Frequency Response of a Six-Electrode MET Sensor at Extremely Low Temperatures
Abstract
:1. Introduction
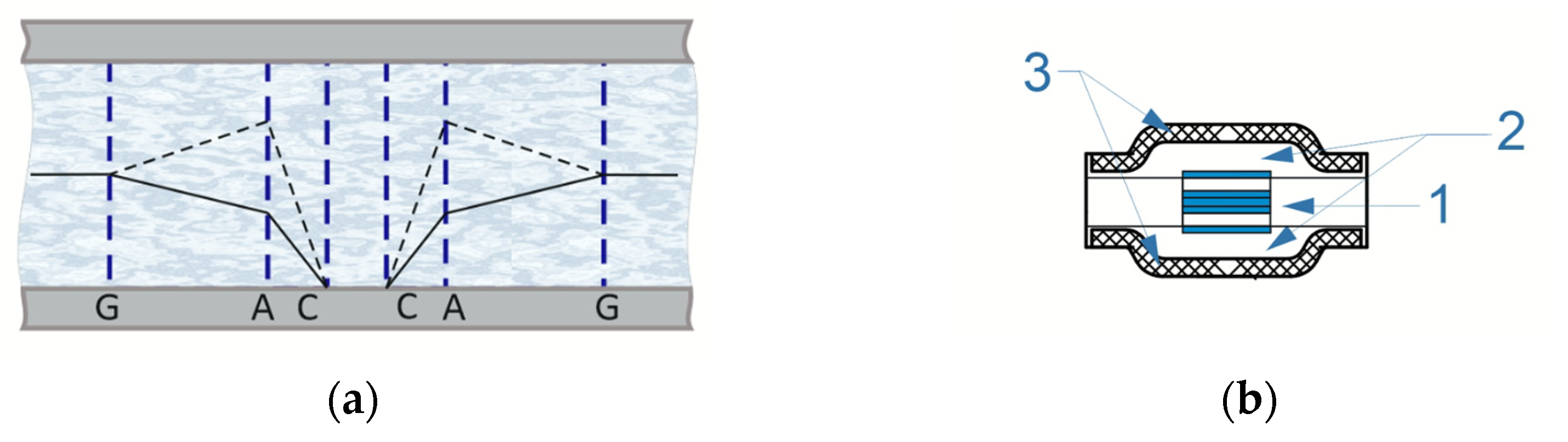
2. Materials and Methods
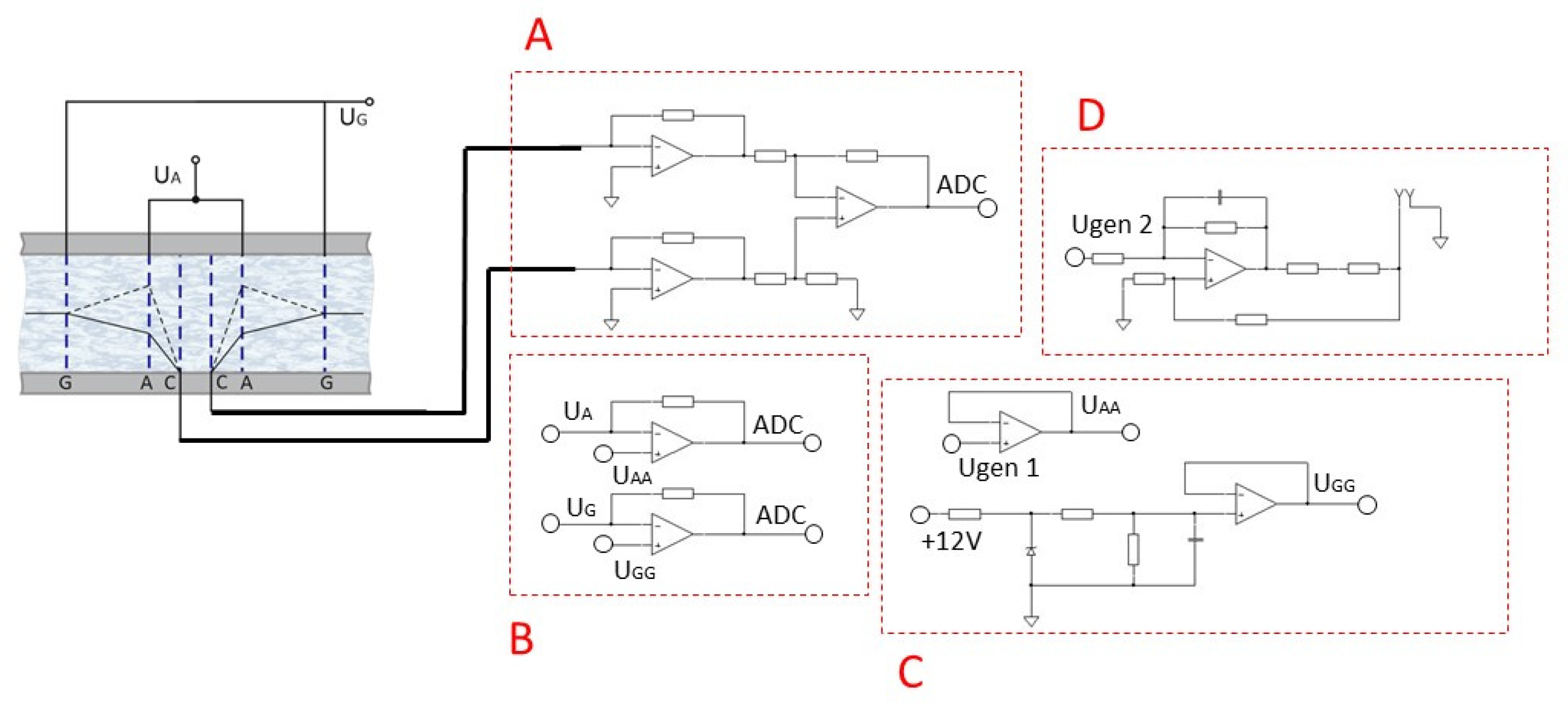
- Block A: converter of the current taken from the cathodes (C) into voltage, which is further digitized using ADC NI USB-6215 and recorded on a PC for further processing;
- Block B: converter of the current taken from the control anodes and anodes into voltage, which is further digitized using ADC NI USB-6215 and recorded on a PC to control the operation of the stand;
- Block C: a circuit that provides constant reference voltage at the anodes A using the built-in NI USB-6215 oscillator, and at the anodes G using resistors. When the four-electrode configuration is enabled, the anodes G are physically disabled.
- The potential was changed automatically using the built-in NI USB-6215 generator in the range of 40–380 mV. The operating mode settling time was 20 min, the data acquisition time was 20 min. The potential was changed manually using resistors.
- Block A: current-to-voltage converter, which is further digitized using ADC NI USB-6215 and recorded on a PC for further processing. The amplitude of the differential cathode current is chosen as the output signal;
- Block B: converter of the current taken from the anodes A and the anodes G into voltage, which is further digitized using ADC NI USB-6215 No. 2 and recorded on a PC to control the operation of the stand;
- Block C: a circuit that provides the required potential value at the anodes A using the built-in NI USB-6215 generator No. 1, and at the anodes G using resistors. When the four-electrode configuration is enabled, the anodes G are physically disabled;
- Block D: a circuit that converts the output signal from the DAC (digital to analog converter) NI USB-6215 No. 2 into current, which is fed to the excitation coil [14,15,16], which interacts with a permanent magnet mounted on the membrane and provides the setting of the external motion on the electrochemical cell.
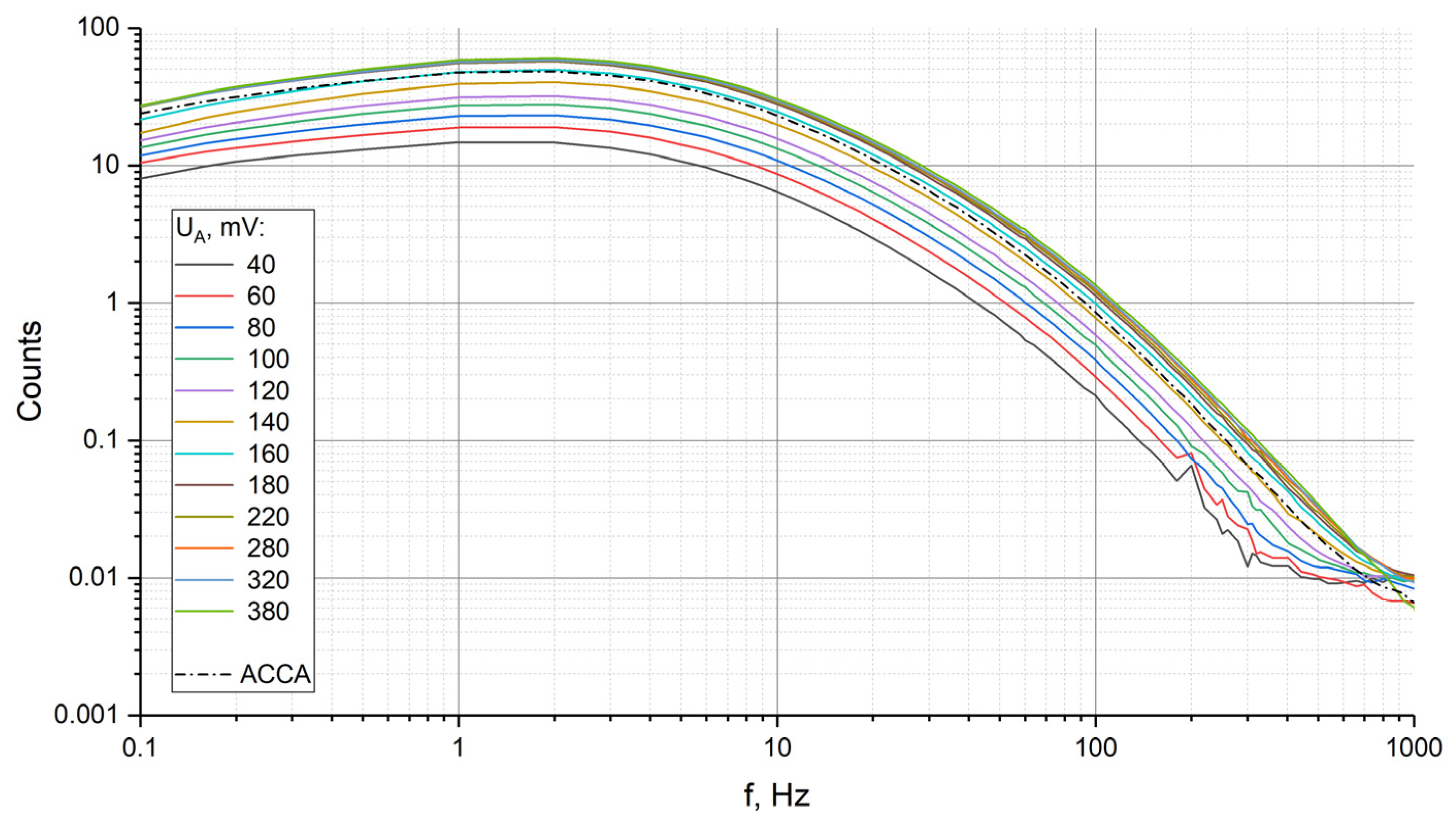
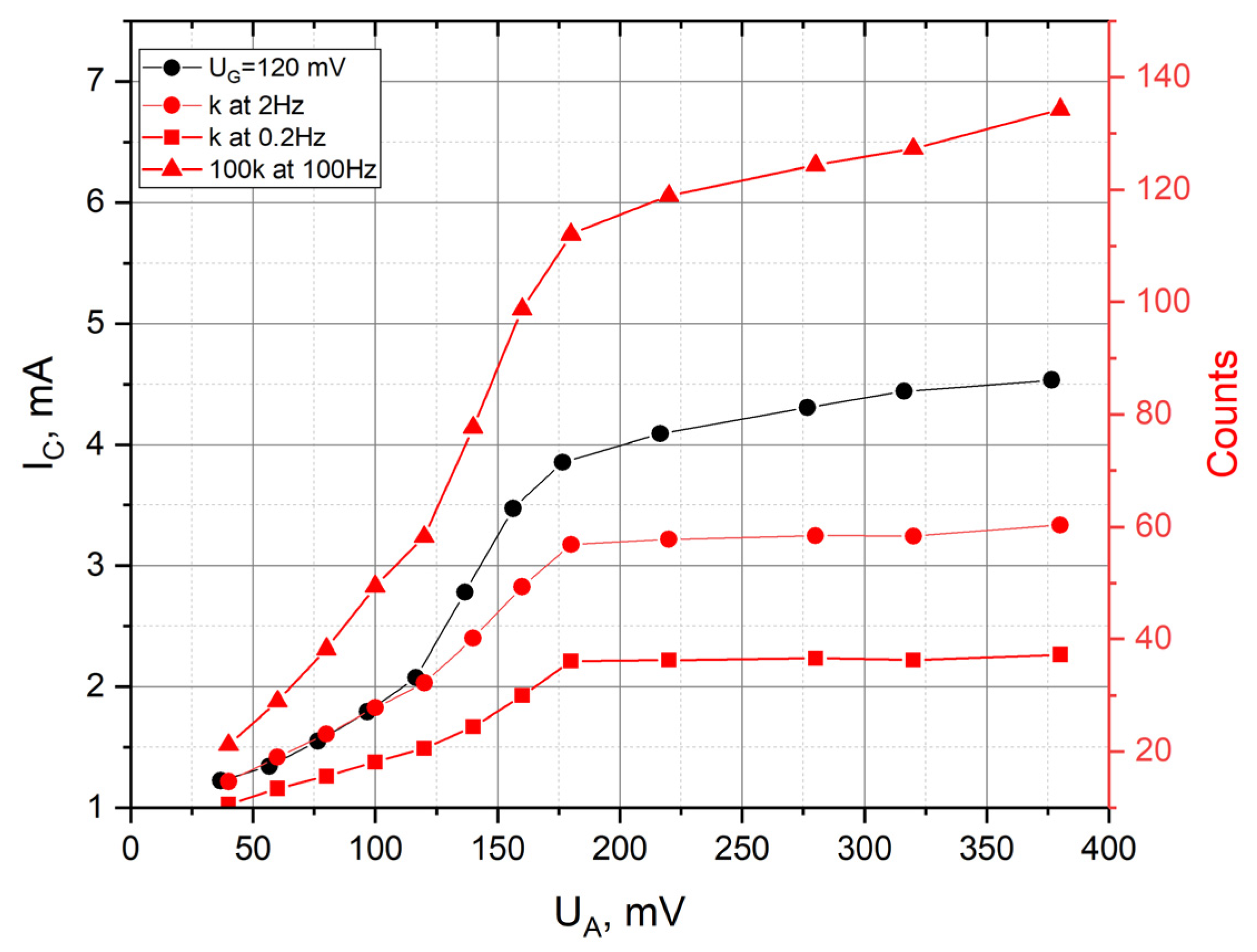
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Presnov, D.A.; Sobisevich, A.L.; Gruzdev, P.D.; Ignatiev, V.I.; Kon’kov, A.I.; Moreev, A.Y.; Tarasov, A.V.; Shuvalov, A.A.; Shurup, A.S. Tomographic Estimation of Waterbody Parameters in the Presence of Ice Cover Using Seismoacoustic Sources. Acoust. Phys. 2019, 65, 593–602. [Google Scholar] [CrossRef]
- Kostylev, D.V.; Bogomolov, L.M.; Boginskaya, N.V. About seismic observations on Sakhalin with the use of molecular-electronic seismic sensors of new type. Sept. 2019 IOP Conf. Ser. Earth Environ. Sci. 2019, 324, 012009. [Google Scholar] [CrossRef]
- Gorbenko, V.I.; Zhostkov, R.A.; Likhodeev, D.V.; Presnov, D.A.; Sobisevich, A.L. Feasibility of using molecular-electronic seismometers in passive seismic prospecting: Deep structure of the Kaluga ring structure from microseismic sounding. Seism. Instruments 2017, 53, 181–191. [Google Scholar] [CrossRef]
- Lavan, O.; de Stefano, M. Seismic Behaviour and Design of Irregular and Complex Civil Structures; Springer: Berlin/Heidelberg, Germany, 2013; Volume 24. [Google Scholar]
- Krylov, A.A.; Egorov, I.V.; Kovachev, S.A.; Ilinskiy, D.A.; Ganzha, O.Y.; Timashkevich, G.K.; Roginskiy, K.A.; Kulikov, M.E.; Novikov, M.A.; Ivanov, V.N.; et al. Ocean-Bottom Seismographs Based on Broadband MET Sensors: Architecture and Deployment Case Study in the Arctic. Sensors 2021, 21, 3979. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, D. Hardware complex for monitoring changes in the state and development of hazardous processes in natural and man-made systems based on molecular electronic technology. In Proceedings of the 22nd International Multidisciplinary Scientific Geo Conference SGEM 2022, Albena, Bulgaria, 2–11 July 2022; Volume 22, Issue 1.1, pp. 533–540. [Google Scholar]
- Zaitsev, D.; Bryksin, V.; Belotelov, K.; Kompaniets, Y.; Iakovlev, R. Algorithms and Measuring Complex for Classification of Seismic Signal Sources, Determination of Distance and Azimuth to the Point of Excitation of Surface Waves. Inform. Autom. 2022, 21, 1211–1239. [Google Scholar]
- Bugaev, A.S.; Antonov, A.; Belotelov, K.; Vergeles, S.; Dudkin, P.; Egorov, E.; Egorov, I.; Zhevnenko, D.; Zhabina, D.; Zaitsev, D.; et al. Measuring Devices Based on Molecular-Electronic Transducers. J. Commun. Technol. Electron. 2018, 63, 1339–1351. [Google Scholar] [CrossRef]
- Egorov, I.V.; Shabalina, A.S.; Agafonov, V.M. Design and Self-Noise of MET Closed-Loop Seismic Accelerometers. IEEE Sens. J. 2017, 17, 2008–2014. [Google Scholar] [CrossRef]
- Antonov, A.; Shabalina, A.; Razin, A.; Avdyukhina, S.; Egorov, I.; Agafonov, V. Low-frequency seismic node based on molecular-electronic transfer sensors for marine and transition zone exploration. J. Atmos. Ocean. Technol. 2017, 34, 1743–1748. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Chen, D.; Chen, J.; Liu, B.; Qi, W.; Zheng, X.; Wei, H.; Zhang, G. The Electrochemical Seismometer Based on a Novel Designed Sensing Electrode for Undersea Exploration. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems and Eurosensors XXXIII, TRANSDUCERS 2019 and EUROSENSORS XXXIII, Berlin, Germany, 23–27 June 2019; pp. 2053–2056. [Google Scholar]
- Deng, T.; Sun, Z.; Li, G.; Chen, J.; Chen, D.; Wang, J. Microelectromechanical system-based electrochemical seismic sensors with an anode and a cathode integrated on one chip. J. Micromech. Microeng. 2017, 27, 025004. [Google Scholar] [CrossRef]
- Chikishev, D.A.; Zaitsev, D.L.; Belotelov, K.S.; Egorov, I.V. The Temperature Dependence of Amplitude- Frequency Response of the MET Sensor of Linear Motion in a Broad Frequency Range. IEEE Sens. J. 2019, 19, 9653–9661. [Google Scholar] [CrossRef]
- Zaitsev, D.; Egorov, I.; Agafonov, V. A Comparative Study of Aqueous and Non-Aqueous Solvents to Be Used in Low-Temperature Serial Molecular– Electronic Sensors. Chemosensors 2022, 10, 111. [Google Scholar] [CrossRef]
- Nickerson, S.D.; Nofen, E.M.; Chen, H.; Ngan, M.; Shindel, B.; Yu, H.; Dai, L.L. A Combined Experimental and Molecular Dynamics Study of Iodide-Based Ionic Liquid and Water Mixtures. J. Phys. Chem. B 2015, 119, 8764–8772. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, D.L.; Agafonov, V.M.; Bugaev, A.S. A Model of Temperature Dependence of the Amplitude-Frequency Response of a Molecular Electronic Sensor Based on a Water-Alcohol Solvent. In Proceedings of the 2022 4th International Conference on Control Systems, Mathematical Modeling, Automation and Energy Efficiency (SUMMA), Lipetsk, Russia, 9–11 November 2022; pp. 97–102. [Google Scholar]
- Krishtop, V.G. Experimental modeling of the temperature dependence of the transfer function of rotational motion sensors based on electrochemical transducers. Russ. J. Electrochem. 2014, 50, 350–354. [Google Scholar] [CrossRef]
- Zaitsev, D.L.; Dudkin, P.V.; Krishtop, T.V.; Neeshpapa, A.V.; Popov, V.G.; Uskov, V.V.; Krishtop, V.G. Experimental Studies of Temperature Dependence of Transfer Function of Molecular Electronic Transducers at High Frequencies. IEEE Sens. J. 2016, 16, 7864–7869. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Chen, D.; Chen, J.; Qi, W.; Liu, B.; Liang, T.; She, X. Temperature compensation of the mems-based electrochemical seismic sensors. Micromachines 2021, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, J.; Chen, D.; Chen, J.; Chen, L.; Xu, C. An electrochemical, low-frequency seismic micro-sensor based on MEMS with a force-balanced feedback system. Sensors 2017, 17, 2103. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, J.; Chen, D.; Sun, Z.; Chen, L.; Chen, J. A force-balanced negative feedback method for MEMS based electrochemical seismic sensor. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017. [Google Scholar]
- Liang, T.; Wang, J.; Chen, D.; Liu, B.; She, X.; Xu, C.; Qi, W.; Agafonov, V.; Egorov, E.; Chen, J. A MEMS-Based Electrochemical Angular Accelerometer with a Force-Balanced Negative Feedback. IEEE Sens. J. 2021, 21, 15972–15978. [Google Scholar] [CrossRef]
- Agafonov, V.; Egorov, I.; Akinina, A. MET Sensor with a Sensitivity Controlled by Electrical Signals. In Proceedings of the 12th International Advances in Applied Physics & Materials Science Congress & Exhibition (APMAS 2022), Oludeniz, Turkey, 13–19 October 2022; p. 87. [Google Scholar]
- Sun, Z.; Agafonov, V.; Egorov, E. The influence of the boundary condition on anodes for solution of convection-diffusion equation with the application to a four-electrode electrochemical cell. J. Electroanal. Chem. 2011, 661, 157–161. [Google Scholar] [CrossRef]
- Bugaev, A.; Agafonova, V.; Egorov, I.; Agafonova, E.; Avdyukhina, S. Influence of the Dielectric Coating of the Outer Side of the Cathode in the Anode–Cathode Pairs of a Molecular Electronic Sensitive Element on the Conversion Coefficient. Micromachines 2022, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Havskov, J.; Alguacil, G. Instrumentation in Earthquake Seismology; Modern Approaches in Geophysics; Springer: New York, NY, USA, 2010; Volume 22, pp. 11–76. [Google Scholar]




| Hz | |
|---|---|
| 0.2 | 3.98 |
| 2 | 3.41 |
| 100 | 6.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agafonov, V.; Egorov, I.; Akinina, A. Frequency Response of a Six-Electrode MET Sensor at Extremely Low Temperatures. Sensors 2023, 23, 4311. https://doi.org/10.3390/s23094311
Agafonov V, Egorov I, Akinina A. Frequency Response of a Six-Electrode MET Sensor at Extremely Low Temperatures. Sensors. 2023; 23(9):4311. https://doi.org/10.3390/s23094311
Chicago/Turabian StyleAgafonov, Vadim, Ivan Egorov, and Anna Akinina. 2023. "Frequency Response of a Six-Electrode MET Sensor at Extremely Low Temperatures" Sensors 23, no. 9: 4311. https://doi.org/10.3390/s23094311






