A Portable Readout System for Biomarker Detection with Aptamer-Modified CMOS ISFET Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of ISFETs with CMOS Readout Circuits
2.3. Characterization of ISFETs with Semiconductor Analyzer
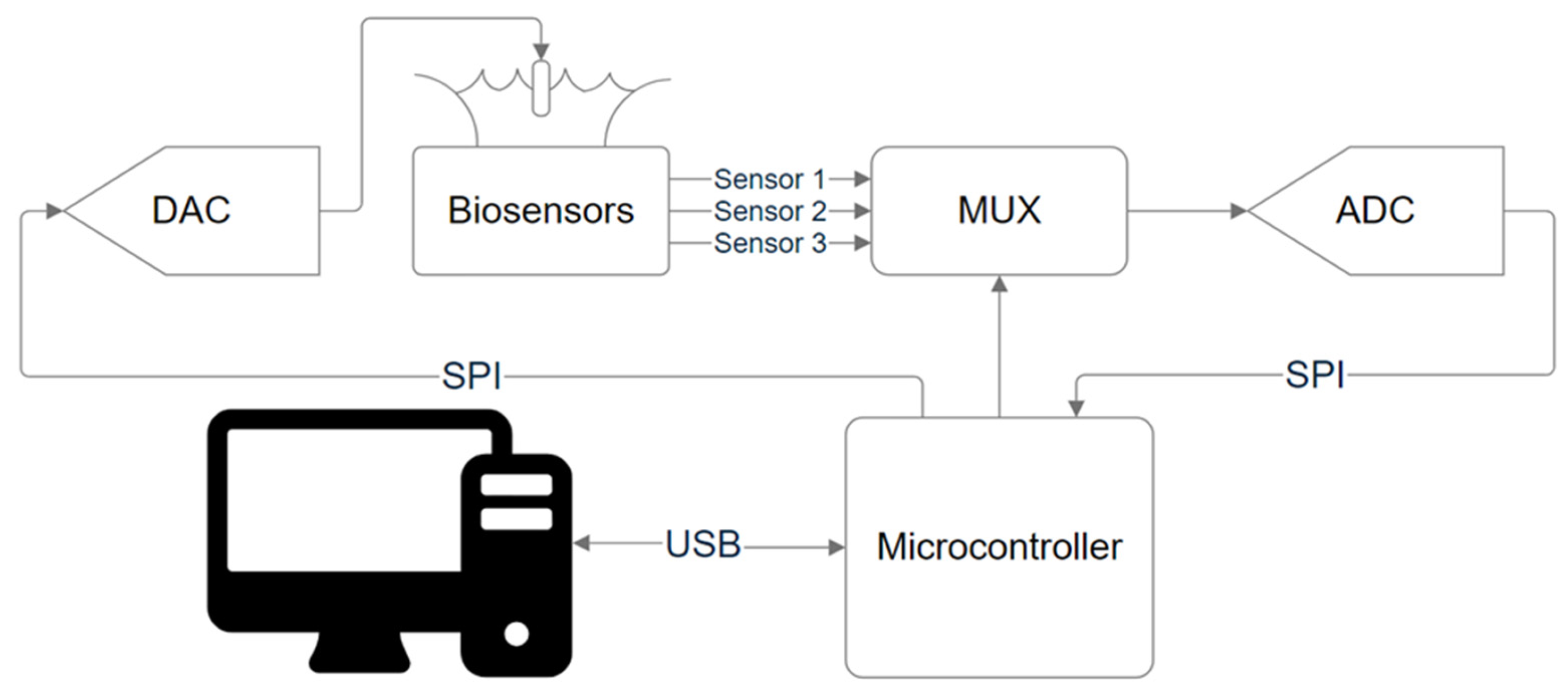
2.4. Assembly of External Readout Circuit
2.5. Software Development
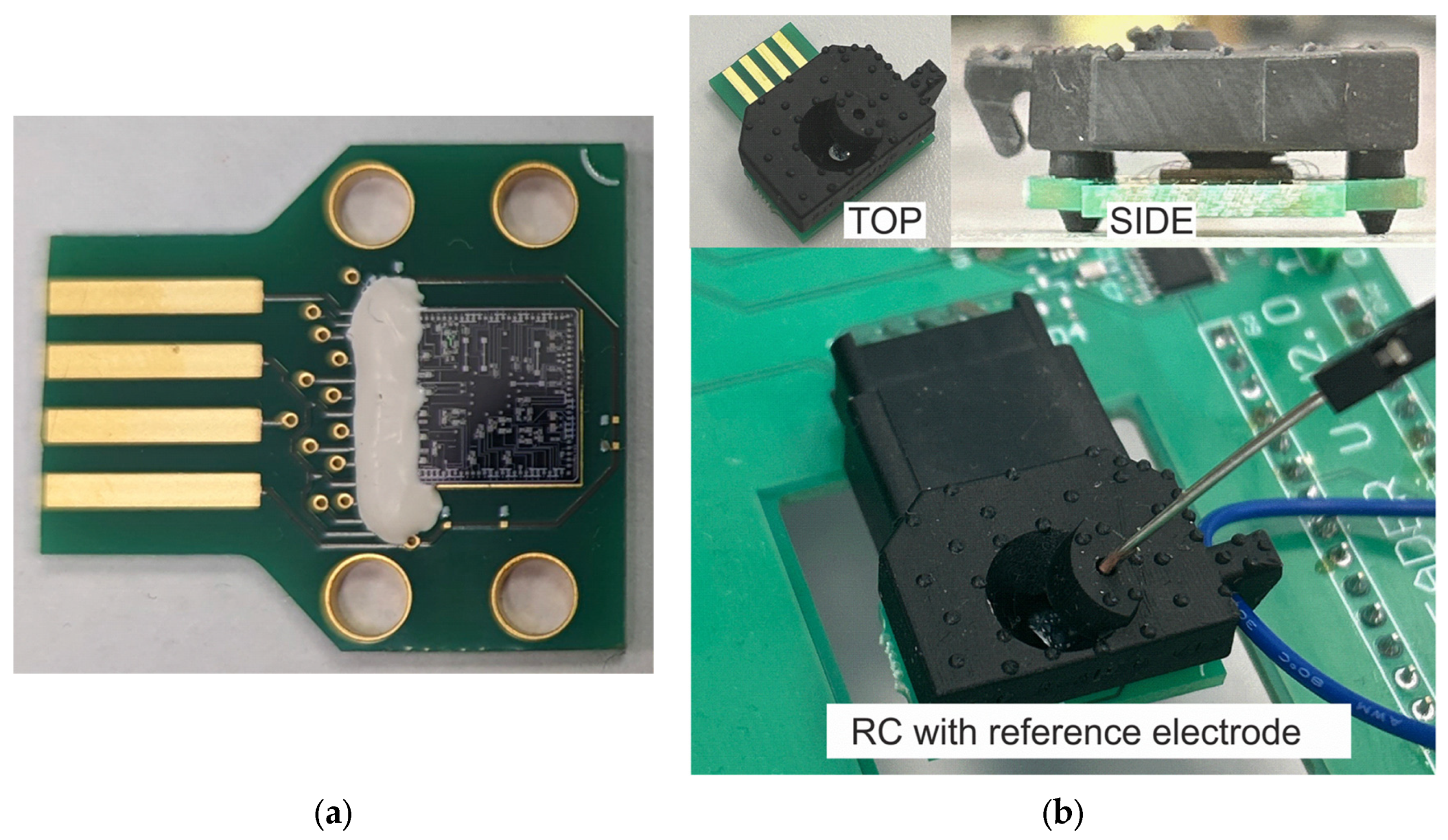
2.6. Formation of Reaction Chamber
2.7. Measurements on the Portable Device
3. Results and Discussion
3.1. The ISFET with CMOS Readout Circuit
3.2. External Readout Circuit
3.3. Software
3.4. Chip Assembly and Reaction Chamber Formation
3.5. Device Testing
3.5.1. Characterization of ISFETs with Readout Circuits on Portable Device
3.5.2. Sensor Modification with Bioreceptor
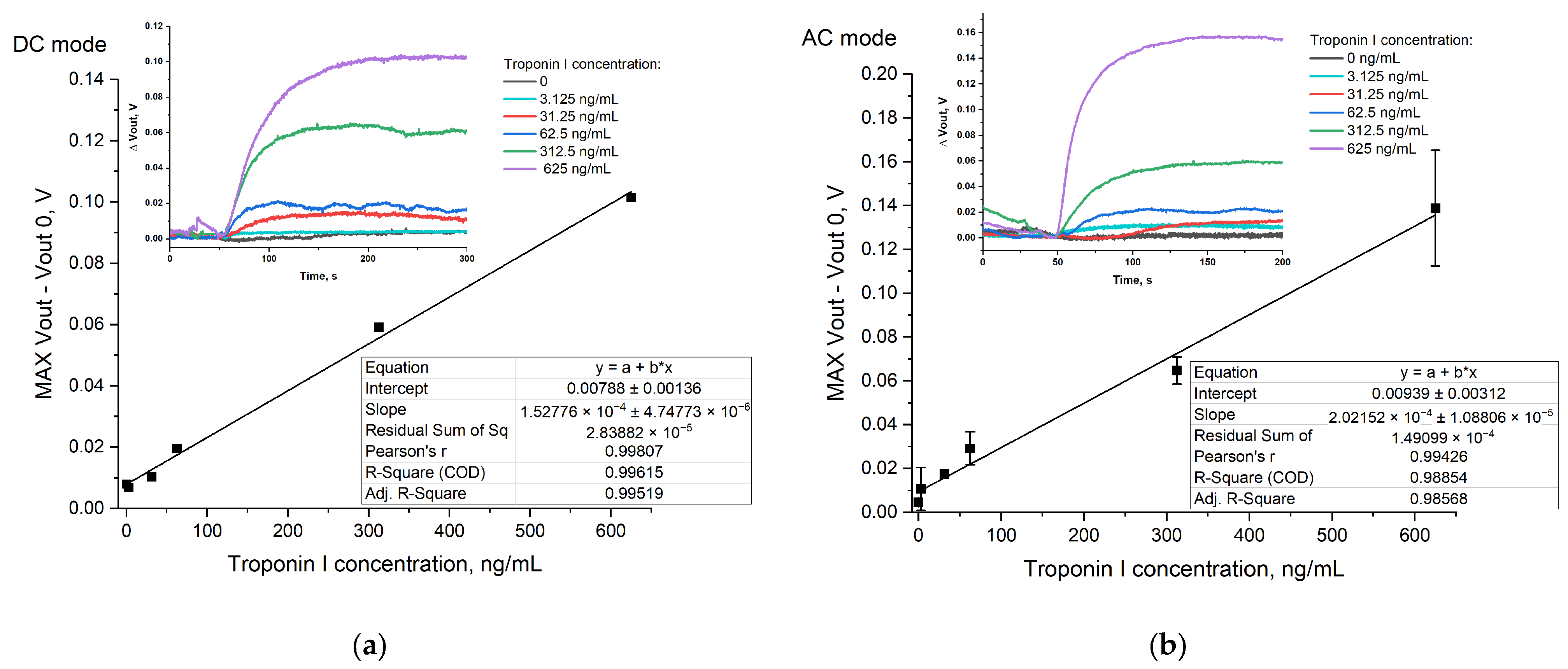
3.5.3. Biosensor Demonstration
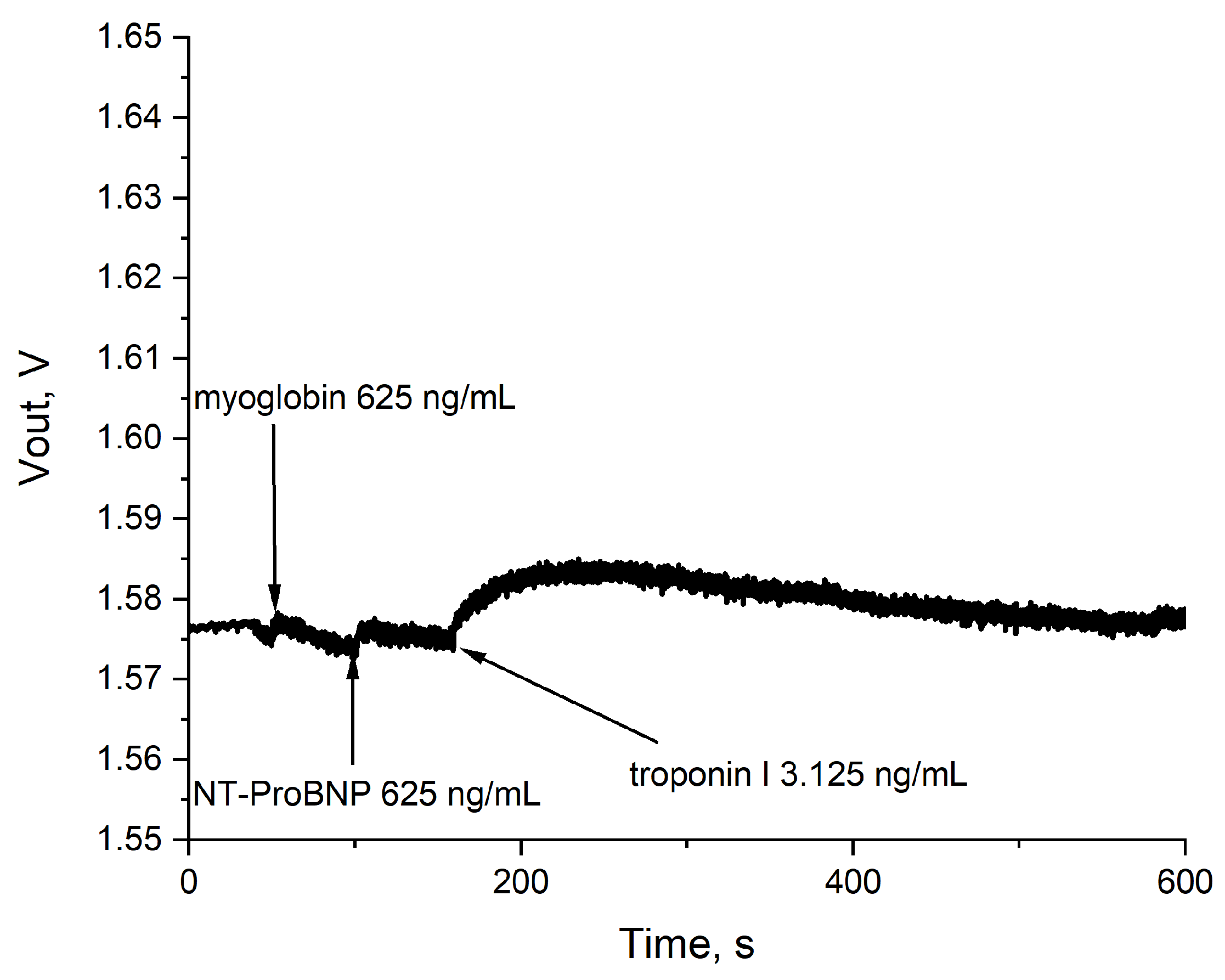
3.5.4. Real Sample Measurements
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. New Challenges in Point of Care Electrochemical Detection of Clinical Biomarkers. Sens. Actuators B Chem. 2021, 345, 130349. [Google Scholar] [CrossRef]
- Duque-Ossa, L.C.; García-Ferrera, B.; Reyes-Retana, J.A. Troponin I as a Biomarker for Early Detection of Acute Myocardial Infarction. Curr. Probl. Cardiol. 2023, 48, 101067. [Google Scholar] [CrossRef] [PubMed]
- Javed, U.; Aftab, W.; Ambrose, J.A.; Wessel, R.J.; Mouanoutoua, M.; Huang, G.; Barua, R.S.; Weilert, M.; Sy, F.; Thatai, D. Frequency of Elevated Troponin I and Diagnosis of Acute Myocardial Infarction. Am. J. Cardiol. 2009, 104, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Amundson, B.E.; Apple, F.S. Cardiac Troponin Assays: A Review of Quantitative Point-of-Care Devices and Their Efficacy in the Diagnosis of Myocardial Infarction. Clin. Chem. Lab. Med. 2015, 53, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Cullen, L.; Collinson, P.O.; Giannitsis, E. Point-of-Care Testing with High-Sensitivity Cardiac Troponin Assays: The Challenges and Opportunities. Emerg. Med. J. 2022, 39, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Jo, H.; Oh, S.S. Detection and beyond: Challenges and Advances in Aptamer-Based Biosensors. Mater. Adv. 2020, 1, 2663–2687. [Google Scholar] [CrossRef]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-Based Rapid Diagnosis for Point-of-Care Application. Microfluid. Nanofluid. 2023, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Citartan, M.; Tang, T.-H. Recent Developments of Aptasensors Expedient for Point-of-Care (POC) Diagnostics. Talanta 2019, 199, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y.; Phang, W.-M.; Hashim, U. Aptamer-Based ‘Point-of-Care Testing’. Biotechnol. Adv. 2016, 34, 198–208. [Google Scholar] [CrossRef]
- Aslan, Y.; Atabay, M.; Chowdhury, H.K.; Göktürk, I.; Saylan, Y.; Inci, F. Aptamer-Based Point-of-Care Devices: Emerging Technologies and Integration of Computational Methods. Biosensors 2023, 13, 569. [Google Scholar] [CrossRef]
- Amen, M.T.; Pham, T.T.T.; Cheah, E.; Tran, D.P.; Thierry, B. Metal-Oxide FET Biosensor for Point-of-Care Testing: Overview and Perspective. Molecules 2022, 27, 7952. [Google Scholar] [CrossRef] [PubMed]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing Based on Field-Effect Transistors (FET): Recent Progress and Challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review—Field-Effect Transistor Biosensing: Devices and Clinical Applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- Thriveni, G.; Ghosh, K. Advancement and Challenges of Biosensing Using Field Effect Transistors. Biosensors 2022, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Martinoia, S.; Rosso, N.; Grattarola, M.; Lorenzelli, L.; Margesin, B.; Zen, M. Development of ISFET Array-Based Microsystems for Bioelectrochemical Measurements of Cell Populations. Biosens. Bioelectron. 2001, 16, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Ingebrandt, S.; Han, Y.; Nakamura, F.; Poghossian, A.; Schöning, M.J.; Offenhäusser, A. Label-Free Detection of Single Nucleotide Polymorphisms Utilizing the Differential Transfer Function of Field-Effect Transistors. Biosens. Bioelectron. 2007, 22, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Moser, N.; Lande, T.S.; Toumazou, C.; Georgiou, P. ISFETs in CMOS and Emergent Trends in Instrumentation: A Review. IEEE Sens. J. 2016, 16, 6496–6514. [Google Scholar] [CrossRef]
- Shah, S.; Christen, J.B. Pulse Width Modulation Circuit for ISFET Drift Reset. In Proceedings of the 2013 IEEE SENSORS, Baltimore, MD, USA, 3–6 November 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1–4. [Google Scholar]
- Welch, D.; Shah, S.; Ozev, S.; Blain Christen, J. Experimental and Simulated Cycling of ISFET Electric Fields for Drift Reset. IEEE Electron. Device Lett. 2013, 34, 456–458. [Google Scholar] [CrossRef]
- Ma, X.; Peng, R.; Mao, W.; Lin, Y.; Yu, H. Recent Advances in Ion-sensitive Field-effect Transistors for Biosensing Applications. Electrochem. Sci. Adv. 2023, 3, e2100163. [Google Scholar] [CrossRef]
- Jo, H.; Gu, H.; Jeon, W.; Youn, H.; Her, J.; Kim, S.-K.; Lee, J.; Shin, J.H.; Ban, C. Electrochemical Aptasensor of Cardiac Troponin I for the Early Diagnosis of Acute Myocardial Infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef]
- Dinar, A.M.; Zain, A.S.M.; Salehuddin, F. Comprehensive Identification of Sensitive and Stable ISFET Sensing Layer High-k Gate Based on ISFET/Electrolyte Models. Int. J. Electr. Comput. Eng. 2019, 9, 926. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. ISFET Characteristics in CMOS and Their Application to Weak Inversion Operation. Sens. Actuators B Chem. 2009, 143, 211–217. [Google Scholar] [CrossRef]
- Andrianova, M.S.; Grudtsov, V.P.; Komarova, N.V.; Kuznetsov, E.V.; Kuznetsov, A.E. ISFET-Based Aptasensor for Thrombin Detection Using Horseradish Peroxidase. Procedia Eng. 2017, 174, 1084–1092. [Google Scholar] [CrossRef]
- Miscourides, N.; Georgiou, P. ISFET Arrays in CMOS: A Head-to-Head Comparison Between Voltage and Current Mode. IEEE Sens. J. 2019, 19, 1224–1238. [Google Scholar] [CrossRef]
- Ryazantsev, D.; Sheshil, A.; Grudtsov, V.; Rybachek, E.; Gabdrakhmanov, A.; Komarova, N. Design of CMOS Readout Circuits for ISFET Aptasensor. In Proceedings of the 2023 IEEE Ural-Siberian Conference on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 15–17 May 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 113–116. [Google Scholar]
- Gubanova, O.; Poletaev, A.; Komarova, N.; Grudtsov, V.; Ryazantsev, D.; Shustinskiy, M.; Shibalov, M.; Kuznetsov, A. A Novel Extended Gate ISFET Design for Biosensing Application Compatible with Standard CMOS. Mater. Sci. Semicond. Process. 2024, 177, 108387. [Google Scholar] [CrossRef]
- Henderson, E. BioForce Nanosciences, Inc. Nanomedicine 2007, 2, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Tai, T.-Y.; Li, K.-H.; Gopinathan, P.; Chung, Y.-D.; Sarangadharan, I.; Ma, H.-P.; Huang, P.-C.; Shiesh, S.-C.; Wang, Y.-L.; et al. An Integrated Microfluidic System with Field-Effect-Transistor Sensor Arrays for Detecting Multiple Cardiovascular Biomarkers from Clinical Samples. Biosens. Bioelectron. 2019, 129, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.M.; Schoch, B.; Schmid, F.; Keller, P.; Sudano, I.; Lüscher, T.F.; Noll, G.; Ruschitzka, F.; Enseleit, F. Takotsubo Cardiomyopathy Has a Unique Cardiac Biomarker Profile: NT-ProBNP/Myoglobin and NT-ProBNP/Troponin T Ratios for the Differential Diagnosis of Acute Coronary Syndromes and Stress Induced Cardiomyopathy. Int. J. Cardiol. 2012, 154, 328–332. [Google Scholar] [CrossRef]
- Apple, F.S.; Christenson, R.H.; Valdes, R.; Andriak, A.J.; Berg, A.; Duh, S.-H.; Feng, Y.-J.; Jortani, S.A.; Johnson, N.A.; Koplen, B.; et al. Simultaneous Rapid Measurement of Whole Blood Myoglobin, Creatine Kinase MB, and Cardiac Troponin I by the Triage Cardiac Panel for Detection of Myocardial Infarction. Clin. Chem. 1999, 45, 199–205. [Google Scholar] [CrossRef]
- Bernstein, L.H.; Zions, M.Y.; Alam, M.E.; Haq, S.A.; Heitner, J.F.; Zarich, S.; Seamonds, B.; Berger, S. What Is the Best Approximation of Reference Normal for NT-ProBNP? Clinical Levels for Enhanced Assessment of NT-ProBNP (CLEAN). J. Med. Lab. Diagn. 2011, 2, 16–21. [Google Scholar]
- de Winter, R.J.; Lijmer, J.G.; Koster, R.W.; Hoek, F.J.; Sanders, G.T. Diagnostic Accuracy of Myoglobin Concentration for the Early Diagnosis of Acute Myocardial Infarction. Ann. Emerg. Med. 2000, 35, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.-A.; Su, Y.-T.; Wang, J.-S.; Chang, C.-Y.; Hu, W.-P.; Huang, C.-J.; Chan, H.W.-H.; Chen, W.-Y. Comparing Surface Modification Methods for Silicon Nanowire Field-Effect Transistor Biosensors for Diagnosis Applications: A Case Study of Cardiac Troponin I. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132146. [Google Scholar] [CrossRef]
- Prajesh, R.; Goyal, V.; Kakkar, S.; Sharma, J.; Alam, M.A.; Maurya, R.K.; Bhalla, V.; Agarwal, A. Polysilicon Field Effect Transistor Biosensor for the Detection of Cardiac Troponin-I (CTnI). J. Electrochem. Soc. 2021, 168, 027501. [Google Scholar] [CrossRef]
- Kutovyi, Y.; Li, J.; Zadorozhnyi, I.; Hlukhova, H.; Boichuk, N.; Yehorov, D.; Menger, M.; Vitusevich, S. Highly Sensitive and Fast Detection of C-Reactive Protein and Troponin Biomarkers Using Liquidgated Single Silicon Nanowire Biosensors. MRS Adv. 2020, 5, 835–846. [Google Scholar] [CrossRef]
- Hlukhova, H.; Menger, M.; Offenhäusser, A.; Vitusevich, S. Highly Sensitive Aptamer-Based Method for the Detection of Cardiac Biomolecules on Silicon Dioxide Surfaces. MRS Adv. 2018, 3, 1535–1541. [Google Scholar] [CrossRef]
- Lee, W.C.; Lee, H.; Lim, J.; Park, Y.J. An Effective Electrical Sensing Scheme Using AC Electrothermal Flow on a Biosensor Platform Based on a Carbon Nanotube Network. Appl. Phys. Lett. 2016, 109, 223701. [Google Scholar] [CrossRef]
- Rodrigues, T.; Mishyn, V.; Leroux, Y.R.; Butruille, L.; Woitrain, E.; Barras, A.; Aspermair, P.; Happy, H.; Kleber, C.; Boukherroub, R.; et al. Highly Performing Graphene-Based Field Effect Transistor for the Differentiation between Mild-Moderate-Severe Myocardial Injury. Nano Today 2022, 43, 101391. [Google Scholar] [CrossRef]
- Mishyn, V.; Rodrigues, T.; Leroux, Y.R.; Butruille, L.; Woitrain, E.; Montaigne, D.; Aspermair, P.; Happy, H.; Knoll, W.; Boukherroub, R.; et al. Electrochemical and Electronic Detection of Biomarkers in Serum: A Systematic Comparison Using Aptamer-Functionalized Surfaces. Anal. Bioanal. Chem. 2022, 414, 5319–5327. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, K.; Ding, Y.; Liu, H.; Xie, S.; Cao, Y.; Yue, W. Magnetically Controlled Graphene Field-Effect Transistor Biosensor for Highly Sensitive Detection of Cardiac Troponin I. Discov. Nano 2023, 18, 106. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, K.; Ren, J.; Wang, H.; Cheng, J. An Electrochemical Metallic Nanowire Aptasensor for Rapid and Ultrasensitive Detection of Cardiac Troponin I. Sens. Actuators B Chem. 2024, 401, 135001. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Huang, W.; Yang, N.; Yuan, Q.; Yang, Y. Aptamer-functionalized Field-effect Transistor Biosensors for Disease Diagnosis and Environmental Monitoring. Exploration 2023, 3, 20210027. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.-A.; Chen, W.-Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors 2019, 19, 4214. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Hochholzer, W.; Stelzig, C.; Laule, K.; Freidank, H.; Morgenthaler, N.G.; Bergmann, A.; Potocki, M.; Noveanu, M.; Breidthardt, T.; et al. Incremental Value of Copeptin for Rapid Rule Out of Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 54, 60–68. [Google Scholar] [CrossRef]
- López-Cuenca, Á.; Manzano-Fernández, S.; Lip, G.Y.H.; Casas, T.; Sánchez-Martínez, M.; Mateo-Martínez, A.; Pérez-Berbel, P.; Martínez, J.; Hernández-Romero, D.; Romero Aniorte, A.I.; et al. Interleukin-6 and High-Sensitivity C-Reactive Protein for the Prediction of Outcomes in Non-ST-Segment Elevation Acute Coronary Syndromes. Rev. Esp. Cardiol. 2013, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Russo-Volpe, S.; Miles, E.A.; Rees, D.; Banerjee, T.; Roynette, C.E.; Wells, S.J.; Goua, M.; Wahle, K.W.J.; Calder, P.C.; et al. Age- and Dose-Dependent Effects of an Eicosapentaenoic Acid-Rich Oil on Cardiovascular Risk Factors in Healthy Male Subjects. Atherosclerosis 2007, 193, 159–167. [Google Scholar] [CrossRef]




| Sensor Type | Aptamer Used | LoD, ng/mL | Linear Range, ng/mL | Reference |
|---|---|---|---|---|
| Polysilicon FET | 55-nt DNA, no sequence provided | 0.1 | 0.1–1000 | [35] |
| Silicon nanowire FET | 79 nt DNA aptamer, no sequence provided | 1 | 1–1000 | [36] |
| Silicon nanowire FET | 79 nt DNA aptamer, 5′-GCCTGTTGTGAGCCTCCTAACTACATGTTCTCAGGGTTGAGGCTGGATGGCGATGGTGGCATGCTTATTCTTGTCTCCC-3′ | 0.0239 | 10–1000 | [37] |
| Silicon nanowire FET | Tro6, 40 nt DNA, 5′-CGCATGCCAAACGTTGCCTCATAGTTCCCTCCCCGTGTCC-3′ | 0.002 | 0.002–0.008 | [34] |
| Ta2O5-gated FET | Tro6, 40 nt DNA, 5′-CGCATGCCAAACGTTGCCTCATAGTTCCCTCCCCGTGTCC-3′ | 3.27 | 3.27–625 | This work |
| Carbon nanotube network FET decorated with gold nanoparticles | Tro6, 40 nt DNA, 5′-CGCATGCCAAACGTTGCCTCATAGTTCCCTCCCCGTGTCC-3′ | 2.4 | 2.4–2400 | [38] |
| Graphene-based FET | Tro4, 40 nt DNA, 5′-CGTGCAGTACGCCAACCTTTCTCATGCGCTGCCCCTCTTA-3′ | 0.00334 | 0.01–0.50 | [39] |
| Graphene-based FET | Tro4, 40 nt DNA, 5′-CGTGCAGTACGCCAACCTTTCTCATGCGCTGCCCCTCTTA-3′ | 0.0033 | 0.0033–0.2 | [40] |
| Magnetic graphene FET | Tro4, 40 nt DNA, 5′-CGTGCAGTACGCCAACCTTTCTCATGCGCTGCCCCTCTTA-3′ | 0.239 | 0.239–23,900 | [41] |
| Metallic nanowire FET | Tro4, 40 nt DNA, 5′-CGTGCAGTACGCCAACCTTTCTCATGCGCTGCCCCTCTTA-3′ data | 0.000013 | 0.0001–10 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryazantsev, D.; Shustinskiy, M.; Sheshil, A.; Titov, A.; Grudtsov, V.; Vechorko, V.; Kitiashvili, I.; Puchnin, K.; Kuznetsov, A.; Komarova, N. A Portable Readout System for Biomarker Detection with Aptamer-Modified CMOS ISFET Array. Sensors 2024, 24, 3008. https://doi.org/10.3390/s24103008
Ryazantsev D, Shustinskiy M, Sheshil A, Titov A, Grudtsov V, Vechorko V, Kitiashvili I, Puchnin K, Kuznetsov A, Komarova N. A Portable Readout System for Biomarker Detection with Aptamer-Modified CMOS ISFET Array. Sensors. 2024; 24(10):3008. https://doi.org/10.3390/s24103008
Chicago/Turabian StyleRyazantsev, Dmitriy, Mark Shustinskiy, Andrey Sheshil, Alexey Titov, Vitaliy Grudtsov, Valerii Vechorko, Irakli Kitiashvili, Kirill Puchnin, Alexander Kuznetsov, and Natalia Komarova. 2024. "A Portable Readout System for Biomarker Detection with Aptamer-Modified CMOS ISFET Array" Sensors 24, no. 10: 3008. https://doi.org/10.3390/s24103008







