Voltammetric Determination of Prochlorperazine and Ethopropazine Using a Gold Electrode Modified with Decanethiol SAM
Abstract
:Introduction
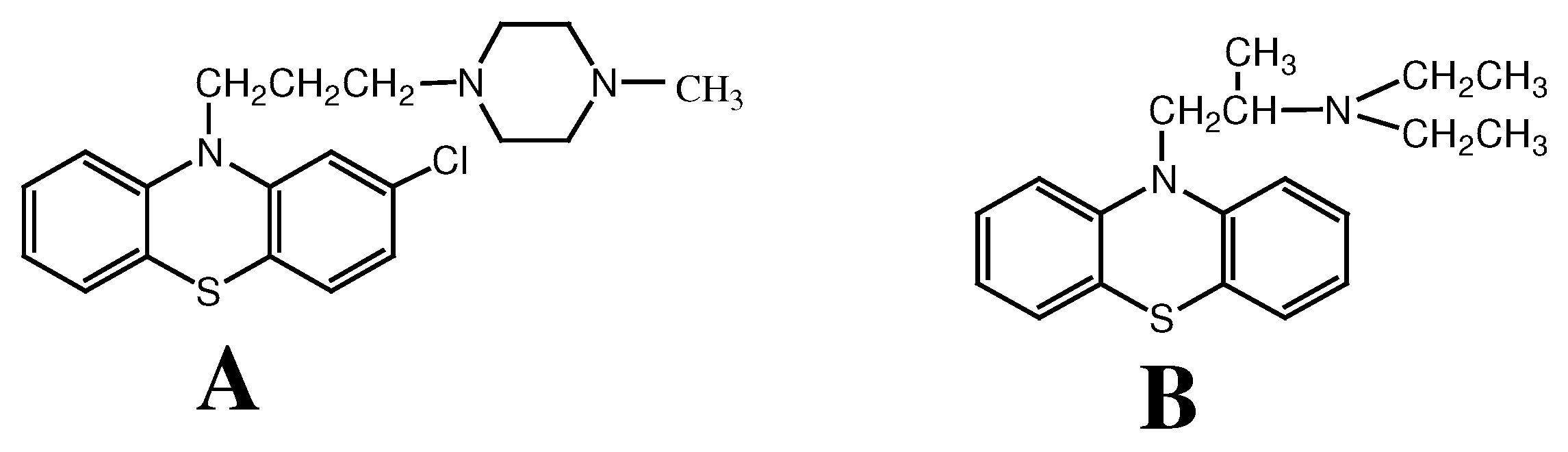
Experimental
Apparatus
Reagents
Electrode preparation
Procedure
Results and Discussion
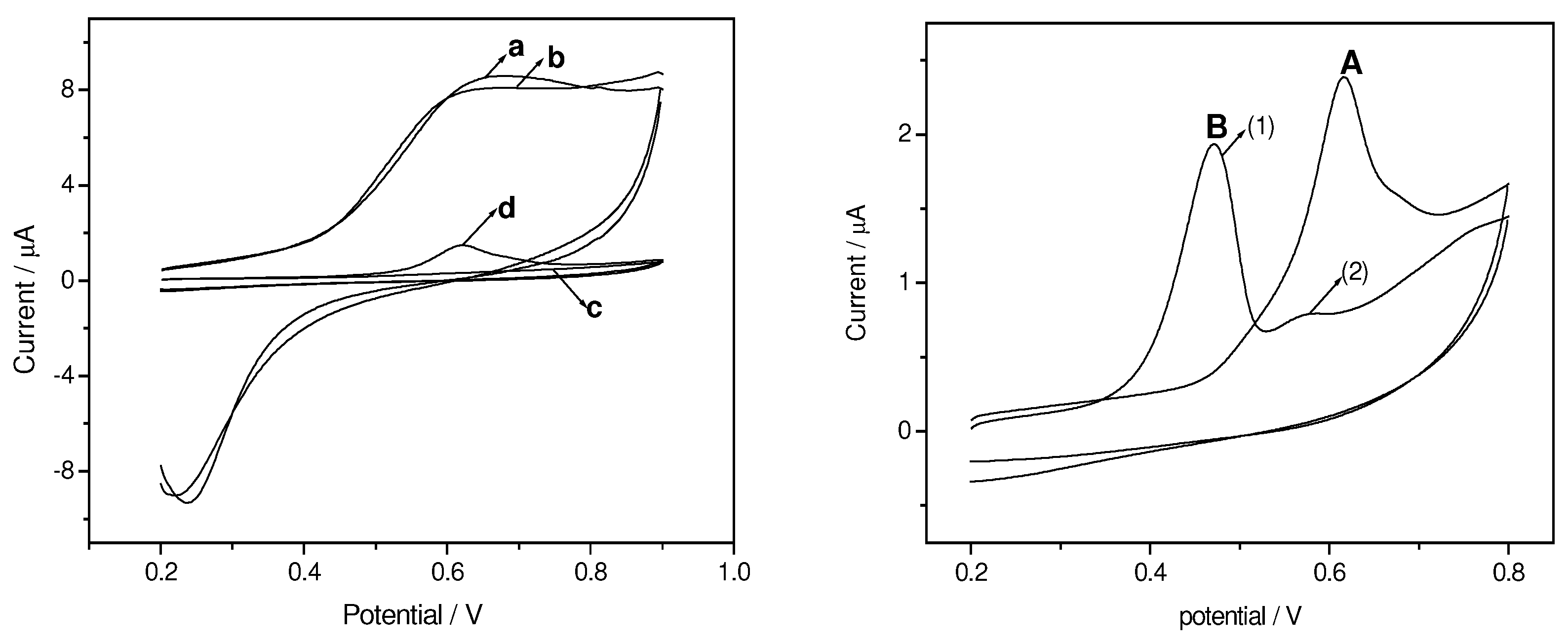
Cyclic Voltammograms
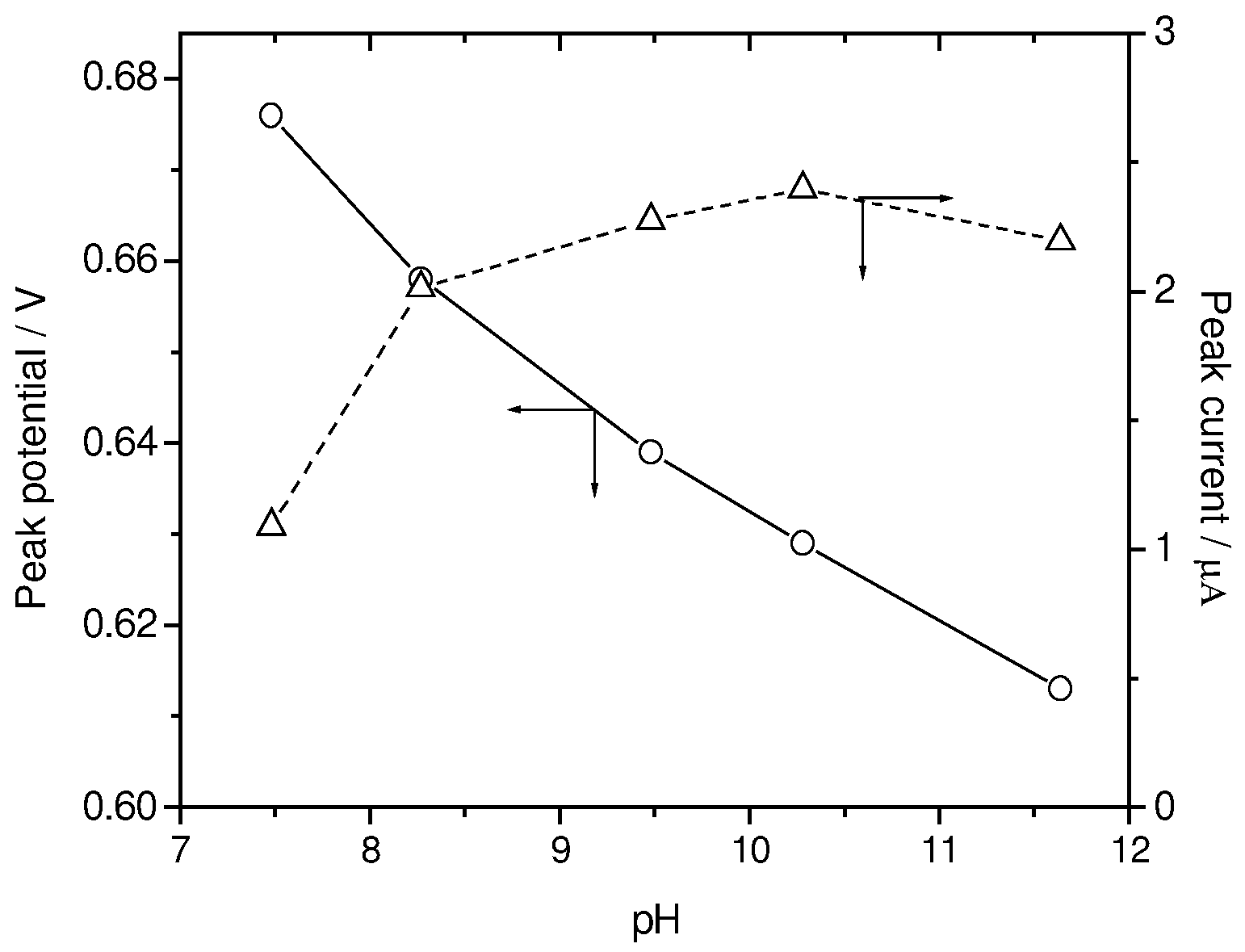
Influence of solution pH
Variation of peak current and potential with scan rate
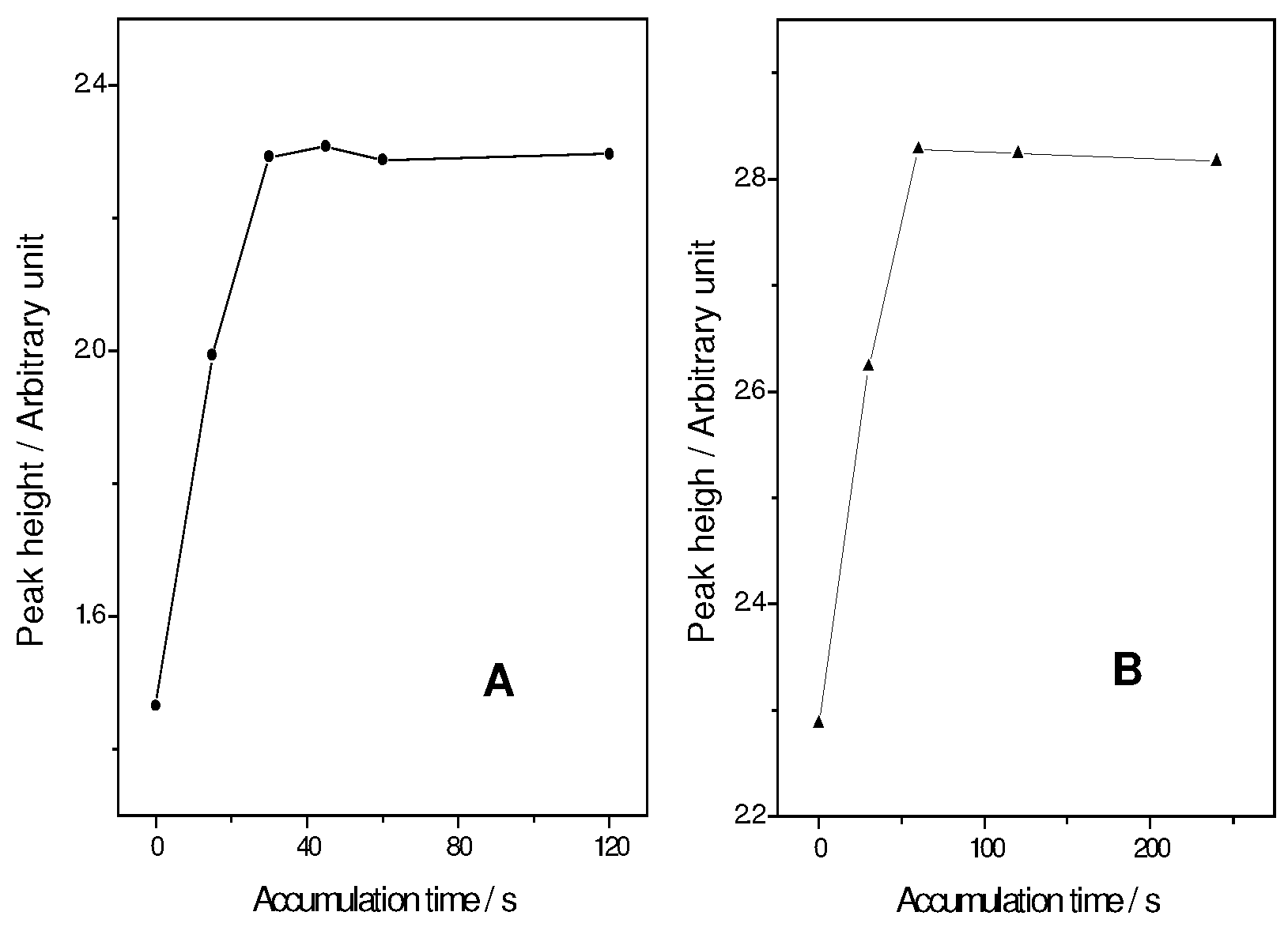
Influence of accumulation potential and time

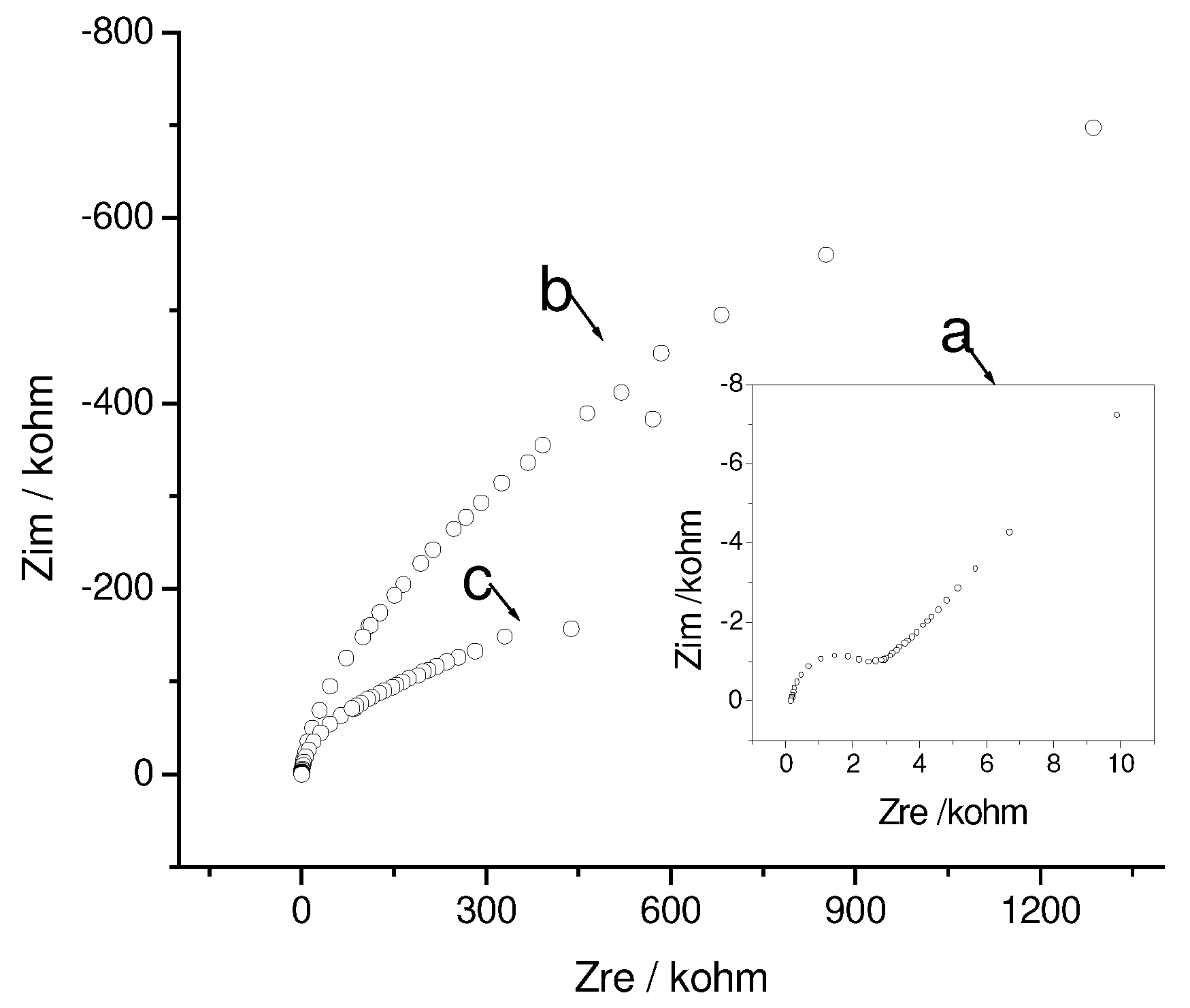
Influence of accumulated phenothiazines on the SAM structure
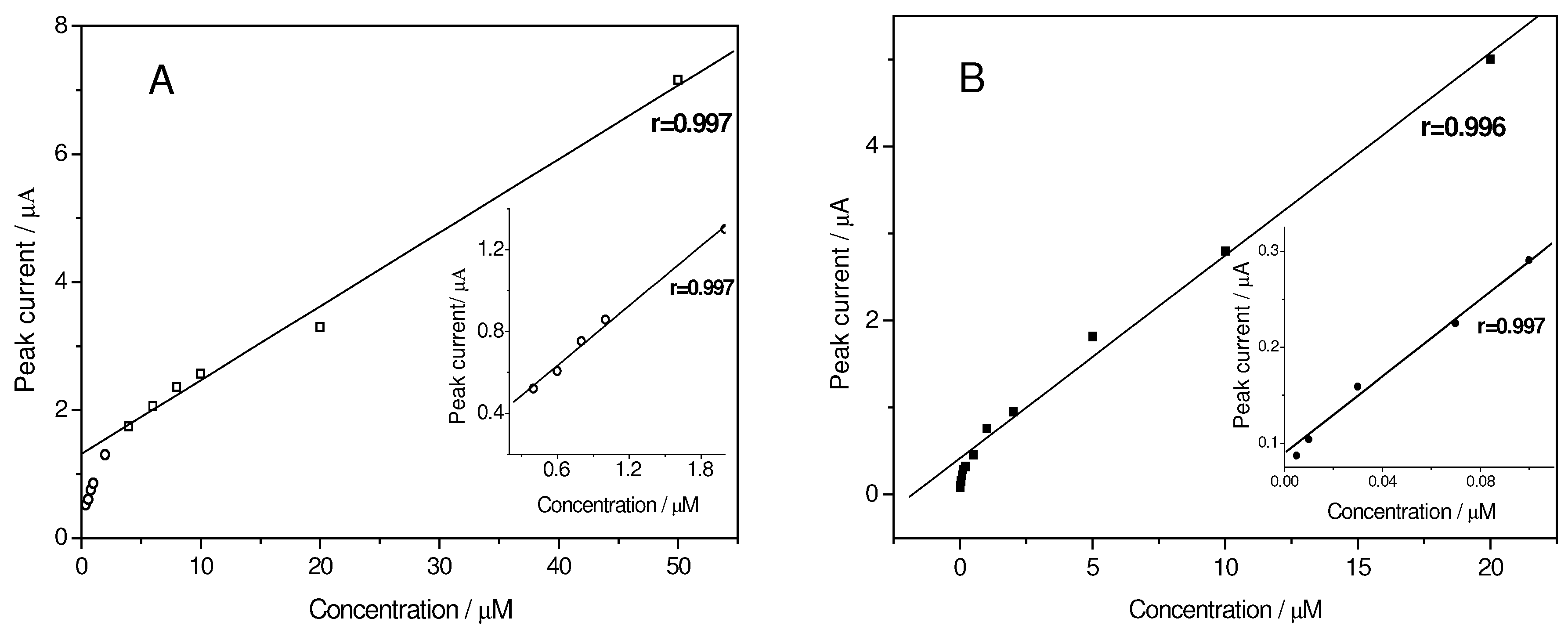
Linear calibration plot
Interference of coexistent matrix
| AA concentration / µM | 0.0 | 1.0 | 5.0 | 10 |
| ip (prochlorperazine) / µA | 2.024 | 2.259 | 3.702 | 6.384 |
| ip (ethopropazine) / µ A | 1.8576 | 2.805 | 6.939 | 9.919 |
Conclusions
Acknowledgement
References
- Belal, F.; El-Ashry, S. M.; Shehata, I, M.; El-Sherbeny, A.; El-Sherbeny, D. T. Differential pulse polarographic determination of some N-substituted phenothiazine derivatives in dosage forms and urine through treatment with nitrous acid. Microchem. Acta 2000, 135, 147–154. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, L.; Kokot, S. Voltammetric determination of chlorpromazine hydrochloride with the use of multivariate calibration. Anal. Chim. Acta 2001, 439, 159–168. [Google Scholar] [CrossRef]
- Tarasiewicz, M.; Kwymicka, L. Extractive spectrophotometric determination of some phenothiazines with picric and flavianic acids. Anal. Lett. 1996, 31, 929–936. [Google Scholar] [CrossRef]
- Basabaiah, K.; Krishmurthy, G. Spectrophotometric assay of some antipsychotropic and anticholinergic phenothiazine drugs using ammonium molybdate. Anal. Lett. 1998, 31, 1037–1046. [Google Scholar] [CrossRef]
- Revanasiddappa, M.; Ramappa, P. G. Spectrophotometric determination of some phenothiazine drugs. Talanta 1996, 43, 1291–1296. [Google Scholar] [CrossRef]
- Lopezpaz, J. L.; Townshend, A. Flow injection chemiluminescence determination of imipramine and chlorpromazine. Anal. Commun. 1996, 33, 31–33. [Google Scholar]
- Stevenson, D.; Reid, E. Determination of chlorpromazine and its sulfoxide and 7-hydroxy metabolites by ion-pair high pressure liquid chromatography. Anal. Lett. 1981, 14, 1785–1805. [Google Scholar] [CrossRef]
- Korpinsk, J.; Starczewska, B.; Puzahowska-Tarasiewicz, H. Analytical properties of 2- and 10-disubstituted phenothiazine derivatives. Anal. Sci. 1996, 12, 161–170. [Google Scholar] [CrossRef]
- Golabi, S. M.; Showkati-Shishvan, M. Potentiometric titration of phenothiazine compounds in chloroform and its use in pharmaceutical analysis. Talanta 1991, 38, 1253–1256. [Google Scholar] [CrossRef]
- Pournaghi-Azar, M. H.; Farhadi, K. Potentiometric study of reaction between tetrabutylammonium periodate and phenothiazines in chloroform: application to the analysis of phenothiazine derivatives. Talanta 1997, 44, 1773–1781. [Google Scholar] [CrossRef]
- Bishop, E.; Hussein, W. Electroanalytical studies of phenothiazine neuroleptics at gold and platinum electrodes. Analyst 1984, 109, 229–234. [Google Scholar] [CrossRef]
- Wang, J.; Rivas, G.; Cai, X.; Shiraishi, H.; Farias, P. A. M.; Dontha, N.; Luo, D. Accumulation and trace measurements of phenothiazine drugs at DNA modified electrodes. Anal. Chim. Acta 1996, 332, 139–144. [Google Scholar] [CrossRef]
- Gooding, J. J.; Mearns, F.; Yang, W.; Liu, J. Self-assembled monolayers into the 21st century: recent advances and applications. Electroanalysis 2003, 15, 81–96. [Google Scholar] [CrossRef]
- Zeng, B.; Ding, X.; Zhao, F. Accumulative and stripping behavior of silver ion at 1,4-dithiothereitol SAM modified gold electrode. Talanta 2003, 59, 510–515. [Google Scholar] [CrossRef]
- Jung, S. K.; Wilson, G. S. Polymeric mercaptosilane-modified platinum electrodes for elimination of interferants in glucose biosensors. Anal. Chem. 1996, 68, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Yang, Y.; Zhao, F. Voltammetric determination of epinephrine with a 3-mercaptopropionic acid self-assembled monolayer modified gold electrode. Electroanalysis 2003, 15, 1054–1059. [Google Scholar] [CrossRef]
- Duan, C.; Meyerhoff, M. E. Separation-free sandwich enzyme immunoassays using microporous gold electrode and self-assembled monolayer/immobilized capture antibodies. Anal. Chem. 1994, 66, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.; Angnes, L. On –line monitoring of hydrophobic compounds at self-assembled monolayer modified amperometric flow detectors. Anal. Chem. 1993, 65, 1893–1896. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yang, Y.; Peng, Y.; Zhao, F.; Zeng, B. Voltammetric Determination of Prochlorperazine and Ethopropazine Using a Gold Electrode Modified with Decanethiol SAM. Sensors 2003, 3, 524-533. https://doi.org/10.3390/s31100524
Yang Y, Peng Y, Zhao F, Zeng B. Voltammetric Determination of Prochlorperazine and Ethopropazine Using a Gold Electrode Modified with Decanethiol SAM. Sensors. 2003; 3(11):524-533. https://doi.org/10.3390/s31100524
Chicago/Turabian StyleYang, Yuxia, Yan Peng, Faqiong Zhao, and Baizhao Zeng. 2003. "Voltammetric Determination of Prochlorperazine and Ethopropazine Using a Gold Electrode Modified with Decanethiol SAM" Sensors 3, no. 11: 524-533. https://doi.org/10.3390/s31100524




