Surface-Enhanced Raman Scattering in Silver-Coated Suspended-Core Fiber
Abstract
:1. Introduction
2. Fabrication and Experimental Setup
2.1. Chemicals and Reagents
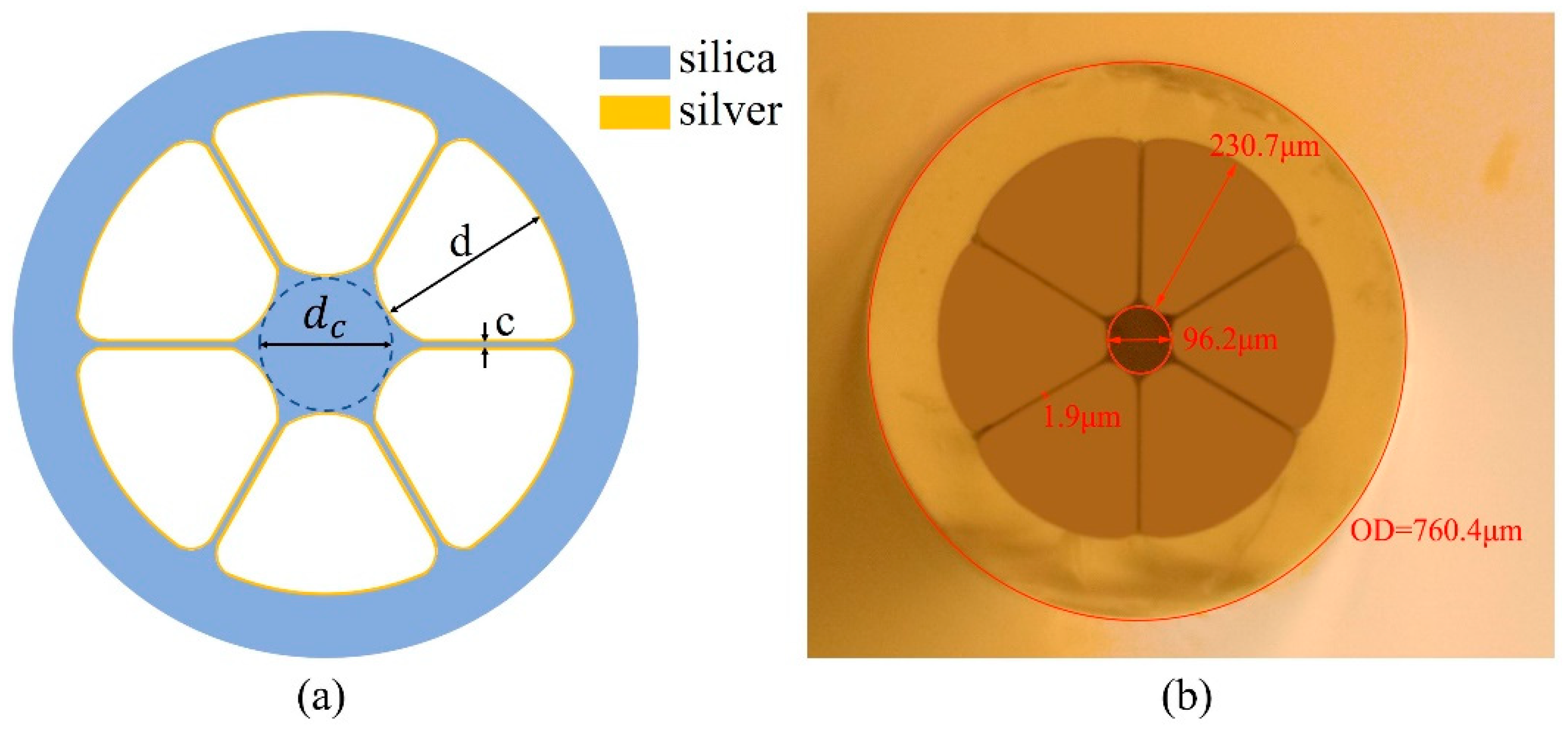
2.2. LSCF Configuration
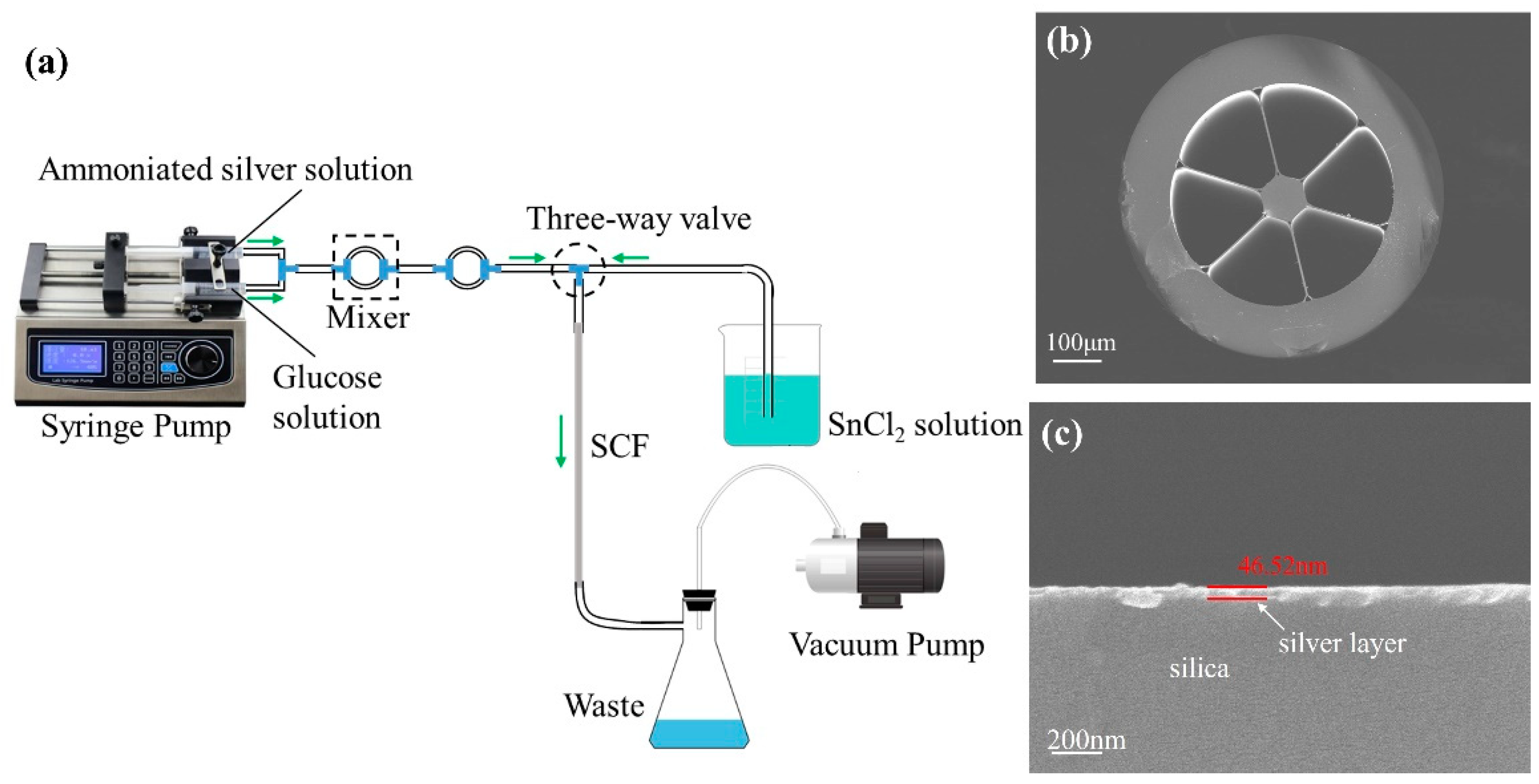
2.3. Preparation of the Ag-Coated LSCF
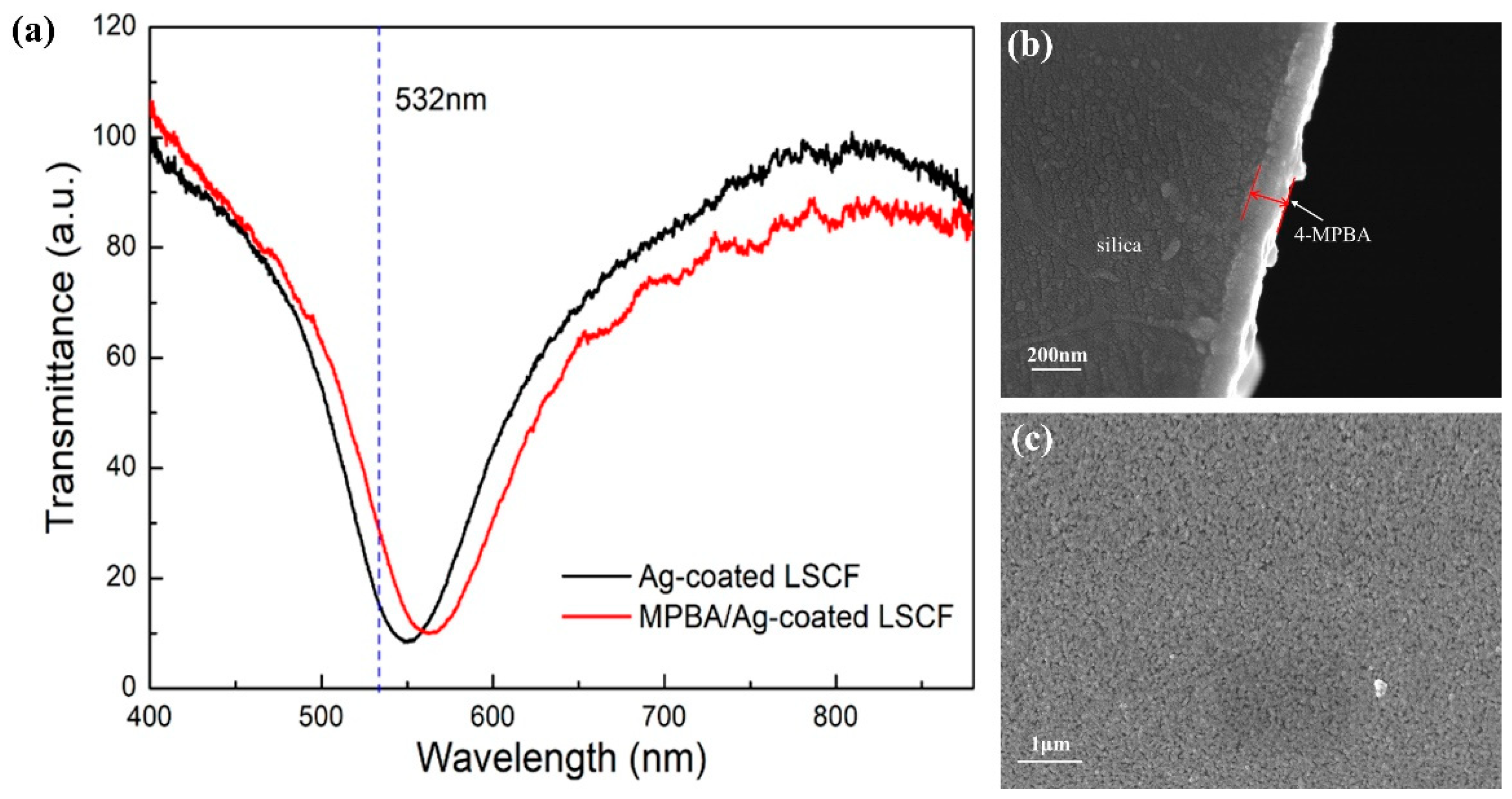
2.4. Preparation of SERS Sensing Surface
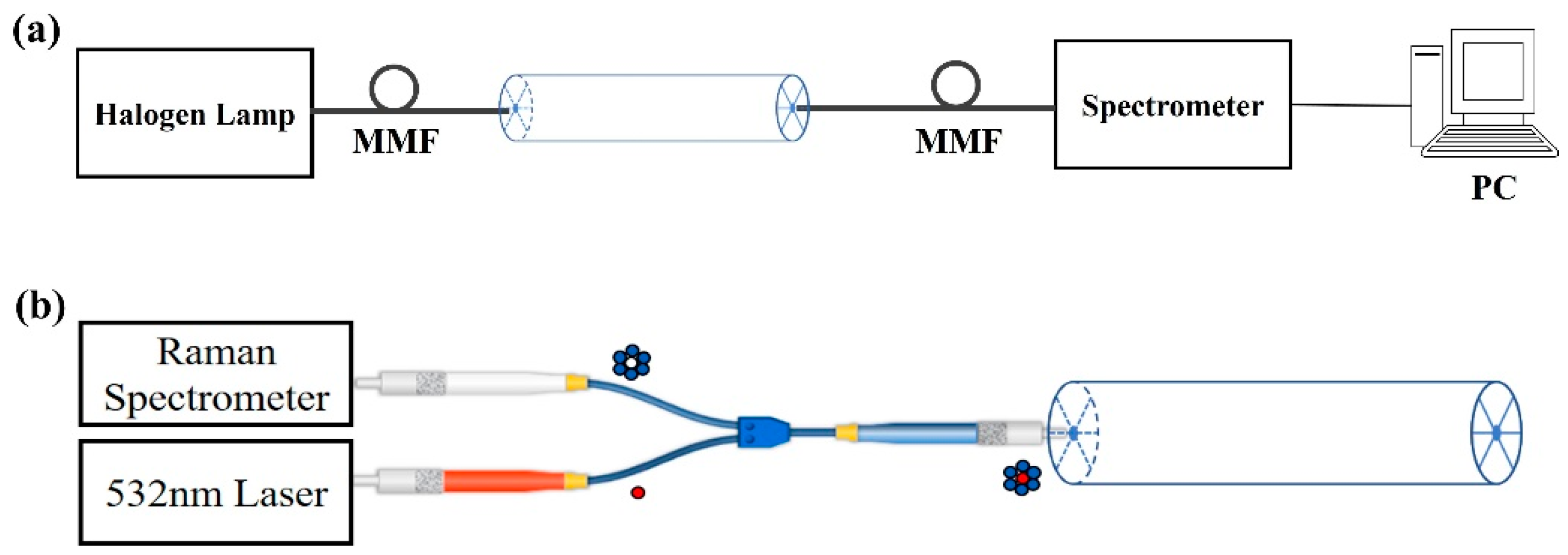
2.5. Experimental Setup
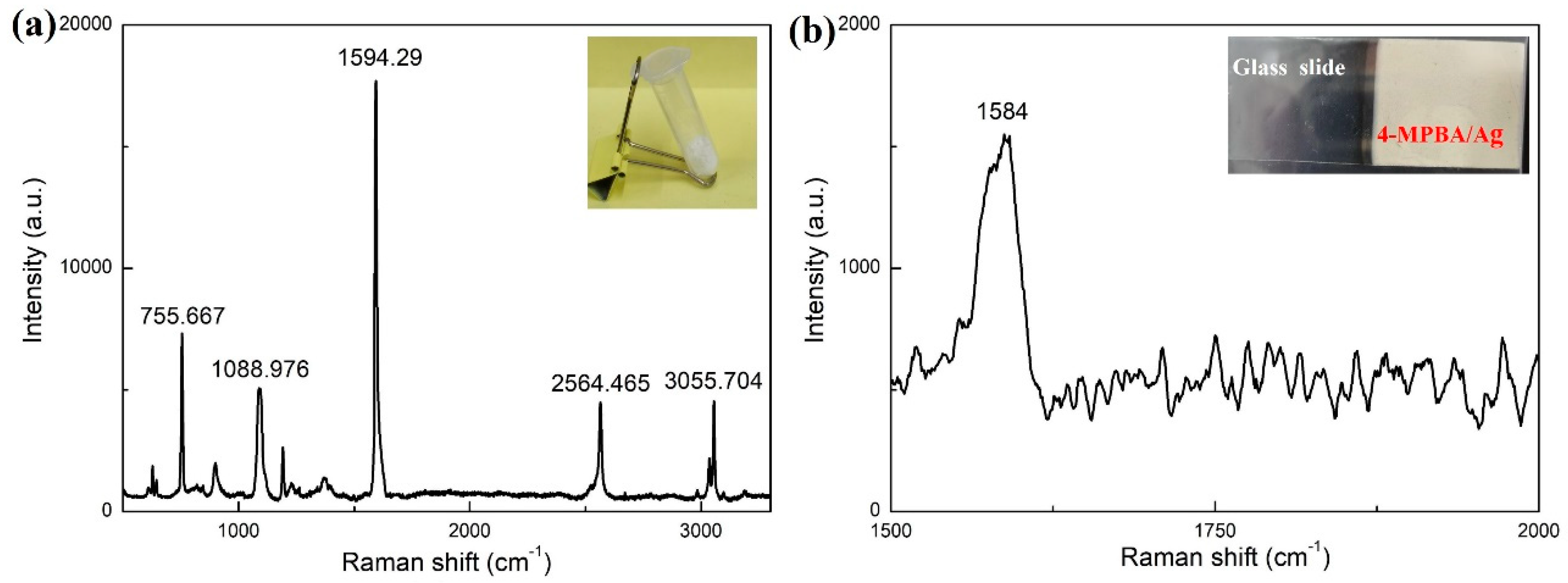
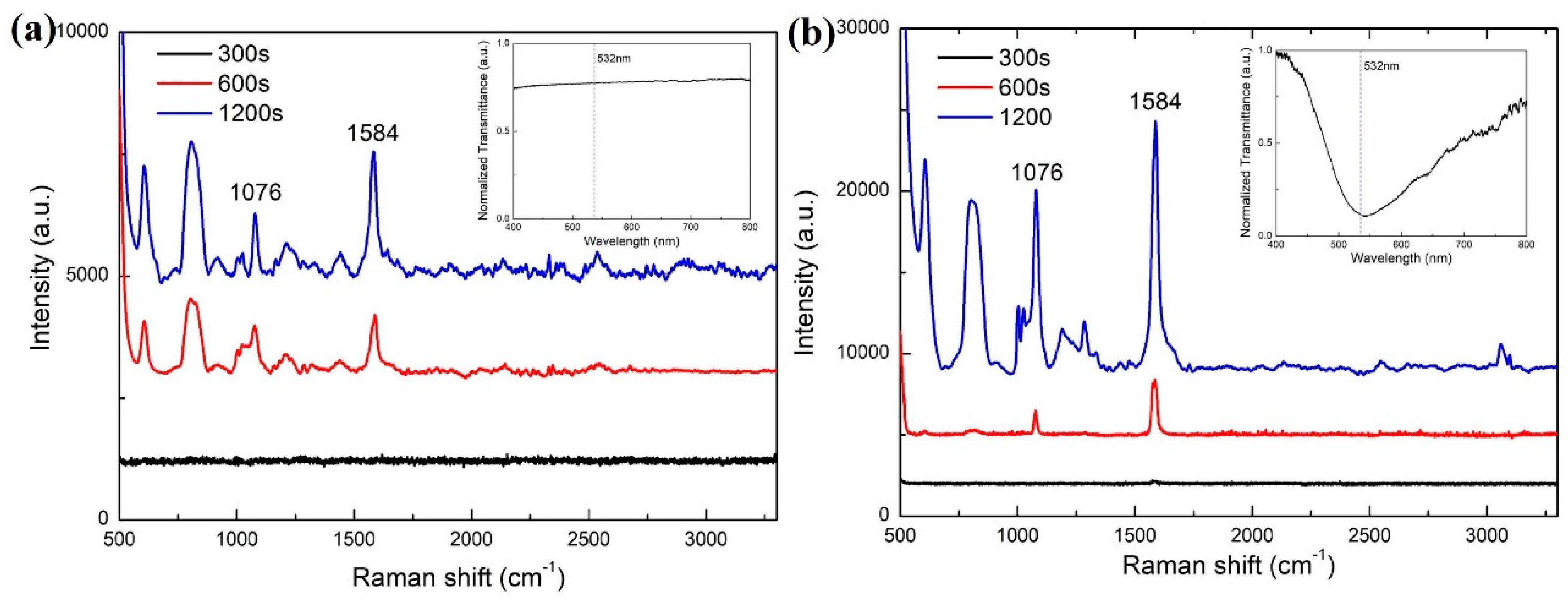
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerrini, L.; Pazos, E.; Penas, C.; Vázquez, M.E.; Mascareñas, J.L.; Alvarez-Puebla, R.A. Highly sensitive SERS quantification of the oncogenic protein c-Jun in cellular extracts. J. Am. Chem. Soc. 2013, 135, 10314–10317. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Chen, S.; Hayashi, M.; Shiu, Y.; Huang, C.; Chuang, W.; Su, C.-J.; Jeng, H.-C.; Chang, J.-W.; Lee, Y.-C.; et al. Mesostructured arrays of nanometer-spaced gold nanoparticles for ultrahigh number density of SERS hot spots. Adv. Funct. Mater. 2014, 24, 2544–2552. [Google Scholar] [CrossRef]
- Liu, X.; Dang, A.; Li, T.; Sun, Y.; Lee, T.C.; Deng, W.; Wu, S.; Zada, A.; Zhao, T.; Li, H. Plasmonic coupling of Au nanoclusters on a flexible MXene/Graphene oxide fiber for ultrasensitive SERS sensing. ACS Sens. 2023, 8, 1287–1298. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, S.; Yi, J.; Luo, S.; Li, C.; Liu, G.; Yan, J.; Li, J.-F.; Mao, B.-W.; Tian, Z.-Q. Nanostructure-Based Plasmon-Enhanced Raman Spectroscopic Strategies for Characterization of the Solid-Electrolyte Interphase: Opportunities and Challenges. J. Phys. Chem. C 2023, 127, 13466–13477. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Ding, Y.; Yang, Z.; Li, S.; Zhou, X.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Hasan, D.; Ruoff, R.S.; Wang, A.; Fan, D. Near-Field Enhanced Plasmonic-Magnetic Bifunctional Nanotubes for Single Cell Bioanalysis. Adv. Funct. Mater. 2013, 23, 4332–4338. [Google Scholar] [CrossRef]
- Nie, S.; Emery, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Gong, X.; Tang, M.; Gong, Z.; Qiu, Z.; Wang, D.; Fan, M. Screening pesticide residues on fruit peels using portable Raman spectrometer combined with adhesive tape sampling. Food Chem. 2019, 295, 254–258. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.; Yu, J.; Li, Z.; Li, W.; Wang, J.; Liu, A.; Man, B.; Yang, S.; Zhang, C. Local hot charge density regulation: Vibration-free pyroelectric nanogenerator for effectively enhancing catalysis and in-situ surface-enhanced Raman scattering monitoring. Nano Energy 2021, 81, 105585. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhai, W.; Li, X.; Li, D.; Lin, H.; Han, S. Electrokinetic preseparation and molecularly imprinted trapping for highly selective SERS detection of charged phthalate plasticizers. Anal. Chem. 2020, 93, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, W. Fiber-optic surface plasmon resonance sensors and biochemical applications: A review. J. Light. Technol. 2020, 39, 3781–3791. [Google Scholar] [CrossRef]
- Yue, X.; Su, Y.; Wang, X.; Li, L.; Ji, W.; Ozaki, Y. Reusable silicon-based SERS chip for ratiometric analysis of fluoride ion in aqueous solutions. ACS Sens. 2019, 4, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Poggesi, S.; Casari Bariani, G.; Mittapalli, R.; Adam, P.M.; Manzano, M.; Ionescu, R.E. Robust SERS platforms based on annealed gold nanostructures formed on ultrafine glass substrates for various (bio) applications. Biosensors 2019, 9, 53. [Google Scholar] [CrossRef]
- Lee, M.; Oh, K.; Choi, H.K.; Lee, S.G.; Youn, H.J.; Lee, H.L.; Jeong, D.H. Subnanomolar sensitivity of filter paper-based SERS sensor for pesticide detection by hydrophobicity change of paper surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Arnob, M.M.P.; Baek, K.M.; Lee, S.Y.; Shih, W.C.; Jung, Y.S. 3D cross-point plasmonic nanoarchitectures containing dense and regular hot spots for surface-enhanced Raman spectroscopy analysis. Adv. Mater. 2016, 28, 8695–8704. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef]
- Kim, W.; Kim, Y.H.; Park, H.K.; Choi, S. Facile fabrication of a silver nanoparticle immersed, surface-enhanced Raman scattering imposed paper platform through successive ionic layer absorption and reaction for on-site bioassays. ACS Appl. Mater. Interfaces 2015, 7, 27910–27917. [Google Scholar] [CrossRef]
- Boginskaya, I.; Sedova, M.; Baburin, A.; Afanas’ev, K.; Zverev, A.; Echeistov, V.; Ryzhkov, V.; Rodionov, I.; Tonanaiskii, B.; Ryzhikov, I.; et al. SERS-Active substrates nanoengineering based on e-beam evaporated self-assembled silver films. Appl. Sci. 2019, 9, 3988. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.; You, J.; Wu, C. Focused-ion-beam-fabricated Au/Ag multilayered nanorod array as SERS-active substrate for virus strain detection. Sens. Actuators B Chem. 2013, 181, 361–367. [Google Scholar] [CrossRef]
- Kozhina, E.P.; Bedin, S.A.; Nechaeva, N.L.; Podoynitsyn, S.N.; Tarakanov, V.P.; Andreev, S.N.; Grigoriev, Y.V.; Naumov, A.V. Ag-nanowire bundles with gap hot spots synthesized in track-etched membranes as effective SERS-substrates. Appl. Sci. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Kozhina, E.; Bedin, S.; Martynov, A.; Andreev, S.; Piryazev, A.; Grigoriev, Y.; Gorbunova, Y.; Naumov, A. Ultrasensitive Optical Fingerprinting of Biorelevant Molecules by Means of SERS-Mapping on Nanostructured Metasurfaces. Biosensors 2022, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Kovalec, N.P.; Kozhina, E.P.; Doludenko, I.M.; Razumovskaya, I.V.; Bedin, S.A.; Grigoriev, Y.V.; Kanevsky, V.M. Agglomeration of Ensembles of Silver Nanowires, Obtained by the Method of Template Synthesis. Bull. Russ. Acad. Sci. Phys. 2021, 85, 854–857. [Google Scholar] [CrossRef]
- Kozhina, E.P.; Andreev, S.N.; Tarakanov, V.P.; Bedin, S.A.; Doludenko, I.M.; Naumov, A.V. Study of local fields of dendrite nanostructures in hot spots formed on SERS-active substrates produced via template-assisted synthesis. Bull. Russ. Acad. Sci. Phys. 2020, 84, 1465–1468. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Y.; Yang, C.; Kong, L. In situ surface-enhanced Raman scattering sensing with soft and flexible polymer optical fiber probes. Opt. Lett. 2018, 43, 5443–5446. [Google Scholar] [CrossRef]
- Gao, D.; Yang, X.; Teng, P.; Liu, Z.; Yang, J.; Kong, D.; Zhang, J.; Luo, M.; Li, Z.; Tian, F.; et al. Optofluidic in-fiber integrated surface-enhanced Raman spectroscopy detection based on a hollow optical fiber with a suspended core. Opt. Lett. 2019, 44, 5173–5176. [Google Scholar] [CrossRef]
- Zhu, Y.; Dluhy, R.A.; Zhao, Y. Development of silver nanorod array based fiber optic probes for SERS detection. Sens. Actuators B Chem. 2011, 157, 42–50. [Google Scholar] [CrossRef]
- Yang, X.; Gu, C.; Qian, F.; Li, Y.; Zhang, J. Highly Sensitive Detection of Proteins and Bacteria in Aqueous Solution Using Surface-Enhanced Raman Scattering and Optical Fibers. Anal. Chem. 2011, 83, 5888–5894. [Google Scholar] [CrossRef]
- Tao, P.; Ge, K.; Dai, X.; Xue, D.; Luo, Y.; Dai, S.; Xu, T.; Jiang, T.; Zhang, P. Fiber Optic SERS Sensor with Silver Nanocubes Attached Based on Evanescent Wave for Detecting Pesticide Residues. ACS Appl. Mater. Interfaces 2023, 15, 30998–33100. [Google Scholar] [CrossRef]
- Sánchez-Solís, A.; Karim, F.; Alam, M.S.; Zhan, Q.; López-Luke, T.; Zhao, C. Print metallic nanoparticles on a fiber probe for 1064-nm surface-enhanced Raman scattering. Opt. Lett. 2019, 44, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dai, Z.; Chen, N.; Liu, S.; Pang, F.; Lu, B.; Wang, T. Gold nanoparticles-modified tapered fiber nanoprobe for remote SERS detection. IEEE Photon. Technol. Lett. 2014, 26, 777–780. [Google Scholar] [CrossRef]
- Xia, M.; Guo, H.; Tang, J.; Li, C.; Zhao, R.; Wang, L.; Liu, W.; Yang, J.; Liu, J. Simple, repeatable and low-cost SERS fiber probe for fluorochrome detection. Micro Nano Lett. 2018, 13, 714–719. [Google Scholar] [CrossRef]
- Oo, M.K.K.; Han, Y.; Martini, R.; Sukhishvili, S.; Du, H. Forward-propagating surface-enhanced Raman scattering and intensity distribution in photonic crystal fiber with immobilized Ag nanoparticles. Opt. Lett. 2009, 34, 968–970. [Google Scholar] [CrossRef]
- Han, Y.; Tan, S.L.; Oo, M.K.K.; Pristinski, D.; Sukhishvili, S.; Du, H. Towards Full-Length Accumulative Surface-Enhanced-Raman Scattering-Active Photonic Crystal Fibers. Adv. Mater. 2010, 22, 2647–2651. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Z.; Li, J. Photonic crystal fiber based surface plasmon resonance chemical sensors. Sens. Actuators B Chem. 2014, 202, 557–567. [Google Scholar] [CrossRef]
- Cubillas, A.; Unterkofler, S.; Euser, T.; Etzold, B.; Jones, A.; Sadler, P.J.; Wasserscheid, P.; Russell, P.S.J. Photonic crystal fibers for chemical sensing and photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef]
- Khaing Oo, M.K.; Han, Y.; Kanka, J.; Sukhishvili, S.; Du, H. Structure fits the purpose: Photonic crystal fibers for evanescent-field surface-enhanced Raman spectroscopy. Opt. Lett. 2010, 35, 466–468. [Google Scholar] [CrossRef]
- Monro, T.M.; Warren-Smith, S.; Schartner, E.P.; François, A.; Heng, S.; Ebendorff-Heidepriem, H.; Afshar, S. Sensing with suspended-core optical fibers. Opt. Fiber Technol. 2010, 16, 343–356. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Shi, Y. Fiber optic surface plasmon resonance sensor based on a silver-coated large-core suspended-core fiber. Opt. Lett. 2019, 44, 4550–4553. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-L.; Zhang, Y.-N.; Li, L.-K.; Li, X.-G.; Zhao, Y. A plug-and-play optical fiber SPR sensor for simultaneous measurement of glucose and cholesterol concentrations. Biosens. Bioelectron. 2022, 198, 113798. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhang, Y.; Yuan, H.; Ji, W.; Liu, Y.; Zhao, J.; Han, M.; Peng, W. Boronic acid functionalized fiber-optic SPR sensors for high sensitivity glycoprotein detection. Sens. Actuators B Chem. 2018, 260, 976–982. [Google Scholar] [CrossRef]
- Bian, Z.; Liu, A.; Li, Y.; Fang, G.; Yao, Q.; Zhang, G.; Wu, Z. Boronic acid sensors with double recognition sites: A review. Analyst 2020, 145, 719–744. [Google Scholar] [CrossRef]
- Afshar, S.; Ruan, Y.; Warren-Smith, S.C.; Monro, T.M. Enhanced fluorescence sensing using microstructured optical fibers: A comparison of forward and backward collection modes. Opt. Lett. 2008, 33, 1473–1475. [Google Scholar] [CrossRef]
- Bi, X.; Du, X.; Jiang, J.; Huang, X. Facile and sensitive glucose sandwich assay using in situ-generated Raman reporters. Anal. Chem. 2015, 87, 2016–2021. [Google Scholar] [CrossRef]
- Gao, D.; Yang, X.; Luo, M.; Teng, P.; Zhang, H.; Liu, Z.; Gao, S.; Li, Z.; Wen, X.; Yuan, L.; et al. Surface-Enhanced Raman Spectroscopy Detection of Cerebrospinal Fluid Glucose Based on the Optofluidic In-Fiber-Integrated Composites of Graphene Oxide, Silver Nanoparticles, and 4-Mercaptophenylboronic Acid. ACS Appl. Nano Mater. 2021, 4, 10784–10790. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhang, X.; Zhu, X.-S.; Shi, Y.-W. Surface-Enhanced Raman Scattering in Silver-Coated Suspended-Core Fiber. Sensors 2024, 24, 160. https://doi.org/10.3390/s24010160
Xu Y, Zhang X, Zhu X-S, Shi Y-W. Surface-Enhanced Raman Scattering in Silver-Coated Suspended-Core Fiber. Sensors. 2024; 24(1):160. https://doi.org/10.3390/s24010160
Chicago/Turabian StyleXu, Yangyang, Xian Zhang, Xiao-Song Zhu, and Yi-Wei Shi. 2024. "Surface-Enhanced Raman Scattering in Silver-Coated Suspended-Core Fiber" Sensors 24, no. 1: 160. https://doi.org/10.3390/s24010160




