Advanced Home-Based Shoulder Rehabilitation: A Systematic Review of Remote Monitoring Devices and Their Therapeutic Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategy
2.3. Eligibility Criteria
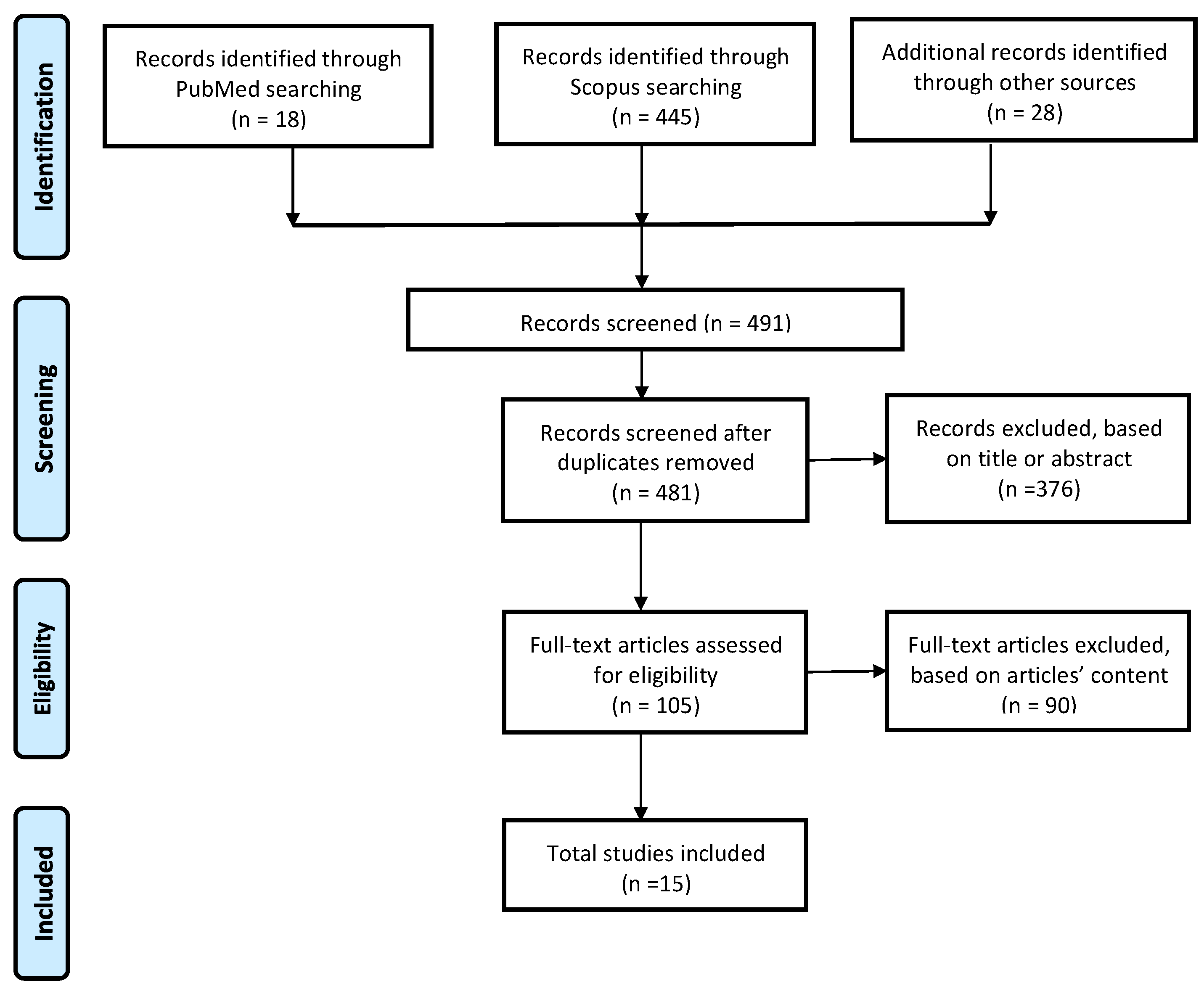
2.4. Study Selection Process
2.5. Data Items
2.6. Data Analysis
2.7. Quantitative Synthesis
2.8. Qualitative Synthesis
2.9. Integration of Findings
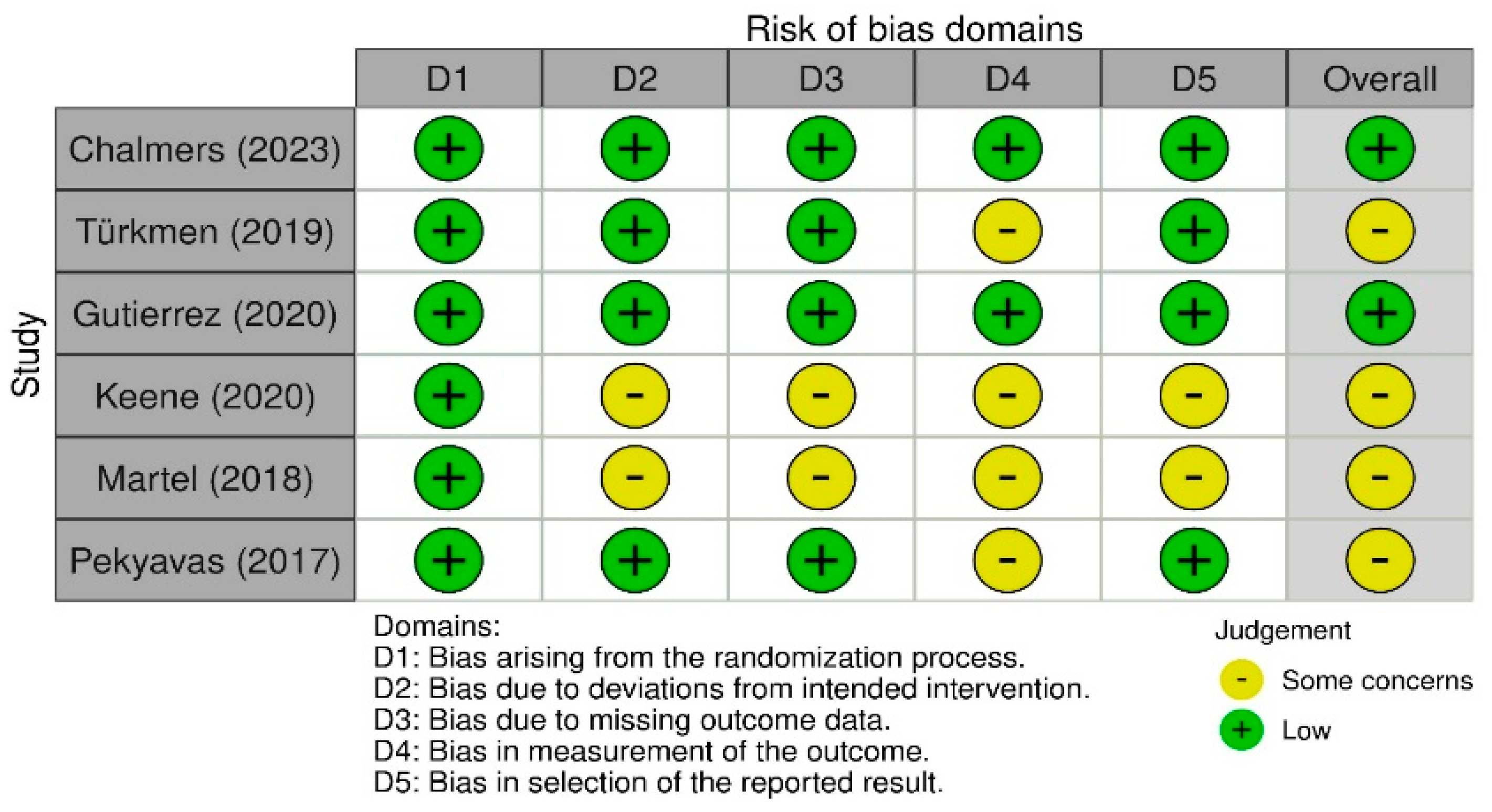
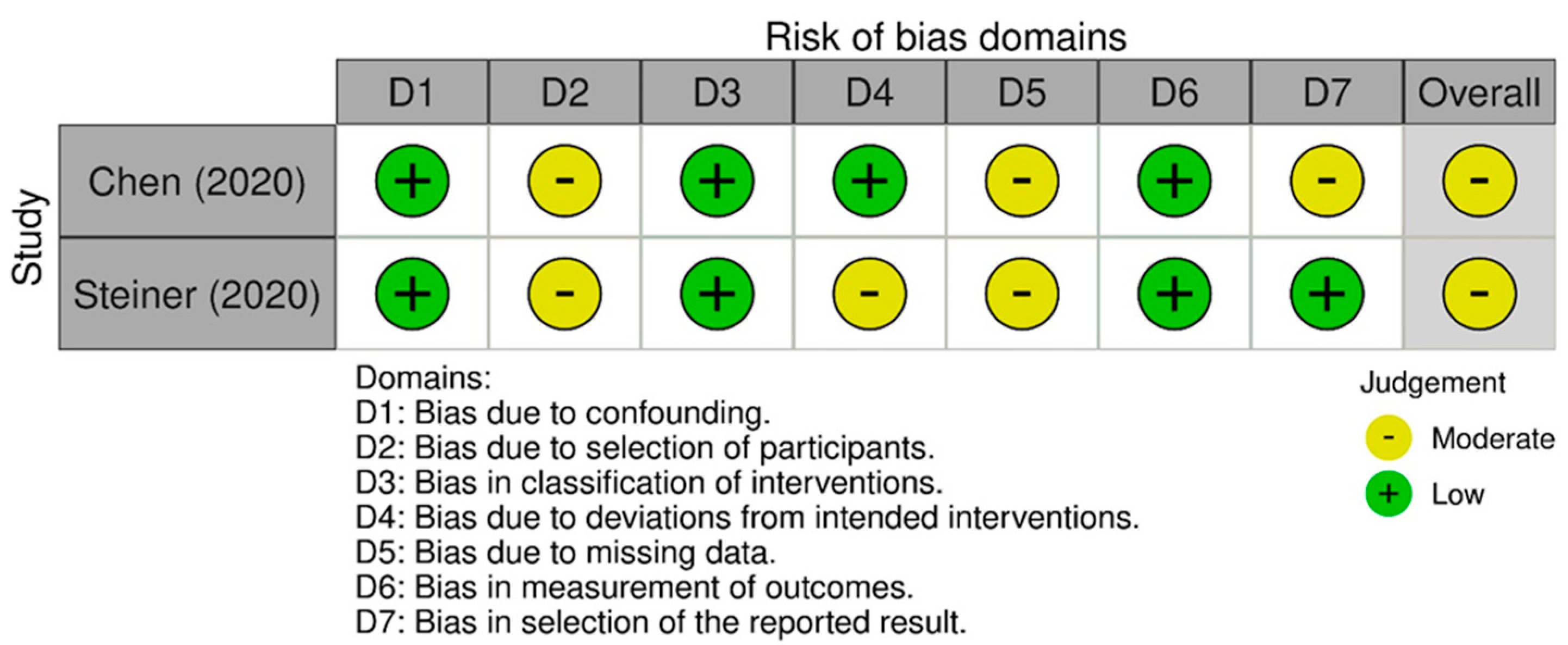
2.10. Risk of Biased Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Monitoring System
3.4. Artificial Intelligence
3.5. Exercises Protocol
3.6. Parameters Monitored during Exercises
3.7. Quality Assessment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdem, E.U.; Ünver, B. Effects of supervised home-based exercise therapy on disability and function in patients with shoulder pain. J. Exerc. Ther. Rehabil. 2018, 5, 143–149. [Google Scholar]
- Steiner, B.A.-O.; Elgert, L.A.-O.; Haux, R.A.-O.; Wolf, K.A.-O.X. AGT-Reha-WK study: Protocol for a non-inferiority trial comparing the efficacy and costs of home-based telerehabilitation for shoulder diseases with medical exercise therapy. BMJ Open 2020, 10, e036881. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Ciuffreda, M.; Locher, J.; Buchmann, S.; Maffulli, N.; Denaro, V. The effectiveness of conservative and surgical treatment for shoulder stiffness: A systematic review of current literature. Br. Med. Bull. 2018, 127, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Elgert, L.; Saalfeld, B.; Schwartze, J.; Borrmann, H.P.; Kobelt-Pönicke, A.; Figlewicz, A.; Kasprowski, D.; Thiel, M.; Kreikebohm, R.; et al. Health-Enabling Technologies for Telerehabilitation of the Shoulder: A Feasibility and User Acceptance Study. Methods Inf. Med. 2020, 59, e90–e99. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Lai, C.C.; Liu, K.C.; Hsieh, C.Y.; Chan, C.T. Wearable-based Frozen Shoulder Rehabilitation Exercise Recognition using Machine Learning Approaches. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023; pp. 1–5. [Google Scholar]
- Cunha, B.; Ferreira, R.; Sousa, A.S.P. Home-Based Rehabilitation of the Shoulder Using Auxiliary Systems and Artificial Intelligence: An Overview. Sensors 2023, 23, 7100. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.A.-O.; Berton, A.; Risi Ambrogioni, L.; Lo Presti, D.A.-O.X.; Carnevale, A.; Candela, V.; Stelitano, G.; Schena, E.A.-O.; Nazarian, A.; Denaro, V. Cost-Effectiveness of Supervised versus Unsupervised Rehabilitation for Rotator-Cuff Repair: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 2852. [Google Scholar] [CrossRef] [PubMed]
- Gava, V.A.-O.X.; Ribeiro, L.P.; Barreto, R.P.G.; Camargo, P.R. Effectiveness of physical therapy given by telerehabilitation on pain and disability of individuals with shoulder pain: A systematic review. Clin. Rehabil. 2022, 36, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Panahi, A.; Andrews, D.; Nelson, A. An FPGA-Based Upper-Limb Rehabilitation Device for Gesture Recognition and Motion Evaluation Using Multi-Task Recurrent Neural Networks. In Proceedings of the 2020 International Conference on Field-Programmable Technology (ICFPT), Maui, HI, USA, 9–11 December 2020; pp. 296–297. [Google Scholar]
- Barzegar Khanghah, A.; Fernie, G.; Roshan Fekr, A. Design and Validation of Vision-Based Exercise Biofeedback for Tele-Rehabilitation. Sensors 2023, 23, 1206. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, I.; Lorussi, F.; Bellizzi, M.; Carbonaro, N.; Casarosa, S.; Trotta, L.; Tognetti, A. Daily Life Self-management and Self-treatment of Musculoskeletal Disorders Through SHOULPHY. In Wireless Mobile Communication and Healthcare: 2018//2018; Springer International Publishing: Cham, Switzerland, 2018; pp. 233–241. [Google Scholar]
- Yeh, S.-C.; Lee, S.; Fank, Y.; Gong, Y.; Lin, J.; Hsieh, Y. A Cloud-Based Tele-Rehabilitation System for Frozen Shoulder. Adv. Mater. Res. 2013, 717, 766–771. [Google Scholar] [CrossRef]
- Hall, K.; Grinstead, A.; Lewis, J.S.; Mercer, C.; Moore, A.; Ridehalgh, C. Rotator cuff related shoulder pain. Describing home exercise adherence and the use of behavior change interventions to promote home exercise adherence: A systematic review of randomized controlled trials. Phys. Ther. Rev. 2021, 26, 299–322. [Google Scholar] [CrossRef]
- Marley, W.D.; Barratt, A.; Pigott, T.; Granat, M.; Wilson, J.D.; Roy, B. A multicenter randomized controlled trial comparing gamification with remote monitoring against standard rehabilitation for patients after arthroscopic shoulder surgery. J. Shoulder Elb. Surg. 2022, 31, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, S.; Liu, Y.; Zhang, S.; Dai, L. Design and Optimization of an Adaptive Knee Joint Orthosis for Biomimetic Motion Rehabilitation Assistance. Biomimetics 2024, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Deen, M.J. Wearable IMU-Based System for Real-Time Monitoring of Lower-Limb Joints. IEEE Sens. J. 2021, 21, 8267–8275. [Google Scholar] [CrossRef]
- Albani, G.; Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Parisi, F.; Ferrari, G.; Cau, N.; Cimolin, V.; Azzaro, C.; et al. An Integrated Multi-Sensor Approach for the Remote Monitoring of Parkinson’s Disease. Sensors 2019, 19, 4764. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yan, J.; Liu, Y.; Ye, M. Noninvasive Estimation of Joint Moments with Inertial Sensor System for Analysis of STS Rehabilitation Training. J. Healthc. Eng. 2018, 2018, 6570617. [Google Scholar] [CrossRef] [PubMed]
- Hafer, J.F.; Vitali, R.; Gurchiek, R.; Curtze, C.; Shull, P.; Cain, S.M. Challenges and advances in the use of wearable sensors for lower extremity biomechanics. J. Biomech. 2023, 157, 111714. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, M.; Inui, A.; Nishimoto, H.; Mifune, Y.; Yoshikawa, T.; Shinohara, I.; Furukawa, T.; Kato, T.; Tanaka, S.; Kuroda, R. Measurement of Shoulder Abduction Angle with Posture Estimation Artificial Intelligence Model. Sensors 2023, 23, 6445. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.; Burns, D.; Whyne, C. Evaluation of at-home physiotherapy. Bone Jt. Res. 2023, 12, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Abdullah, R.; Jamal, L. STPT: Spatio-Temporal Polychromatic Trajectory Based Elderly Exercise Evaluation System. IEEE Access 2023, 11, 37958–37975. [Google Scholar] [CrossRef]
- Ino, T.; Samukawa, M.; Ishida, T.; Wada, N.; Koshino, Y.; Kasahara, S.; Tohyama, H. Validity of AI-Based Gait Analysis for Simultaneous Measurement of Bilateral Lower Limb Kinematics Using a Single Video Camera. Sensors 2023, 23, 9799. [Google Scholar] [CrossRef]
- Zhang, X.; Rong, X.; Luo, H. Optimizing lower limb rehabilitation: The intersection of machine learning and rehabilitative robotics. Front. Rehabil. Sci. 2024, 5, 1246773. [Google Scholar] [CrossRef] [PubMed]
- Cóias, A.R.; Lee, M.H.; Bernardino, A. A low-cost virtual coach for 2D video-based compensation assessment of upper extremity rehabilitation exercises. J. NeuroEngineering Rehabil. 2022, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Scronce, G.; Finetto, C.; Coupland, K.; Zhong, M.; Lambert, M.E.; Baker, A.; Luo, F.; Seo, N.J. Application of Deep Learning Algorithm to Monitor Upper Extremity Task Practice. Sensors 2023, 23, 6110. [Google Scholar] [CrossRef] [PubMed]
- Arntz, A.A.-O.; Weber, F.A.-O.; Handgraaf, M.A.-O.; Lällä, K.A.-O.; Korniloff, K.A.-O.; Murtonen, K.A.-O.; Chichaeva, J.A.-O.; Kidritsch, A.A.-O.; Heller, M.A.-O.; Sakellari, E.A.-O.X.; et al. Technologies in Home-Based Digital Rehabilitation: Scoping Review. JMIR Rehabil. Assist. Technol. 2023, 10, e43615. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, S.; Cinquini, M.; Gianola, S.; Gonzalez-Lorenzo, M.; Banzi, R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J. Clin. Epidemiol. 2020, 126, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Igelström, E.; Campbell, M.; Craig, P.; Katikireddi, S.V. Cochrane’s risk of bias tool for non-randomized studies (ROBINS-I) is frequently misapplied: A methodological systematic review. J. Clin. Epidemiol. 2021, 140, 22–32. [Google Scholar] [CrossRef]
- Bavan, L.; Surmacz, K.; Beard, D.; Mellon, S.; Rees, J. Adherence monitoring of rehabilitation exercise with inertial sensors: A clinical validation study. Gait Posture 2019, 70, 211–217. [Google Scholar] [CrossRef]
- Burns, D.; Boyer, P.; Razmjou, H.; Richards, R.; Whyne, C. Adherence Patterns and Dose Response of Physiotherapy for Rotator Cuff Pathology: Longitudinal Cohort Study. JMIR Rehabil. Assist. Technol. 2021, 8, e21374. [Google Scholar] [CrossRef]
- Burns, D.; Razmjou, H.; Shaw, J.; Richards, R.; McLachlin, S.; Hardisty, M.; Henry, P.; Whyne, C. Adherence Tracking With Smart Watches for Shoulder Physiotherapy in Rotator Cuff Pathology: Protocol for a Longitudinal Cohort Study. JMIR Res. Protoc. 2020, 9, e17841. [Google Scholar] [CrossRef]
- Chalmers, P.N.; Tashjian, R.Z.; Keener, J.D.; Sefko, J.A.; Da Silva, A.; Morrissey, C.; Presson, A.P.; Zhang, C.; Chamberlain, A.M. Active physical therapy does not improve outcomes after reverse total shoulder arthroplasty: A multi-center, randomized clinical trial. J. Shoulder Elb. Surg. 2023, 32, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, E.; Analay Akbaba, Y.; Altun, S. Effectiveness of video-based rehabilitation program on pain, functionality, and quality of life in the treatment of rotator cuff tears: A randomized controlled trial. J. Hand Ther. 2020, 33, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Espinoza, H.; Quintanilla, F.; Pinto, S.; Zavala-Gonzalez, J.; Gana-Hervias, G.; Cavero-Redondo, I.; Alvarez-Bueno, C. Effectiveness of supervised early exercise program in patients with arthroscopic rotator cuff repair: Study protocol clinical trial. Medicine 2020, 99, e18846. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.J.; Soutakbar, H.; Hopewell, S.; Heine, P.; Jaggi, A.; Littlewood, C.; Hansen, Z.; Barker, K.; Hamilton, W.; Carr, A.J.; et al. Development and implementation of the physiotherapy-led exercise interventions for the treatment of rotator cuff disorders for the ‘Getting it Right: Addressing Shoulder Pain’ (GRASP) trial. Physiotherapy 2020, 107, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Lin, C.-Y.; Tsai, M., Jr.; Chuang, T.-Y.; Lee, O.K.-S. Wearable Motion Sensor Device to Facilitate Rehabilitation in Patients With Shoulder Adhesive Capsulitis: Pilot Study to Assess Feasibility. J. Med. Internet Res. 2020, 22, e17032. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chiang, S.Y.; Lee, K.; Kan, Y.C. An activity recognition model using inertial sensor nodes in a wireless sensor network for frozen shoulder rehabilitation exercises. Sensors 2015, 15, 2181–2204. [Google Scholar] [CrossRef] [PubMed]
- Pekyavas, N.; Ergun, N. Comparison of virtual reality exergaming and home exercise programs in patients with subacromial impingement syndrome and scapular dyskinesis: Short term effect. Acta Orthop. Et Traumatol. Turc. 2017, 51, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Antón, D.; Goni, A.; Illarramendi, A. Exercise Recognition for Kinect-based Telerehabilitation. Methods Inf. Med. 2015, 54, 145–155. [Google Scholar] [CrossRef]
- Hua, A.; Chaudhari, P.; Johnson, N.; Quinton, J.; Schatz, B.; Buchner, D.; Hernandez, M.E. Evaluation of Machine Learning Models for Classifying Upper Extremity Exercises Using Inertial Measurement Unit-Based Kinematic Data. IEEE J. Biomed. Health Inform. 2020, 24, 2452–2460. [Google Scholar] [CrossRef]
- Martel, D.; Lauzé, M.; Agnoux, A.; Fruteau de Laclos, L.; Daoust, R.; Émond, M.; Sirois, M.-J.; Aubertin-Leheudre, M. Comparing the effects of a home-based exercise program using a gerontechnology to a community-based group exercise program on functional capacities in older adults after a minor injury. Exp. Gerontol. 2018, 108, 41–47. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.; Sing, D.C.; Beeram, I.; Puvanesarajah, V.; Tornetta, P.; Fritz, J.; Yi, P.H. Detecting upper extremity native joint dislocations using deep learning: A multicenter study. Clin. Imaging 2022, 92, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Harini, N.; Ramji, B.; Sriram, S.; Sowmya, V.; Soman, K.P. Chapter five-Musculoskeletal radiographs classification using deep learning. In Deep Learning for Data Analytics; Das, H., Pradhan, C., Dey, N., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 79–98. [Google Scholar] [CrossRef]
- Kunze, K.N.; Jang, S.J.; Li, T.Y.; Pareek, A.; Finocchiaro, A.; Fu, M.C.; Taylor, S.A.; Dines, J.S.; Dines, D.M.; Warren, R.F.; et al. Artificial intelligence for automated identification of total shoulder arthroplasty implants. J. Shoulder Elb. Surg. 2023, 32, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Marigi, E.M.; Sanchez-Sotelo, J. Research on artificial intelligence in shoulder and elbow surgery is increasing. JSES Int. 2023, 7, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.; Owais, M.; Park, C.; Mahmood, T.; Haider, A.; Park, K.R. Artificial Intelligence-Based Recognition of Different Types of Shoulder Implants in X-ray Scans Based on Dense Residual Ensemble-Network for Personalized Medicine. J. Pers. Med. 2021, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liang, X.; Guo, D.; Xie, D. Application of Artificial Intelligence in Shoulder Pathology. Preprints 2024. [Google Scholar] [CrossRef]
- McLendon, P.B.; Christmas, K.N.; Simon, P.; Plummer, O.R.; Hunt, A.; Ahmed, A.S.; Mighell, M.A.; Frankle, M.A. Machine Learning Can Predict Level of Improvement in Shoulder Arthroplasty. JBJS Open Access 2021, 6, e20. [Google Scholar] [CrossRef]
- Siddiqui, H.U.; Saleem, A.A.; Raza, M.A.; Villar, S.G.; Lopez, L.A.; Diez, I.D.; Rustam, F.; Dudley, S. Empowering Lower Limb Disorder Identification through PoseNet and Artificial Intelligence. Diagnostics 2023, 13, 2881. [Google Scholar] [CrossRef]

| Groups | Search Parameters |
|---|---|
| Medical application | “rehabilitation” OR “physiotherapy” OR “physiotherapy exercise *” OR “physical therapy” OR “rehabilitation exercise *” OR “telerehabilitation” OR “tele-rehabilitation” OR “tele rehabilitation” OR “tele-monitoring” OR “remote monitoring” OR “patient monitoring” OR “home-based” |
| Technology and kinematic/physiological data | “smartwatch” OR “motion tracking system *” OR “motion capture” OR “mocap” OR “contactless” OR “markerless” OR “camera-based” OR “camera *” OR “depth camera” OR “RGB-D” OR “RGBD” OR “RGB” OR “video” OR “video-based” OR “Kinect” OR “marker-less” OR “markerless” OR “wearable” OR “wearable sensor” OR “inertial sensor” OR “inertial measurement unit” OR “IMU” OR “MIMU” OR “accelerometer” OR “acceleration” OR “gyroscope” OR “IMU-based” OR “electromyography” OR “EMG” OR “surface electromyography” OR “surface electromyogram” OR “sEMG” OR “body-worn sensor” OR “skeletonization” OR “stress” OR “heart rate variability *” OR “hrv” |
| Artificial intelligence | “classification” OR “recognition” OR “pattern recognition” OR “unsupervised” OR “supervised” OR “deep learning” OR “spectrogram” OR “neural network” OR “artificial neural network” OR “ANN” OR “machine learning” OR “ML” OR “AI” OR “artificial intelligence” OR “Convolutional Neural Network” OR “CNN” OR “transformer” OR “classifier” OR “YOLO” OR “decision tree” OR “DT” OR “random forest” OR “RF” OR “k-nearest neighbors” OR “kNN” OR “k-NN” OR “Naive Bayes” OR “NB” OR “support vector machine” OR “SVM” OR “support vector machine classifier” OR “SVC” |
| Body segment | “shoulder” OR “rotator-cuff” OR “rotator-cuff” OR “shoulder pain” OR “shoulder injur *” OR “shoulder surgery” OR “rotator cuff injury” OR “frozen shoulder” OR “shoulder impingement” OR “upper extremity” OR “adhesive capsulitis” OR “dislocation” OR “Tendinitis” OR “Bursitis” OR “Fractures” OR “Arthritis” OR “Arthrosis” |
| First Author, Year | Study Design | Level of Evidence | Sample Size | Mean Age (SD) | Female Patients % | Shoulder Disease |
|---|---|---|---|---|---|---|
| Antón, 2015 [41] | Case series | IV | 15 | 66 | - | - |
| Bavan, 2019 [31] | Case series | IV | 20 | 58.7 | 60% | RC |
| Boyer, 2023 [21] | Case series | IV | 42 | 45 (13) | 64.30% | RC |
| Burns, 2020 [33] | Cohort study (case series) | IV | 140 | - | - | RC |
| Burns, 2021 [32] | Prospective cohort study (case series) | II | 42 | 45 (13) | 64% | RC |
| Chalmers, 2023 [34] | Randomized clinical trial | I | HEP group: 46 PT group: 43 | HEP group: 71.5 (7.9) PT group: 69.1 (7.5) | HEP group: 63% PT group: 54% | Osteoarthritis, inflammatory conditions, or RCTA |
| Türkmen, 2019 [35] | Randomized Controlled Trial | I | 30 | 50.60 (8.54) | 33% | RC |
| Chen, 2020 [38] | Prospective Control trial (case control) | II | 24 | MSR group: 53 (6.2) HEP group: 56.1 (13.3) | MSR group: 42.9% HEP group: 28.6% | AC |
| Gutiérrez-Espinoza, 2020 [36] | Randomized Control Trial | I | 118 | - | - | RC |
| Hua, 2020 [42] | Case series | IV | 50 | 21.9 (4.0) | 40% | - |
| Keene, 2020 [37] | Randomized Control Trial | I | 708 | - | - | RC |
| Lin, 2015 [39] | Case series | IV | 13 | - | - | AC |
| Martel, 2018 [43] | Randomized Control Trial | I | 48 | HEP group: 74.9 (7.1) YMCA group: 72.9 (6.7) Control Group: 72.7 (6.5) | HEP group: 75% YMCA group: 63%Control Group: 75% | - |
| Pekyavas, 2017 [40] | Randomized Control Trial-Therapeutic study | I | 30 | HEP group: 40.6 (11.7) Wii group: 40.33 (13.2) | HEP group: 86.7% Wii group: 93.3% | SAIS (type 2) |
| Steiner, 2020 [2] | Prospective comparative study (case control) | II | 84 | - | - | - |
| First Author, Year | Input Variable | Output Variable |
|---|---|---|
| Antón, 2015 [41] | Age; shoulder disease; rehabilitation duration | Movement recognition performance |
| Bavan, 2019 [31] | Sex; age; arm dominance | Movement recognition performance |
| Boyer, 2023 [21] | Sex; age; rotator cuff tear thickness | Movement recognition performance |
| Burns, 2020 [33] | Age; BMI; arm dominance; symptoms duration; mechanism of injury; rotator cuff tear thickness; operative procedures; comorbidities; smoking; alcohol; opioid and cannabinoid intake; physical activity level; education; marital status; job demands; socioeconomic status; social support; patient self-efficacy | Adherence |
| Burns, 2021 [32] | Sex; age; BMI; Baseline pain level; physical activity level; job demands; education; socioeconomical status; patient self-efficacy | Adherence; Dose-response between physiotherapy activity and recovery; Movement recognition performance |
| Chalmers, 2023 [34] | Sex; age; affected arm; hand dominance; BMI; work status; comorbidities; ethnicity; smoking | ROM; patient-reported outcomes. |
| Türkmen, 2019 [35] | Sex; age; affected arm | Movement recognition performance |
| Chen, 2020 [38] | Sex; age; education | Adherence; Movement recognition performance |
| Gutiérrez-Espinoza, 2020 [36] | Age; BMI; dominant shoulder; duration of symptoms; socioeconomical status; occupation; education; previous treatment | Functional improvement; pain relief; ROM |
| Hua, 2020 [42] | Sex; age; BMI; History of upper extremity injury; physical abilities; health condition | Movement recognition performance |
| Keene, 2020 [37] | Age; rotator cuff disorder | Functional improvement; pain relief; adherence |
| Lin, 2015 [39] | Shoulder disease | Movement recognition performance |
| Martel, 2018 [43] | Sex; age; BMI | Functional capacities, cognitive function, health status, adherence, and acceptability |
| Pekyavas, 2017 [40] | Sex; age; diagnosis of type 2 SAIS and scapular dyskinesis | Efficacy of home exercise program and virtual reality exergaming; shoulder pain |
| Steiner, 2020 [2] | Age; shoulder complaints; ability to perform exercises without health risk; BMI | Efficacy |
| First Author, Year | AI Model | Metrics | Cross-Validation Technique |
|---|---|---|---|
| Antón, 2015 [41] | DTW | CM; Accuracy | |
| Bavan, 2019 [31] | DT SVM k-NN RF | CM; Accuracy; Sensitivity; Precision; Specificity | 10 folds CV; LOSOV |
| Boyer, 2023 [21] | k-NN FCN RF | Accuracy; Sensitivity; Specificity; AUROC; F1 score | 5 folds CV |
| Burns, 2020 [33] | CRNN | Accuracy; Precision; Sensitivity; F1 score | - |
| Burns, 2021 [32] | FCN | Accuracy; Sensitivity; Specificity; AUROC; F1 score | - |
| Chalmers, 2023 [34] | - | - | - |
| Türkmen, 2019 [35] | - | - | - |
| Chen, 2020 [38] | - | - | - |
| Gutiérrez-Espinoza, 2020 [36] | - | - | - |
| Hua, 2020 [42] | RF (300 trees) LinearSVC k-NN MLP | CM; Accuracy; Precision; Sensitivity; F1 score; Speed; Support | - |
| Keene, 2020 [37] | - | - | - |
| Lin, 2015 [39] | BPNN | Accuracy | - |
| Martel, 2018 [43] | - | - | - |
| Pekyavas, 2017 [40] | - | - | - |
| Steiner, 2020 [2] | - | - | - |
| First Author, Year | Monitoring System (Type and Brand) | Number, Placement, and Wearability of Sensors | Task Executed | Recognition of Movement | |
|---|---|---|---|---|---|
| Shoulder Rehabilitation Exercise | Number, Repetitions, Protocol | ||||
| Antón, 2015 [41] | Camera (Microsoft Kinect system) | - | Shoulder abduction; Hands to mouth; Shoulder extension; Shoulder flexion; Hands to head; Shoulder rotation | N = 6 | ✓ |
| Bavan, 2019 [31] | M-IMU (MetaMotion R) | N = 1; Upper arm (above elbow); Arm sleeve. | Shoulder abduction; Shoulder flexion; Wall slide; Wall press; Shoulder rotation | N = 5; Rept = 10 | ✓ |
| Boyer, 2023 [21] | IMU | N = 1; Wrist; Smartwatches (Huawei Watch 2 smartwatches) | 8 motions: Flexion; Abduction; ER; IR; Row; Elbow extension; Pull-down; Press-up6 simple motions: Elevation; Rotation; Row; Elbow flexion; Pull-down; Press-up | N = 18 | ✓ |
| Burns, 2020 [33] | M-IMU | N = 1; Wrist; Smartwatches (Huawei Watch 2 smartwatches) | - | - | ✓ |
| Burns, 2021 [32] | M-IMU | N = 1; Wrist; Smartwatches (Huawei Watch 2 smartwatches) | 9 motion types: Flexion; ER; IR; Press-up; Pull-down; Row; Abduction; Elbow flexion; Extension | N = 19; P = Patients were asked to complete their assigned exercises each day that they were not attending in-person physiotherapy. | ✓ |
| Chalmers, 2023 [34] | Camera | - | Active abduction; Active forward elevation; Active Internal rotation in adduction; Active External rotation in adduction | N = 4 | - |
| Türkmen, 2019 [35] | Camera | - | Examples: Shoulder flexion with a stick; Scapular retraction; External rotation; Scapular adduction | P = Every day 3 sessions of 10 repetitions each session | - |
| Chen, 2020 [38] | IMU (BoostFix, COMPAL Electronics Inc). | N = 3; Sternum, upper arm, and dorsal wrist. Elastic straps | Shoulder Pendulum Exercise; Forward wall walking stretch; Lateral wall walking stretch; Cane stretch for shoulder flexion; Cane stretch for shoulder abduction; Cane stretch for shoulder external rotation; Cane stretch for shoulder internal rotation; Cane stretch for shoulder extension. | N = 8 P = Daily, 10 times each exercise, hold for 10 sec each exercise | - |
| Gutiérrez-Espinoza, 2020 [36] | EMG | - | Isometric Scapular Depression; Isometric Scapular orientation; External Rotation; Passive Flexion | - | - |
| Hua, 2020 [42] | M-IMU (Adafruit BNO055) | N = 4; Torso, upper arm, forearm, and hand; Elastic straps | Standing row; External rotation with arm abducted 90°; External rotation; Bicep curl; Forearm pronation/supination; Wrist curl; Lateral arm raise; Front arm raise; Horizontal Abduction | N = 9. Rept = 10 | ✓ |
| Keene, 2020 [37] | - | - | External rotation; Flexion; Abduction of the Shoulder | N = 22 | - |
| Lin, 2015 [39] | IMU | N = 2; Upper arm, and wrist; Elastic straps | Scapula exercise; Codman’s pendulum exercise; Finger wall-climbing exercise; Back-shoulder circling exercise; Towel exercise; Spiral rotation exercise in four steps | N = 6 Rept = 60 s | ✓ |
| Martel, 2018 [43] | Camera (Microsoft Kinect system) | - | Butt kicks, high knees, lateral launches, side steps;squats; leg extension; lateral shifting; balance; shoulder abduction/adduction; horizontal flexion and extension | P = Each exercise session lasted 55 min and included different exercises | ✓ |
| Pekyavas, 2017 [40] | Nintendo Wii | - | Posterior, anterior, and inferior capsule stretching; pectoral muscle stretching; serratus anterior muscle strengthening; bilateral shoulder elevation, and scapular mobility exercises. Bilateral shoulder elevation, boxing, bowling, and tennis games. | P = exercise program for 6 weeks, 2 days a week, and 45 min per day | ✓ |
| Steiner, 2020 [2] | Camera (Microsoft Kinect system) | - | Examples: Shoulder abduction/adduction; Shoulder flexion/extension; Shoulder external/internal rotation; Shoulder external rotation/internal rotation at 90° abduction. | N = 10 Rept = 5 days a week, 30 min per exercise, 6 months of training in total | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassi, M.; Villa Corta, M.; Pisani, M.G.; Nicodemi, G.; Schena, E.; Pecchia, L.; Longo, U.G. Advanced Home-Based Shoulder Rehabilitation: A Systematic Review of Remote Monitoring Devices and Their Therapeutic Efficacy. Sensors 2024, 24, 2936. https://doi.org/10.3390/s24092936
Sassi M, Villa Corta M, Pisani MG, Nicodemi G, Schena E, Pecchia L, Longo UG. Advanced Home-Based Shoulder Rehabilitation: A Systematic Review of Remote Monitoring Devices and Their Therapeutic Efficacy. Sensors. 2024; 24(9):2936. https://doi.org/10.3390/s24092936
Chicago/Turabian StyleSassi, Martina, Mariajose Villa Corta, Matteo Giuseppe Pisani, Guido Nicodemi, Emiliano Schena, Leandro Pecchia, and Umile Giuseppe Longo. 2024. "Advanced Home-Based Shoulder Rehabilitation: A Systematic Review of Remote Monitoring Devices and Their Therapeutic Efficacy" Sensors 24, no. 9: 2936. https://doi.org/10.3390/s24092936








