P.; Picken, S.; Schoonman, J. Carbon-based Composite Electrodes: Preparation, Characterization and Application in Electroanalysis. Sensors 2007, 7, 2626-2635. https://doi.org/10.3390/s7112626
Corb I, Manea F, Radovan C, Pop A, Burtica G, Malchev P, Picken S, Schoonman J. Carbon-based Composite Electrodes: Preparation, Characterization and Application in Electroanalysis. Sensors. 2007; 7(11):2626-2635. https://doi.org/10.3390/s7112626
Chicago/Turabian StyleCorb, Ioana, Florica Manea, Ciprian Radovan, Aniela Pop, Georgeta Burtica, Plamen Malchev, Stephen Picken, and Joop Schoonman. 2007. "Carbon-based Composite Electrodes: Preparation, Characterization and Application in Electroanalysis" Sensors 7, no. 11: 2626-2635. https://doi.org/10.3390/s7112626
APA StyleCorb, I., Manea, F., Radovan, C., Pop, A., Burtica, G., Malchev, P., Picken, S., & Schoonman, J. (2007). Carbon-based Composite Electrodes: Preparation, Characterization and Application in Electroanalysis. Sensors, 7(11), 2626-2635. https://doi.org/10.3390/s7112626




