Influence of Different Pt Functionalization Modes on the Properties of CuO Gas-Sensing Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization of the Samples
2.3. Fabrication of Gas Sensor and Testing Method
3. Results
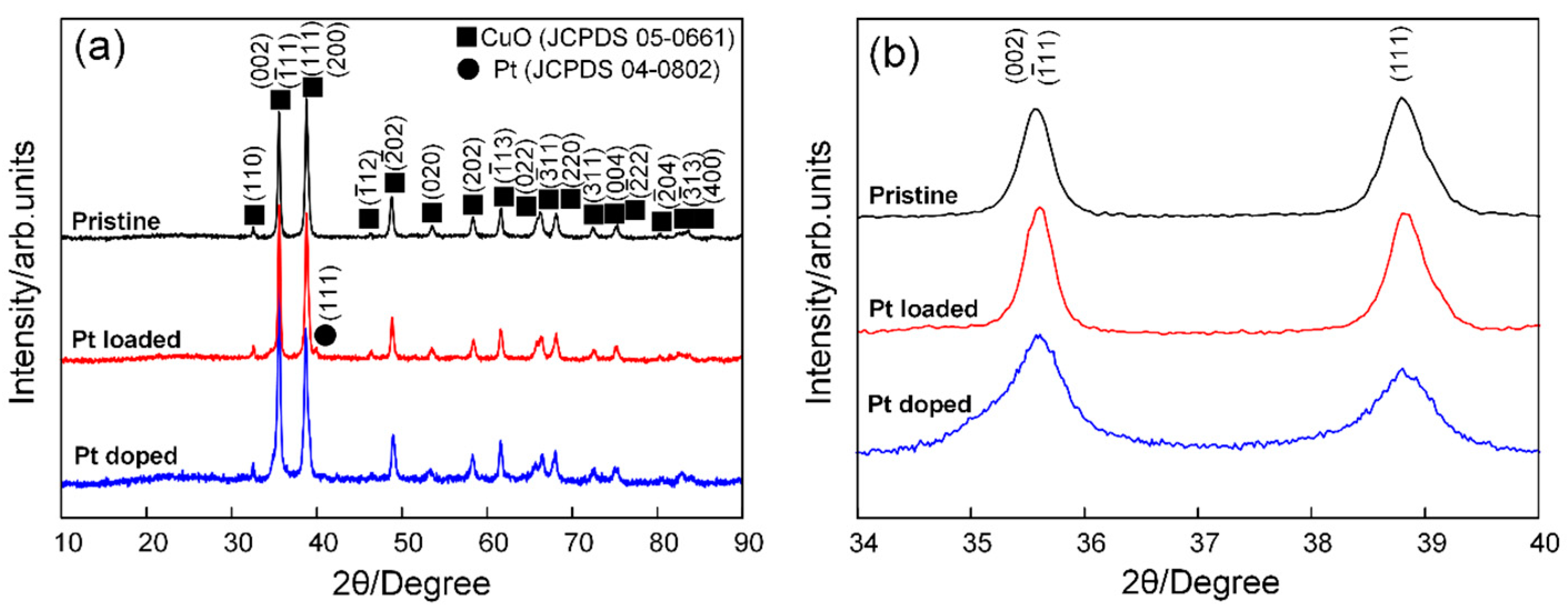
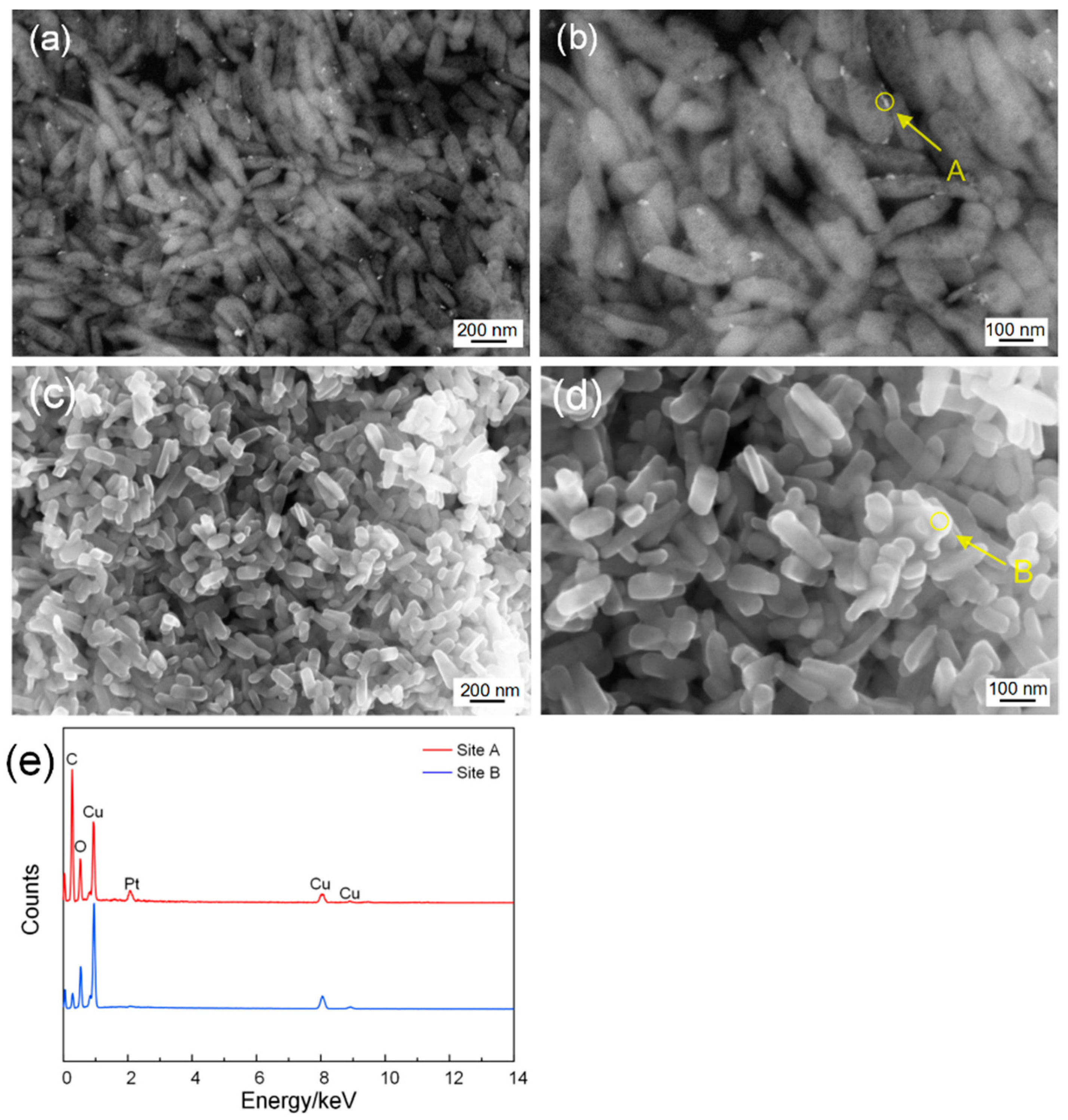
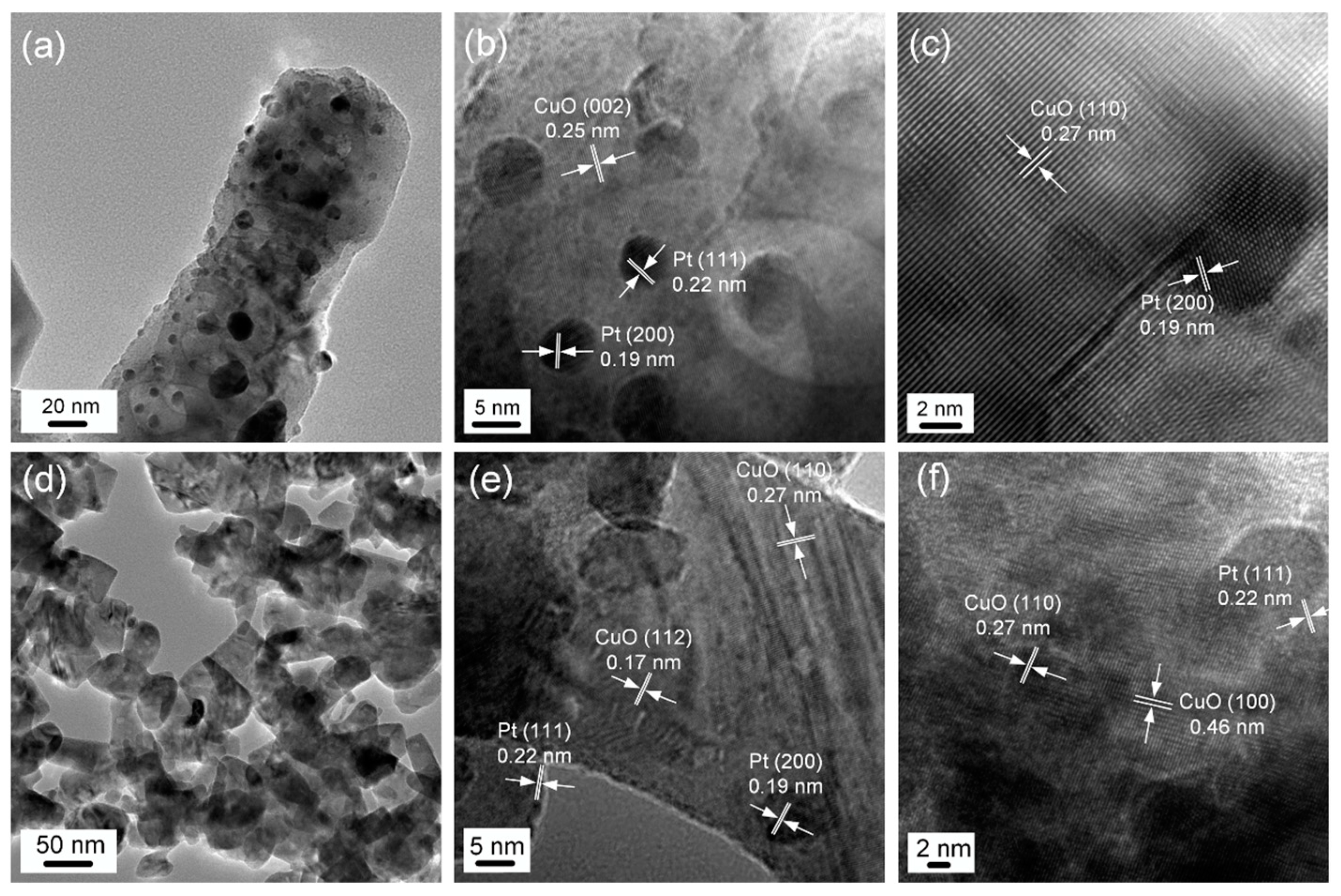
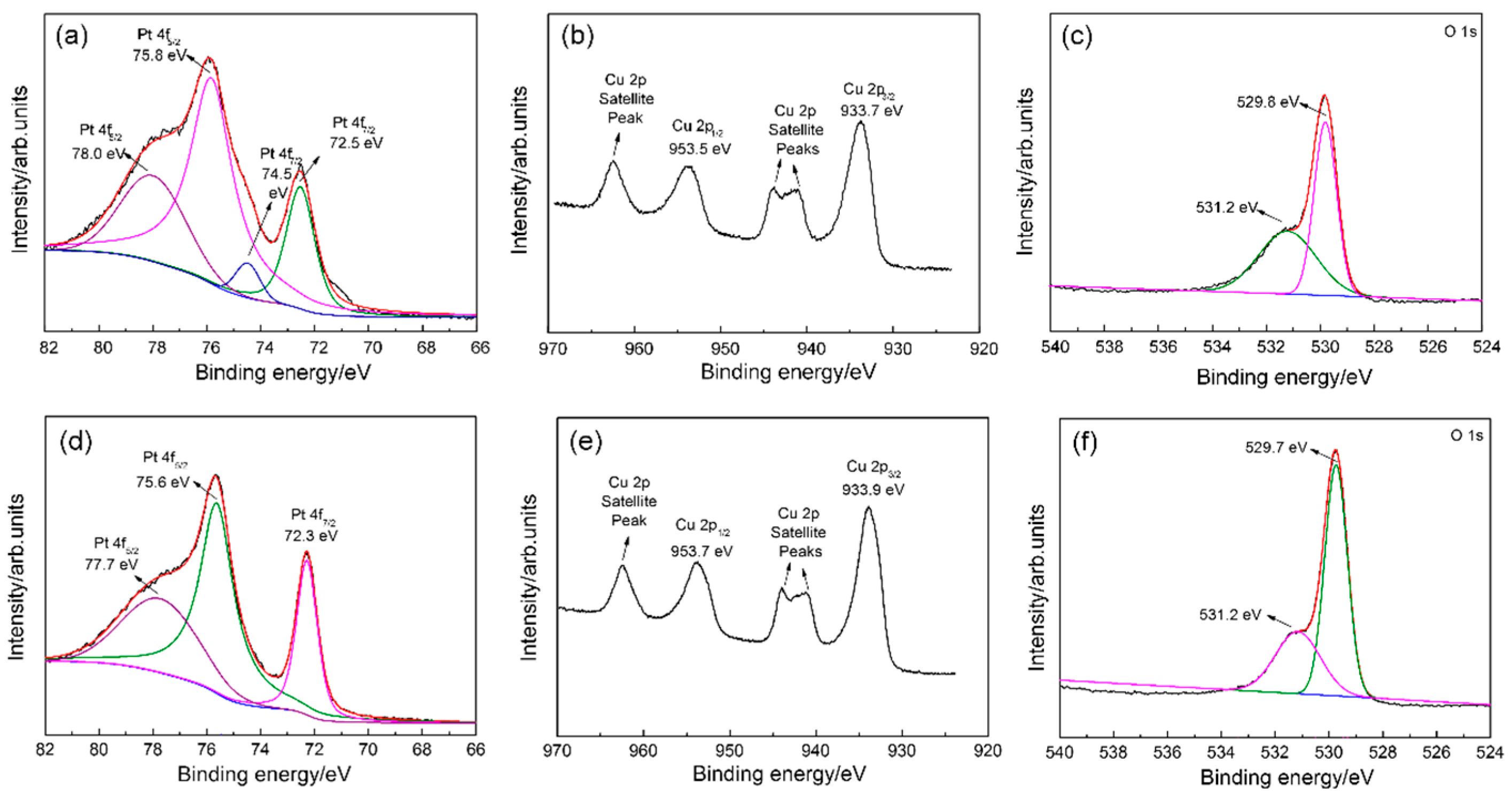
3.1. Structural and Morphological Characteristics
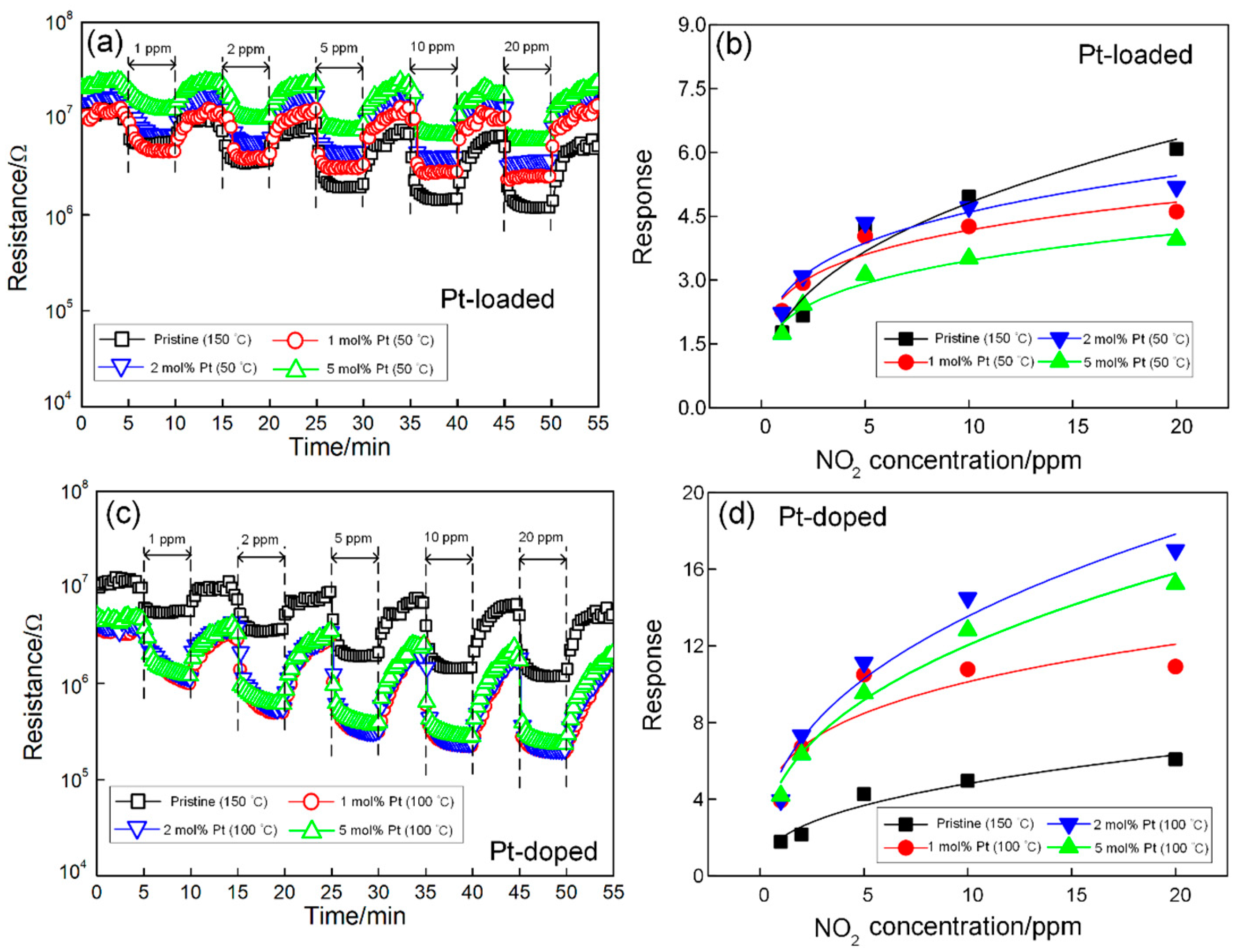
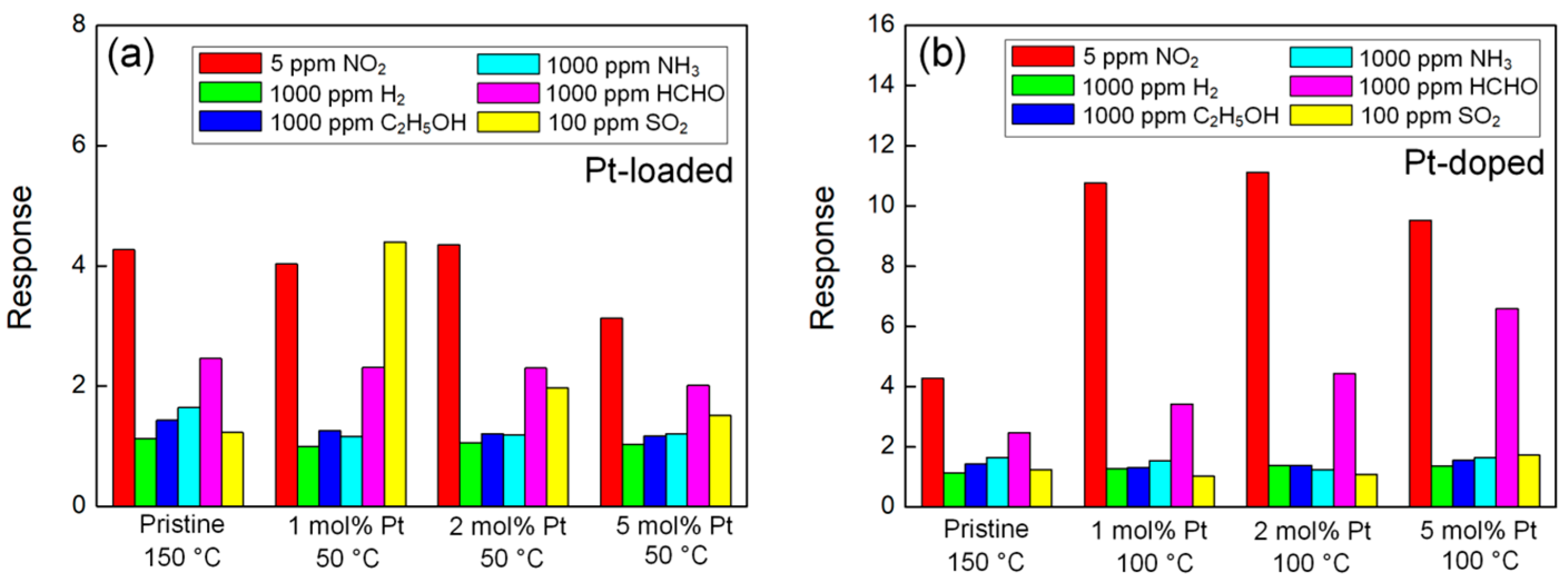
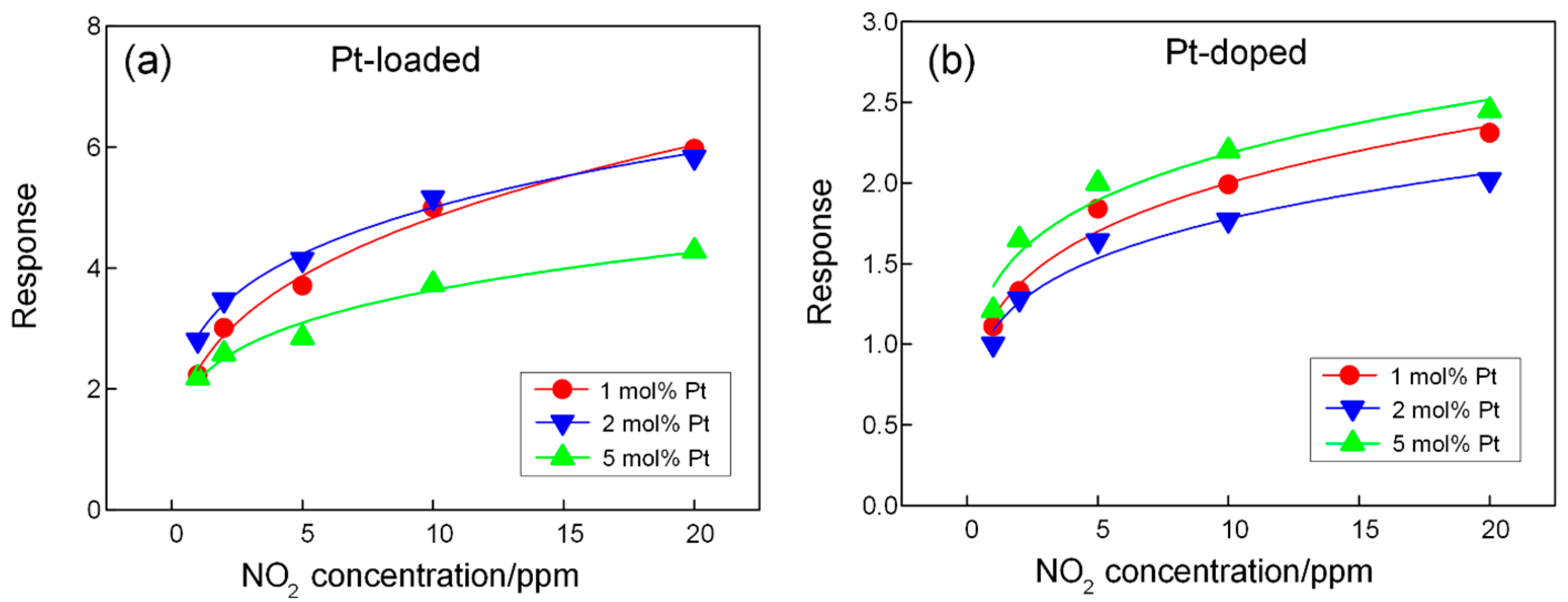
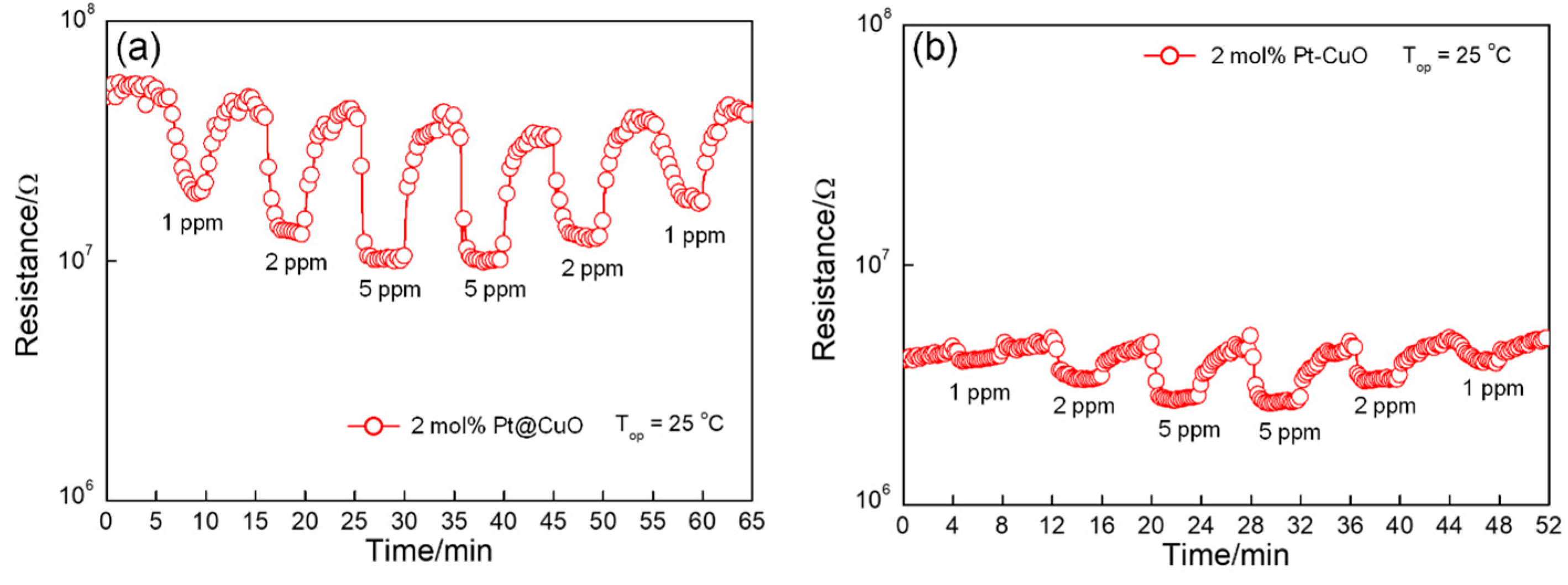
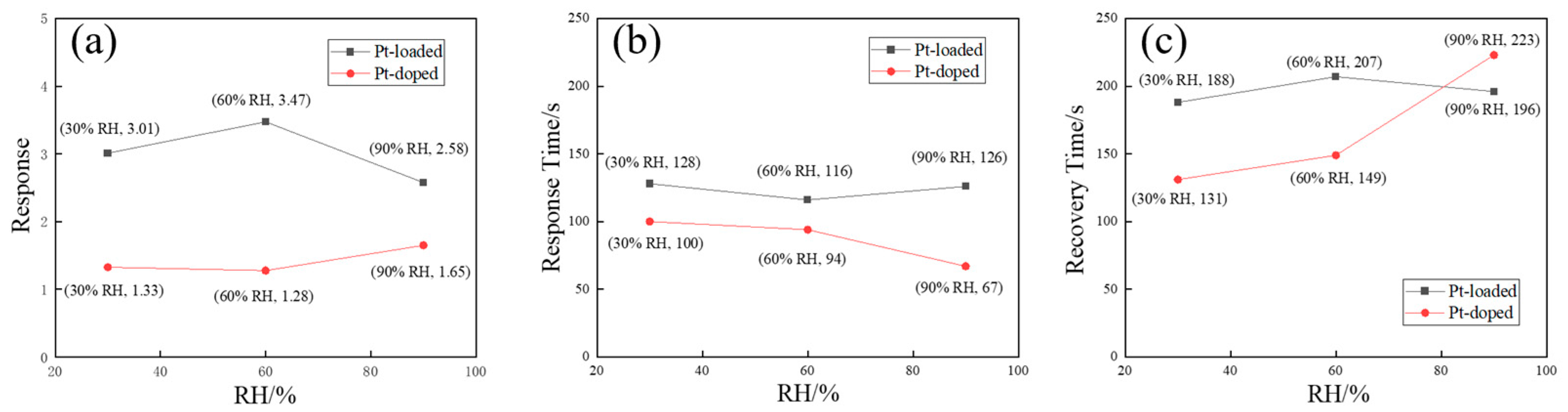
3.2. Gas-Sensing Properties
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, H.; Guo, H.; Feng, C.; Wang, J.; Liu, B.; Xie, Z.; Zheng, Y. Light-activated ultrasensitive NO2 gas sensor based on heterojunctions of CuO nanospheres/MoS2 nanosheets at room temperature. Sens. Actuators B Chem. 2022, 368, 132131. [Google Scholar] [CrossRef]
- Inaba, M.; Oda, T.; Kono, M.; Phansiri, N.; Morita, T.; Nakahara, S.; Nakano, M.; Suehiro, J. Effect of mixing ratio on NO2 gas sensor response with SnO2-decorated carbon nanotube channels fabricated by one-step dielectrophoretic assembly. Sens. Actuators B Chem. 2021, 344, 130257. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Peterson, P.J.; Aujla, A.; Grant, K.H.; Brundle, A.G.; Thompson, M.R.; Vande Hey, J.; Leigh, R.J. Practical use of metal oxide semiconductor gas sensors for measuring nitrogen dioxide and ozone in urban environments. Sensors 2017, 17, 1653. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.-S.; Li, C.; Li, Y.-N.; Zhang, X.-F.; Deng, Z.-P.; Cheng, X.-L.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Facilely controlled synthesis of porous ZnO nanotubes with rich oxygen vacancies for highly sensitive and selective detection of NO2 at low temperature. Sens. Actuators B Chem. 2023, 375, 132865. [Google Scholar] [CrossRef]
- Hingangavkar, G.M.; Navale, Y.H.; Nimbalkar, T.M.; Mulik, R.N.; Patil, V.B. Hydrothermally engineered WO3 nanoflowers: A selective detection towards toxic NO2 gas. Sens. Actuators B Chem. 2022, 371, 132584. [Google Scholar] [CrossRef]
- Patil, S.; Patil, V.; Vanalakar, S.; Shendage, S.; Pawar, S.; Kim, J.; Ryu, J.; Patil, D.R.; Patil, P. Porous In2O3 thick films as a low temperature NO2 gas detector. Mater. Lett. 2021, 306, 130916. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Karakocak, B.B.; Kavadiya, S.; Soundappan, T.; Biswas, P. A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sens. Actuators B Chem. 2018, 259, 745–752. [Google Scholar] [CrossRef]
- Ansari, H.R.; Kordrostami, Z.; Mirzaei, A. In-vehicle wireless driver breath alcohol detection system using a microheater integrated gas sensor based on Sn-doped CuO nanostructures. Sci. Rep. 2023, 13, 7136. [Google Scholar] [CrossRef]
- Güldüren, M.E.; İskenderoğlu, D.; Güney, H.; Gür, E.; Acar, M.; Karadeniz, S.M. Investigating the influence of Ni doping on the CuO thin films deposited via ultrasonic spray pyrolysis: Structural, optical and H2 gas sensing analyses. Int. J. Hydrogen Energy 2023, 48, 828–839. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, H.; Tao, T.; Xia, X.; Bao, Y.; Lourenço, M.; Gao, Y. A CuO/TiO2 Heterojunction based CO Sensor with High Response and Selectivity. Energy Environ. Mater. 2023, 6, e12570. [Google Scholar] [CrossRef]
- Navale, Y.; Navale, S.; Galluzzi, M.; Stadler, F.; Debnath, A.; Ramgir, N.; Gadkari, S.; Gupta, S.; Aswal, D.; Patil, V. Rapid synthesis strategy of CuO nanocubes for sensitive and selective detection of NO2. J. Alloys Compd. 2017, 708, 456–463. [Google Scholar] [CrossRef]
- Han, T.-H.; Bak, S.-Y.; Kim, S.; Lee, S.H.; Han, Y.-J.; Yi, M. Decoration of CuO NWs gas sensor with ZnO NPs for improving NO2 sensing characteristics. Sensor 2021, 21, 2103. [Google Scholar] [CrossRef]
- Nakate, U.T.; Lee, G.H.; Ahmad, R.; Patil, P.; Hahn, Y.-B.; Yu, Y.; Suh, E.-K. Nano-bitter gourd like structured CuO for enhanced hydrogen gas sensor application. Int. J. Hydrogen Energy 2018, 43, 22705–22714. [Google Scholar] [CrossRef]
- Nha, H.T.; Van Tong, P.; Van Duy, N.; Hung, C.M.; Hoa, N.D. Facile Synthesis of Pd-CuO Nanoplates with Enhanced SO2 and H2 Gas-Sensing Characteristics. J. Electron. Mater. 2021, 50, 2767–2778. [Google Scholar] [CrossRef]
- Matsushima, S.; Teraoka, Y.; Miura, N.; Yamazoe, N. Electronic Interaction between Metal Additives and Tin Dioxide in Tin Dioxide-Based Gas Sensors. Jpn. J. Appl. Phys. 1988, 27, 1798–1802. [Google Scholar] [CrossRef]
- Yamazoe, N.J.S. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Morrison, S. Selectivity in semiconductor gas sensors. Sens. Actuators 1987, 12, 425–440. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal-oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Ouyang, Y.; Liu, T.; Chen, Y.; Song, Y.; Shen, Y. Fabrication of CuO nanorod loaded with a single gold nanoparticle for low-temperature NO2 detection. Mater. Lett. 2022, 328, 133115. [Google Scholar] [CrossRef]
- Lee, J.S.; Katoch, A.; Kim, J.H.; Kim, S.S. Effect of Au nanoparticle size on the gas-sensing performance of p-CuO nanowires. Sens. Actuators B Chem. 2016, 222, 307–314. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, H.K.; Park, H.J.; Ma, A.; Choi, S.Y.; Lee, D.S. Sonochemical synthesis of HKUST-1-based CuO decorated with Pt nanoparticles for formaldehyde gas-sensor applications. Sens. Actuators B Chem. 2019, 292, 289–296. [Google Scholar] [CrossRef]
- Hübner, M.; Koziej, D.; Bauer, M.; Barsan, N.; Kvashnina, K.; Rossell, M.D.; Grunwaldt, J.D. The Structure and Behavior of Platinum in SnO2 Based Sensors under Operating Conditions. Angew. Chem. Int. Ed. 2011, 12, 2841–2844. [Google Scholar] [CrossRef]
- Koziej, D.; Hübner, M.; Barsan, N.; Weimar, U.; Sikora, M.; Grunwaldt, J.-D. Operando X-ray absorption spectroscopy studies on Pd-SnO2 based sensors. Phys. Chem. Chem. Phys. 2009, 11, 8620–8625. [Google Scholar] [CrossRef]
- Varma, G.D. Compounds, Enhanced room temperature sensitivity of Ag-CuO nanobrick/reduced graphene oxide composite for NO2. J. Alloys Compd. 2019, 806, 1469–1480. [Google Scholar]
- Devi, L.V.; Selvalakshmi, T.; Sellaiyan, S.; Uedono, A.; Sivaji, K.; Sankar, S. Effect of La doping on the lattice defects and photoluminescence properties of CuO. J. Alloys Compd. 2017, 709, 496–504. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures. Appl. Catal. B Environ. 2014, 162, 122–132. [Google Scholar] [CrossRef]
- Khasevani, S.G.; Taheri, M.; Gholami, M.R. Green synthesis of Ag–Pt bimetallic nanoparticles supported on the Metal–Organic framework (MOF)–Derived metal oxides (γ-Fe2O3/CuO) nanocomposite as a reusable heterogeneous nanocatalyst and nanophotocatalyst. Mater. Chem. Phys. 2021, 261, 124218. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, X.-B.; He, M.; Xie, L.-L.; Zhu, Z.-G.; Wu, J.-Q. Effect of platinum doping on the morphology and sensing performance for CuO-based gas sensor. Appl. Sci. 2018, 8, 1091. [Google Scholar] [CrossRef]
- Cheng, Z.; Feng, B.; Chen, Z.; Zheng, J.; Li, J.; Zuo, S. La2O3 modified silica-pillared clays supported PtOx nanocrystalline catalysts for catalytic combustion of benzene. Chem. Eng. J. 2020, 392, 123747. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Chen, L.; Xu, J.; Ou, J.; Ye, M. Porous styryl-linked polyhedral oligomeric silsesquioxane (POSS) polymers used as a support for platinum catalysts. Mater. Chem. Front. 2019, 3, 851–859. [Google Scholar]
- Li, J.; Wang, X.; Liu, W.; Li, X.; Yang, L.; Ma, H.; Wu, R.; Wei, Q. Highly selective electrochemiluminescence aptasensor coupled with mesoporous Fe3O4@ Cu@ Cu2O as co-reaction accelerator for ATP assay based on target-triggered emitter release. Sens. Actuators B Chem. 2021, 346, 130581. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez-Salido, I.; Kim, Y.D. Size selectivity for CO-oxidation of Ag nanoparticles on highly ordered pyrolytic graphite (HOPG). Surf. Sci. 2005, 598, 96–103. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Van Dao, D.; Ha, N.T.T.; Van Tran, T.; Kim, D.-S.; Yoon, J.-W.; Yu, Y.T. Superhigh sensing response and selectivity for hydrogen gas using PdPt@ ZnO core-shell nanoparticles: Unique effect of alloyed ingredient from experimental and theoretical investigations. Sens. Actuators B Chem. 2022, 354, 131083. [Google Scholar] [CrossRef]
- Gupta, V.; Jain, A.; Maheshwari, G.; Lang, H.; Ishtaiwi, Z.J.S. Copper (II)-selective potentiometric sensors based on porphyrins in PVC matrix. Sens. Actuators B Chem. 2006, 117, 99–106. [Google Scholar] [CrossRef]
- Wu, R.; Tian, L.; Li, H.; Liu, H.; Luo, J.; Tian, X.; Hua, Z.; Wu, Y.; Fan, S. A selective methane gas sensor based on metal oxide semiconductor equipped with an on-chip microfilter. Sens. Actuators B Chem. 2022, 359, 131557. [Google Scholar] [CrossRef]
- Li, T.; Shen, Y.; Zhong, X.; Zhao, S.; Li, G.; Cui, B.; Wei, K. Effect of noble metal element on microstructure and NO2 sensing properties of WO3 nanoplates prepared from a low-grade scheelite concentrate. J. Alloys Compd. 2020, 818, 152927. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Zhong, X.; Han, C.; Zhao, Q.; Wei, D. Design of Au@ WO3 core−shell structured nanospheres for ppb-level NO2 sensing. Sens. Actuators B Chem. 2019, 282, 917–926. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, L.; Zou, Y.; Li, Y.; Zhang, H.; Lu, Q.; Sun, M.; Yin, G.; Zhou, A. Room-temperature gas sensor based on in situ grown, etched and W-doped ZnO nanotubes functionalized with Pt nanoparticles for the detection of low-concentration H2S. J. Alloys Compd. 2022, 922, 166158. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The key role of active sites in the development of selective metal oxide sensor materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Cui, X.; Gao, Y.; Ma, J.; Sun, Y.; Sun, P.; Liu, F.; Liang, X.; Zhang, T.; et al. NH3 gas sensing performance enhanced by Pt-loaded on mesoporous WO3. Sens. Actuators B Chem. 2017, 238, 473–481. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, C.; Zheng, L.; Zheng, W.; Liu, X.; Kumar, M.; Zhang, J. Highly sensitive and selective detection of acetone based on platinum sensitized porous tungsten oxide nanospheres. Sens. Actuators B Chem. 2020, 307, 127616. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.H.; Lee, G.; Kim, J.; Seong, J.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous sensitization of metal–organic framework driven metal@ metal oxide complex catalysts on an oxide nanofiber scaffold toward superior gas sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, Y.; Zhang, W.; Zhang, J.; Wei, D.; Lu, R.; Zhu, L.; Li, H.; Shen, Y. In-situ growth of ZnO nanowire arrays on the sensing electrode via a facile hydrothermal route for high-performance NO2 sensor. Appl. Surf. Sci. 2017, 435, 1096–1104. [Google Scholar] [CrossRef]
- Wu, J.; Yin, B.; Wu, F.; Myung, Y.; Banerjee, P. Charge transport in single CuO nanowires. Appl. Phys. Lett. 2014, 105, 183506. [Google Scholar] [CrossRef]
- Mahmood, K.; Abbasi, S.; Zahra, R.; Rehman, U. Investigation of large Seebeck effect by charge mobility engineering in CuAlO2 thin films grown on Si substrate by thermal evaporation. Ceram. Int. 2018, 44, 17905–17908. [Google Scholar] [CrossRef]
- Schwarze, M.; Gaul, C.; Scholz, R.; Bussolotti, F.; Hofacker, A.; Schellhammer, K.S.; Leo, K. Molecular parameters responsible for thermally activated transport in doped organic semiconductors. Nat. Mater. 2019, 18, 242–248. [Google Scholar] [CrossRef]
- Choi, S.W.; Katoch, A.; Kim, J.H.; Kim, S.S. A novel approach to improving oxidizing-gas sensing ability of p-CuO nanowires using biased radial modulation of a hole-accumulation layer. J. Mater. Chem. C 2014, 2, 8911–8917. [Google Scholar] [CrossRef]
- Yoosefian, M.; Zahedi, M.; Mola, A.; Naserian, S. A DFT comparative study of single and double SO2 adsorption on Pt-doped and Au-doped single-walled carbon nanotube. Appl. Surf. Sci. 2015, 349, 864–869. [Google Scholar] [CrossRef]
- Demir, S.; Fellah, M.F. A DFT study on Pt doped (4,0) SWCNT: CO adsorption and sensing. Appl. Surf. Sci. 2019, 504, 144141. [Google Scholar] [CrossRef]
- Huda, M.N.; Yan, Y.; Moon, C.Y.; Wei, S.H.; Al-Jassim, M.M. Density-functional theory study of the effects of atomic impurity on the band edges of monoclinic WO3. Phys. Rev. B 2008, 77, 195102. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.H.; Lee, H.M.; Kim, T.; Choi, J.H.; Seo, D.K.; Yoo, J.-B.; Hong, S.-H.; Kang, T.J.; Kim, Y.H. Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens. Actuators B Chem. 2012, 166, 172–176. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhao, S.; Zhong, X.; Li, T.; Han, C.; Wei, D.; Meng, D. NO2 sensing properties of one-pot-synthesized ZnO nanowires with Pd functionalization. Sens. Actuators B Chem. 2019, 280, 151–161. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhong, X.; Li, G.; Han, C.; Wei, D.; Li, S. Bimetallic Au/Pd nanoparticles decorated ZnO nanowires for NO2 detection. Sens. Actuators B Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef]


| Sensing Material | Operating Temperature/°C | Concentration/ppm | Response | Response Time/s | Recovery Time/s |
|---|---|---|---|---|---|
| Au@CuO nanorods [21] | 100 | 5 | 4.3 | - | - |
| Ag-CuO nanobricks [26] | 22 | 20 | 67.2 | 35 | 900 |
| Pd-WO3 nanoplates [38] | 150 | 5 | 283.96 | 26 | 66 |
| Au@WO3 core–shell nanospheres [39] | 100 | 5 | 88 | 4 | 59 |
| Pt-CuO nanorods in this work | 25 | 2 | 1.33 | 125 | 188 |
| Pt@CuO nanorods in this work | 25 | 2 | 3.01 | 100 | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, T.; Ouyang, Y.; Huang, S.; Zhang, Z.; Liu, F.; Qiu, L.; Wang, C.; Lin, X.; Chen, J.; et al. Influence of Different Pt Functionalization Modes on the Properties of CuO Gas-Sensing Materials. Sensors 2024, 24, 120. https://doi.org/10.3390/s24010120
Chen X, Liu T, Ouyang Y, Huang S, Zhang Z, Liu F, Qiu L, Wang C, Lin X, Chen J, et al. Influence of Different Pt Functionalization Modes on the Properties of CuO Gas-Sensing Materials. Sensors. 2024; 24(1):120. https://doi.org/10.3390/s24010120
Chicago/Turabian StyleChen, Xiangxiang, Tianhao Liu, Yunfei Ouyang, Shiyi Huang, Zhaoyang Zhang, Fangzheng Liu, Lu Qiu, Chicheng Wang, Xincheng Lin, Junyan Chen, and et al. 2024. "Influence of Different Pt Functionalization Modes on the Properties of CuO Gas-Sensing Materials" Sensors 24, no. 1: 120. https://doi.org/10.3390/s24010120





