Abstract
Linear scan voltammetry (LSV), differential pulse voltammetry (DPV) and voltammetry with adsorptive accumulation (AAV) at a glassy carbon paste electrode (GCPE) based on mixing glassy carbon spherical microparticles with an organic pasting liquid were used for the determination of trace amounts of carcinogenic 1-nitropyrene (1-NP) (the limit of determination around 2.10-5 mol L-1 for LSV and 2.10-6 mol L-1 for DPV) and 1-aminopyrene (1-AP) (the limit of determination around 3.10-6 mol L-1 for LSV, 1.10-6 mol L-1 for DPV and 1.10-7 mol L-1 for AAV) using a Britton-Robinson buffer – methanol mixture as a base electrolyte. The main advantage of this new type of electrode is its full compatibility with media containing a high amount of organic solvent (methanol, acetonitrile)
Introduction
1-nitropyrene is one of the most abundant representatives of nitroaromatic compounds in the environment which has also been detected in automobile exhaust, urban air, incinerators exhalations and in certain food items such as grilled meats or teas (see reviews [1,2,3]). It is listed in the IARC Monographs on the Evaluation of Carcinogenic Risks to Humans [4]. 1-Aminopyrene was found to be 1-nitropyrene's most frequent cell metabolite [5]. Therefore, there is an ever-increasing demand for the determination of trace amounts of these substances. So far, mostly chromatographic methods such as GC-MS or HPLC with fluorimetric detection are used for these purposes [3]. However, these methods are characterized by high investment and running costs. Modern electroanalytical methods, especially differential pulse voltammetry (DPV) and adsorptive stripping voltammetry (AdSV) at mercury [6] or carbon paste [7] electrodes satisfy even the highest demands on sensitivity required for the determination of extremely dangerous chemical carcinogens. Their main advantage being much lower investment and running costs.
Carbon paste electrodes earned wide use as working electrodes for voltammetric techniques [7,8]. A new carbon paste electrode material combining the electrochemical properties of glassy carbon with the attractive advantages of composite paste electrode materials based on mixing glassy carbon microparticles, is originally employed for FIA (flow-injection analysis) as a disposable and renewable electrode system [9] with an oil binder and was introduced recently [10,11]. The resulting glassy carbon paste electrode (GCPE) combines the electrochemical properties of glassy carbon with the various advantages of composite electrodes. Glassy carbon pastes offer high electrochemical reactivity, a wide accessible potential window, a low background current, and are inexpensive, easy to prepare, modify, and renew. The new material has a lower double-layer capacitance compared to conventional carbon pastes [11]. Scanning electron microscopy (SEM) images indicated significant differences in the structure of the spherical glassy carbon paste and classical graphite carbon paste electrodes [10,11]. Glassy carbon electrodes offer attractive electrochemical reactivity, negligible porosity, and good mechanical rigidity, while carbon paste electrodes are popular due to their low background current, simplicity of modification, renewal, and miniaturization. Girault [12] proposed a composite based on glassy carbon microparticles and a polystyrene polymer which exhibits electrochemical properties dependent on the carbon/polymer ratio and ranging from insulating films through isolated and closely spaced random array microelectrodes to microporous macro-electrode. Rivas [13] used a glassy carbon paste electrode modified with polyphenol oxidase for the determination of dopamine, acetaminophen and polyphenols. Quite recently, two types of glassy carbon powder (i.e., Sigradur K and Sigradur G) have been used to obtain a glassy carbon paste electrode which was then modified with Prussian Blue [14].
The main drawback of classical carbon paste electrodes is the fact that they can not be used in a medium with high content of organic solvents (such as methanol or acetonitrile), which dissolve the organic pasting liquid thus, making the paste friable. However, we have found [15] that this new type of GCPE is much more resistant towards organic solvents then classical carbon pastes. The applicability of GCPE for the determination of trace amounts of 1-NP and 1-AP in mixed water-methanol medium is demonstrated in this paper. The possibility to use GCPE for both oxidation and reduction of organic substances is another advantage of this type of electrode. It enables to use a single electrode for the determination of both 1-NP and its reduction product 1-AP.
Experimental
Reagents
1-nitropyrene (97%, C.A.S. Name: Pyrene,1-nitro-, C.A.S. Reg.number: [5522-43-0], abbreviation: 1-NP) and 1-aminopyrene (99%, C.A.S. Name: 1-Pyrenamine, C.A.S. Reg.number: [1606-67-3], abbreviation: 1-AP) were obtained from Sigma-Aldrich. 1.10-3 mol.L-1 methanolic stock solutions of the analytes were kept refrigerated in dark. More diluted solutions were prepared by exact dilution of the stock solution with methanol. Mineral oil (Fluka) and spherical glassy carbon microparticles powder 0.4-12 μm type 2 (Alfa Aesar, Ward Hill, MA, USA) were mixed in 20:80 mass ratio for the preparation of the glassy carbon paste electrode. Sodium hydroxide, glacial acetic acid, phosphoric acid, and methanol (all p.a. purity) were obtained from Lachema (Brno, Czech Republic). Solutions of Britton-Robinson buffers were prepared in 0.2 mol. L-1 concentration. Deionized water (Millipore Q-plus System, Millipore, USA) was used for all experiments.
Apparatus
A polarographic analyzer EcoTriboPolarograph controlled by PolarPro v. 2.0 software (both PolaroSensors, Prague, Czech Republic) was used. A three-electrode arrangement was used with a saturated calomel reference and a platinum foil auxiliary electrode. A scan rate of 10 mV/s was used for all experiments. The pulse height was 50 mV for DPV experiments. A lab-made working glassy carbon paste electrode was used with a 2 mm inner diameter Teflon tube holder of the carbon paste. The contact was established via a steel screw dipped by its end into the carbon paste. The GCPE was stored after preparation and experiments for several days in a 50 mL beaker under a thin layer of water. The electrode was polished prior to use by a wetted filtration paper. A prebubbler containing a methanolic solution of the same water: methanol ratio as the analyzed solution was placed prior the voltammetric cell. pH of the solutions was measured by a Conductivity and pH meter 4330 (Jenway, Great Britain) with a combined glass electrode. All experiments were carried out under laboratory temperature.
Procedures
The general procedure to obtain corresponding voltammograms was as follows: A required amount of the stock solution of the test substance in methanol was placed in a 10 ml volumetric flask, an appropriate volume of methanol was added and the system was diluted to volume with a Britton-Robinson buffer of the required pH. (A different order of mixing the solutions resulted in a precipitation of the test substance.) The solution was transferred into a voltammetric vessel, oxygen was removed from the measured solution by bubbling with nitrogen for five minutes and the voltammogram was recorded. Calibration curves were measured in triplicate and their statistical parameters (e.g., slope, intercept, limit of decision, limit of detection, limit of determination LQ) were calculated from the confidence bands (α=0.05) according to Oppenhelmer [16], Schwartz [17], and Ebel [18] using statistic software Adstat, version. 2.0 (TriloByte, Czech Republic).
Results and Discussion
Linear scan voltammetry of 1-aminopyrene
The influence of pH on the LS voltammetric behavior of 1.10-4 mol.L-11-AP at GCPE in mixtures of Britton-Robinson buffer : methanol (9:1 v/v) was investigated for a pH range of the buffer 2-13. It can be seen from Fig. 1 that 1-AP provides one well-resolved irreversible anodic peak. Its position shifts towards negative potentials with increasing pH and its height is not significantly influenced by the change of pH. In the region of higher potentials, another smaller peak can be observed, which can not be evaluated. For pH 2-6, a third anodic peak can be found.
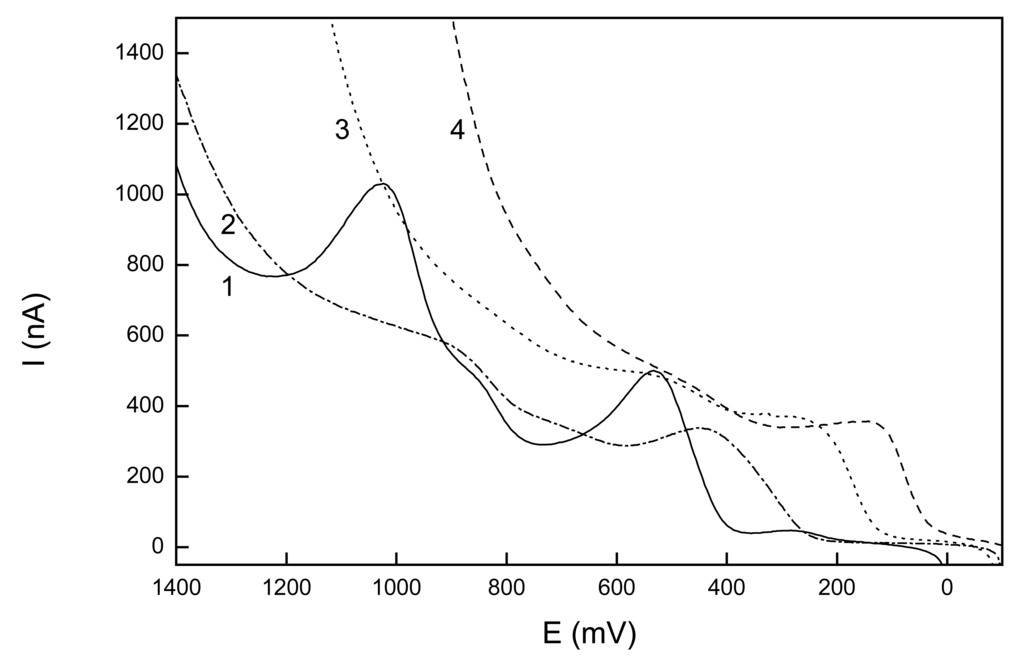
Figure 1.
Selected LS voltammograms of 1-AP (c=1.10-4 mol.L-1) at GCPE in mixture of BR buffer : MeOH (9:1) at buffer pH 2 (1), 6 (2), 10 (3), 12 (4).
Best-developed and most easily evaluated LS voltammograms were obtained for BR buffer pH 2 : methanol (9:1 v/v), where a peak-shaped signal was obtained. Calibration curves were measured under these conditions in the concentration ranges of (2-10).10-5 mol.L-1 and (2-10).10-6 mol.L-1. The first peak was used for a quantitative evaluation (its height was measured from the straight line parallel with the potential axis constructed as a prolongation of the linear part of the LS voltammogram in the region 200-400 mV, i.e. before the onset of the peak.) Parameters of the calibration curves are summarized in Table 1.

Table 1.
Calibration data for voltammetric determination of 1-AP at GCPE in a BR buffer : MeOH mixture.
Differential pulse voltammetry of 1-aminopyrene
The influence of pH on the DP voltammetric behavior of 1.10-4 mol.L-11-AP at GCPE in mixtures of Britton-Robinson buffer : methanol (9:1 v/v) was again investigated for a buffer pH range 2-13 (Fig 2).
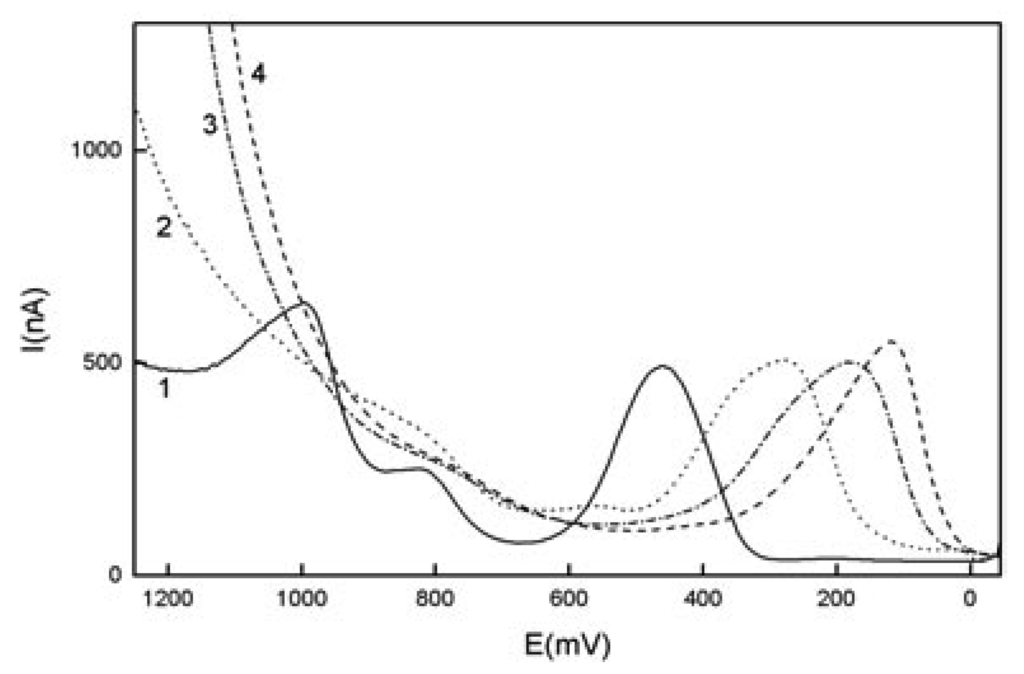
Figure 2.
Selected DP voltammograms of 1-AP (c=1.10-4 mol.L-1) at GCPE in a mixture of BR buffer : MeOH (9:1) at buffer pH 2(1), 6 (2), (3) 10, 12 (4).
It can be seen from Fig. 2 that 1-AP gives one well-developed irreversible anodic peak, which shifts towards positive potentials with decreasing pH, its height being practically independent of pH. For pH 2-6, there is a second peak, followed by a third peak that is observable only in the region of pH 2-4 (see Fig. 2.).
Best-developed and most easily evaluated DP voltammograms were obtained for a BR buffer, pH 2 : methanol (9:1 v/v). Calibration curves were measured under these conditions in the concentration ranges of (2-10).10-5 mol.L-1 and (2-10).10-6 mol.L-1. The first peak was used for the quantitative evaluation. Parameters of the calibration curves are also summarized in Table 1.
Voltammetry of 1-aminopyrene with adsorptive accumulation
Based on the above described DPV experiments, a Britton-Robinson buffer pH 2: methanol (9:1 v/v) mixture was selected for first measurements of 1-AP by voltammetry with adsorptive accumulation at GCPE. Under these conditions, 1-AP gave a well-developed anodic peak observed at Ep = 430 mV. First, the influence of the accumulation potential Eacc on the peak current Ip of 2.10-7 mol.L-1 1-AP was studied for accumulation potentials from -200 to +200 mV in a BR buffer pH 2:methanol (9:1 v/v) mixture using accumulation time tacc = 60s in a stirred solution. Eacc = 200 mV was found optimal. Further, the influence of tacc for the voltammetric determination of 2.10-7 mol.L-1 1-AP with adsorptive accumulation was studied for Eacc = 200 mV and tacc = 0, 30, 60, 120, 180 and 300 s in Britton-Robinson buffer pH 2 : methanol (9:1 v/v) (see Fig. 3).
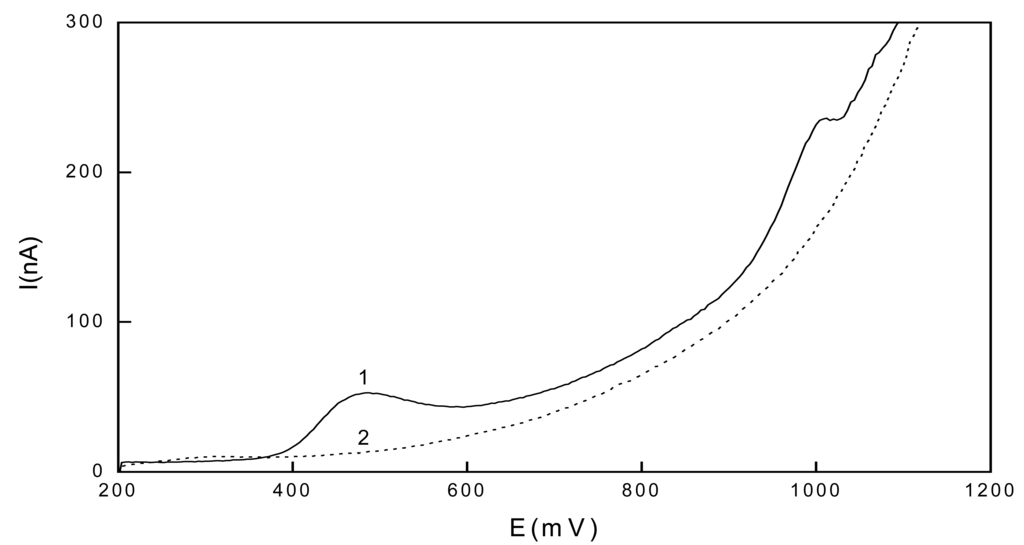
Figure 3.
Influence of the tacc for AAV of 2.10-7 mol.L-1 1-AP in stirred solution of a BR buffer pH 2 : MeOH (9:1 v/v), Eacc = 200 mV.
For the sake of illustration, voltammogram of 2.10-7 mol.l-1 1-AP with adsorptive accumulation is depicted in Fig. 4. To achieve higher sensitivity, 180 s accumulation time was selected for the measuring calibration curves. The peaks were well developed and reproducible under these conditions. A further increase in the accumulation time did not enhance the signal. Calibration curves were measured in the range of (2-10).10-7 mol.L-1 (see also Table 1).
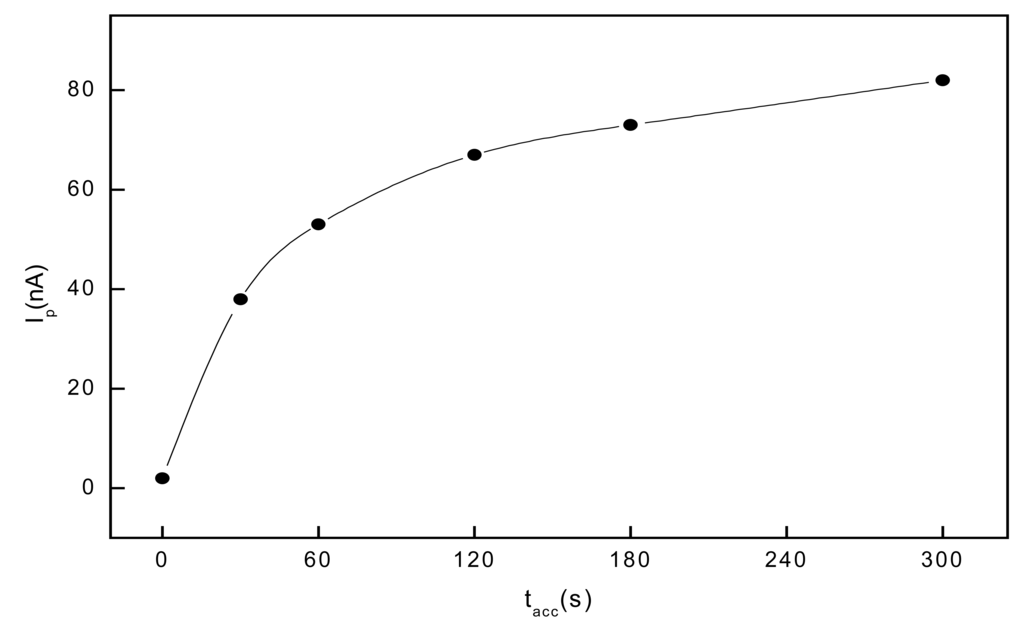
Figure 4.
Voltammogram of 2.10-7 mol.L-1 1-AP with adsorptive accumulation in stirred solution of a BR buffer pH 2 : MeOH (9:1 v/v) (1) and of the supporting electrolyte (2), Eacc = 200 mV, tacc = 60 s.
Linear sweep voltammetry of 1-nitropyrene
The influence of pH on the LS voltammetric behavior of 1.10-4 mol.L-11-NP at GCPE in mixtures of Britton-Robinson buffer pH 2-13 : MeOH (1:9 v/v) was investigated (see Fig. 5).
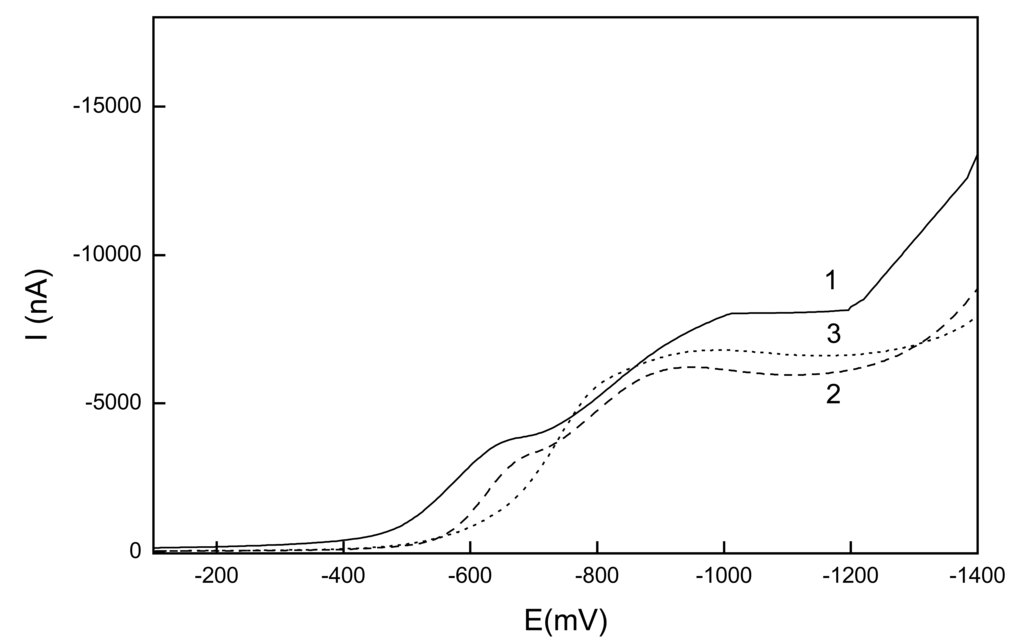
Figure 5.
Selected LS voltammograms of 1-NP (c=1.10-4 mol.L-1) at GCPE in mixture of a BR buffer : MeOH (1:9 v/v) with buffer pH 3 (1), 5 (2) and 8 (3).
Precipitation of the analyte from the solution was observed for solutions with lower content of methanol. It was found that 1-NP gives two well-developed waves. Their half-wave potential E1/2 shifts towards negative potentials with increasing pH and the dependence of their limiting current on pH is not too pronounced. At higher pH, the two waves merge. Best-developed voltammograms were obtained for a BR buffer pH 3 : methanol (1:9 v/v) mixture. Calibration curves were measured under these conditions in the concentration range of (2-10).10-5 mol.L-1 and their parameters are summarized in Table 2.

Table 2.
Calibration data for voltammetric determination of 1-NP at GCPE.
Differential pulse voltammetry of 1-nitropyrene
It was found that 1-NP gives one well-resolved peak. Its Ep shifts towards negative potentials with increasing pH and its current Ip does not change with pH too much (see Fig. 6).
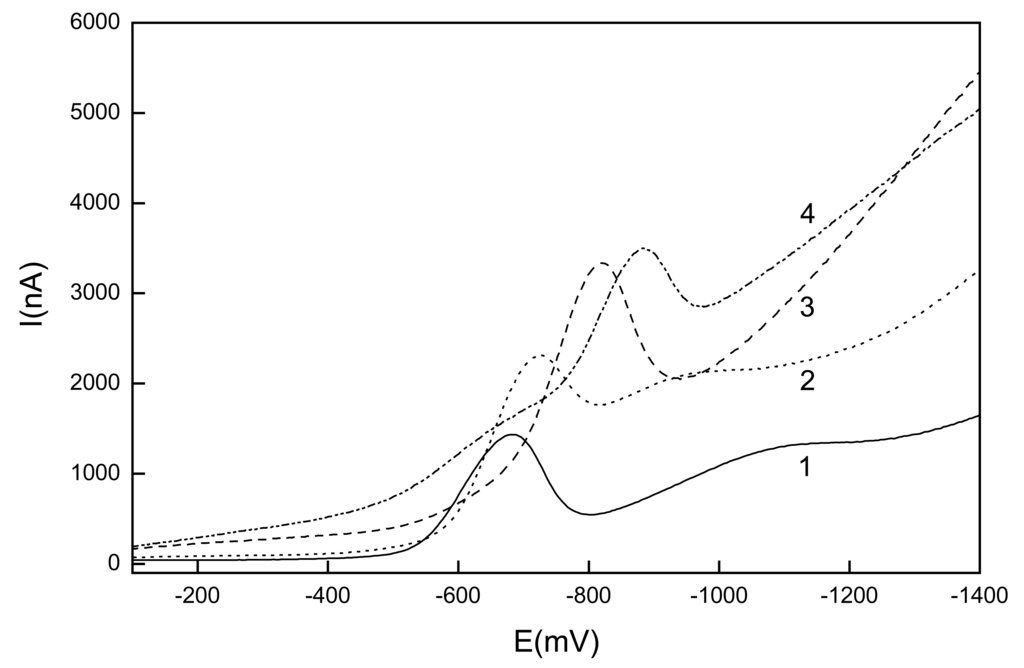
Figure 6.
Selected DP voltammograms of 1-NP (c=1.10-4 mol.L-1) at GCPE in mixture of a BR buffer : MeOH (1:9 v/v) mixture with buffer pH 3 (1), 5 (2), 8 (3) and 12 (4).
Best-developed voltammograms were obtained for a BR buffer pH 3 : methanol (1:9 v/v). Calibration curves were measured under these conditions in the concentration ranges of (2-10).10-5 mol.L-1 and (2-10).10-6 mol.L-1. Parameters of the calibration curves are summarized in Table 2, too. The attempt to increase the sensitivity of the determination by adsorptive stripping voltammetry was not successful.
Conclusions
Glassy carbon paste represents a versatile electrode material offering a variety of applications for electrochemical detection of environmentally significant organic compounds. The main advantage of this type of electrode material is its compatibility with organic solvents. It has been shown that this electrode can be used for the determination of 1-AP based on its anodic oxidation using either LSV, DPV or AAV with a limit of determination around 3.10-6 mol.L-1, 1.10-6 mol.L-1, and 1.10-7 mol.L-1, respectively. Determination of 1-NP based on its cathodic reduction signal can be successfully carried out thanks to the high stability of this type of electrode in methanolic solutions. Under optimal conditions, 1.10-6 mol.L-1 detection limit was achieved for 1-NP using DPV. The higher detection limit in the cathodic region is obviously connected with a relatively high background current connected with traces of oxygen entrapped in the paste during its preparation. In the cathodic region, the carbon paste electrodes are outperformed by mercury electrodes. Nevertheless, in some cases (such as monitoring of the destruction of nitro compounds based on their reduction to amino compounds followed by their oxidation with permanganate, etc.) they provide an advantageous tool to determine both oxidizable and reducible substances with a single electrode. Similarly, the application of this type of electrode for monitoring the efficiency of the photolytic destruction of 1-NP and 1-AP is under investigation.
Acknowledgments
J.B. thanks the Grant Agency of the Czech Republic (grant no. 203/03/0182). J.Z. thanks the Czech Ministry of Education, Youth and Sports (research project 113100002).
References
- Moreira, J.C.; Barek, J. Analysis of carcinogenic nitrated polycyclic aromatic hydrocarbons. Quimica Nova. 1995, 18, 362. [Google Scholar]
- Jacob, J.; Karcher, W.; Belliardo, J.J.; Dumler, R.; Boenke, A. Review. Polycyclic aromatic compounds of environmental and occupational importance - their occurrence, toxicity and the development of high-purity certified reference materials. Part III. Fresenius J. Anal. Chem. 1991, 340, 755. [Google Scholar]
- Cvacka, J.; Barek, J.; Fogg, A.G.; Moreira, J.C.; Zima, J. Review. HPLC of nitrated polycyclic aromatic hydrocarbons. Analyst. 1998, 123, 9. [Google Scholar]
- http://www-cie.iarc.fr/htdocs/monographs/vol46/46-14.htm (January 28, 2004).
- Stanton, C.A.; Chow, F.L.; Phillips, D.H.; Grover, P.L.; Garner, R.C.; Martin, C.N. Evidence for n-(deoxyguanosin-8-yl)-1-aminopyrene as a major DNA adduct in female rats treated with 1-nitropyrene. Carcinogenesis 1985, 6, 535. [Google Scholar]
- Barek, J.; Fogg, A.G.; Muck, A.; Zima, J. Polarography and voltammetry at mercury electrodes. Crit. Rev. Anal. Chem. 2001, 31, 291. [Google Scholar]
- Svancara, I.; Vytras, K.; Barek, J.; Zima, J. Carbon paste electrodes in modern electroanalysis. Crit. Rev. Anal. Chem. 2001, 31, 311. [Google Scholar]
- Kalcher, K.; Kauffmann, J.-M.; Wang, J.; Svancara, I.; Vytras, K.; Neuhold, C.; Yang, Z. Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990-1993. Electroanalysis. 1995, 7, 5. [Google Scholar]
- Mayer, M.; Ruzicka, J. Flow injection based renewable electrochemical sensor system. Anal.Chem. 1996, 68, 3808. [Google Scholar]
- Svancara, I.; Hvizdalova, M.; Vytras, K.; Kalcher, K.; Novotny, R. A microscopic study on carbon paste electrodes. Electroanalysis 1996, 8, 61. [Google Scholar]
- Wang, J.; Kirgöz, U.A.; Mo, J.W.; Lu, J.; Muck, A. Glassy carbon paste electrodes. Electrochem.Commun. 2001, 3, 203. [Google Scholar]
- Seddon, B.J.; Osborne, M.D.; Lagger, G.; Dryfe, R.A.W.; Loyal, U.; Schäfer, H.; Girault, H.H. Micro-glassy carbon inks for thick-film electrodes. Electrochim. Acta. 1997, 42, 1883. [Google Scholar]
- Rodriguez, M.C.; Rivas, G.A. Glassy carbon paste electrodes modified with polyphenol oxidase. Anal. Chim. Acta. 2002, 459, 43. [Google Scholar]
- Ricci, F.; Goncalves, C.; Amine, A.; Gorton, L.; Palleschi, G.; Moscone, D. Electroanalytical study of Prussian Blue modified glassy carbon paste electrodes. Electroanalysis. 2003, 15, 1204. [Google Scholar]
- Muck, A.; Barek, J.; Zima, J.; Wang, J. Determination of 1-nitropyrene and 1-aminopyrene using a glassy carbon paste electrode. In US-CZ Workshop on Electrochemical Sensors, Book of abstracts; p. p.23. Czech Chemical Society: Prague, 2001. [Google Scholar]
- Oppenhelmer, L.; Cappizi, T.P.; Weppelman, R.M.; Metha, H. Determining the lowest limit of reliable assay measurement. Anal. Chem. 1983, 55, 638. [Google Scholar]
- Schwartz, L.M. Lowest limit of reliable assay measurement with nonlinear calibration. Anal. Chem. 1983, 55, 1424. [Google Scholar]
- Ebel, S.; Kamm, U. Statistical definition of the limit of determination. Fresenius Z. Anal. Chem. 1984, 318, 293. [Google Scholar]
© 2004 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.