Plasmonic Nanostructure Biosensors: A Review
Abstract
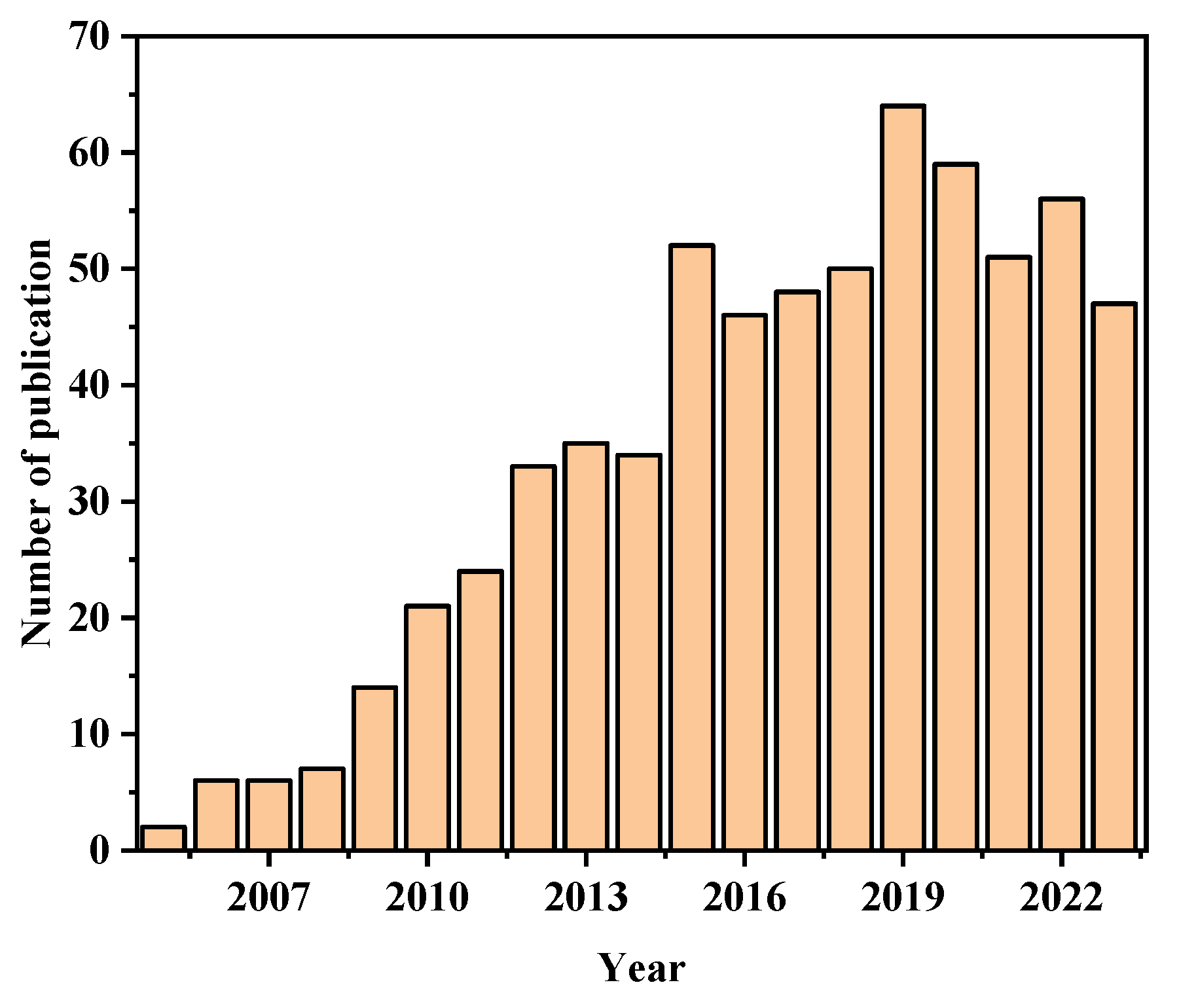
:1. Introduction
2. Basic Principles of Surface Plasmon
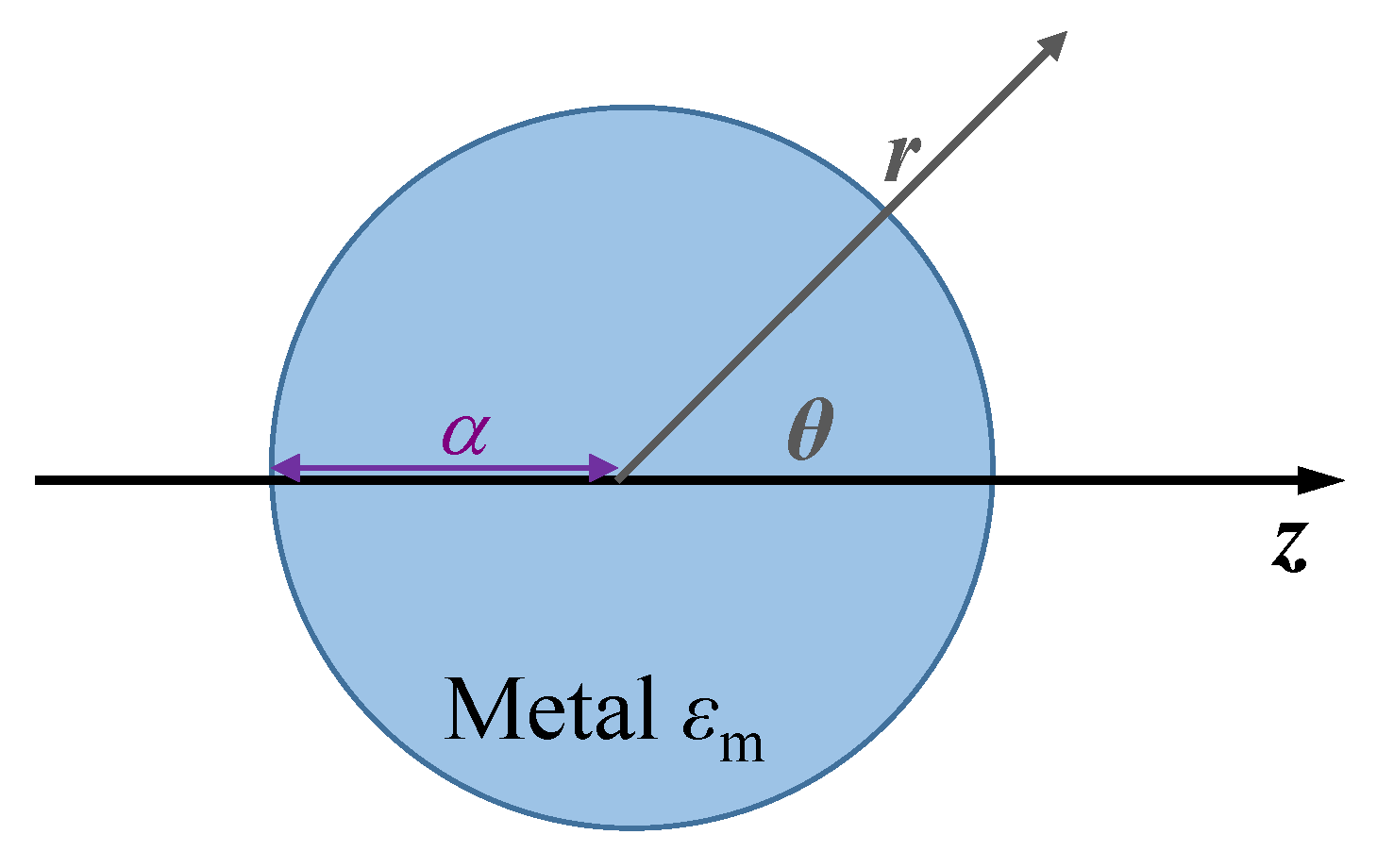
2.1. Physics of LSPR
2.2. Physics of PSPP
3. Performance Characteristics of Plasmonic Bioensors
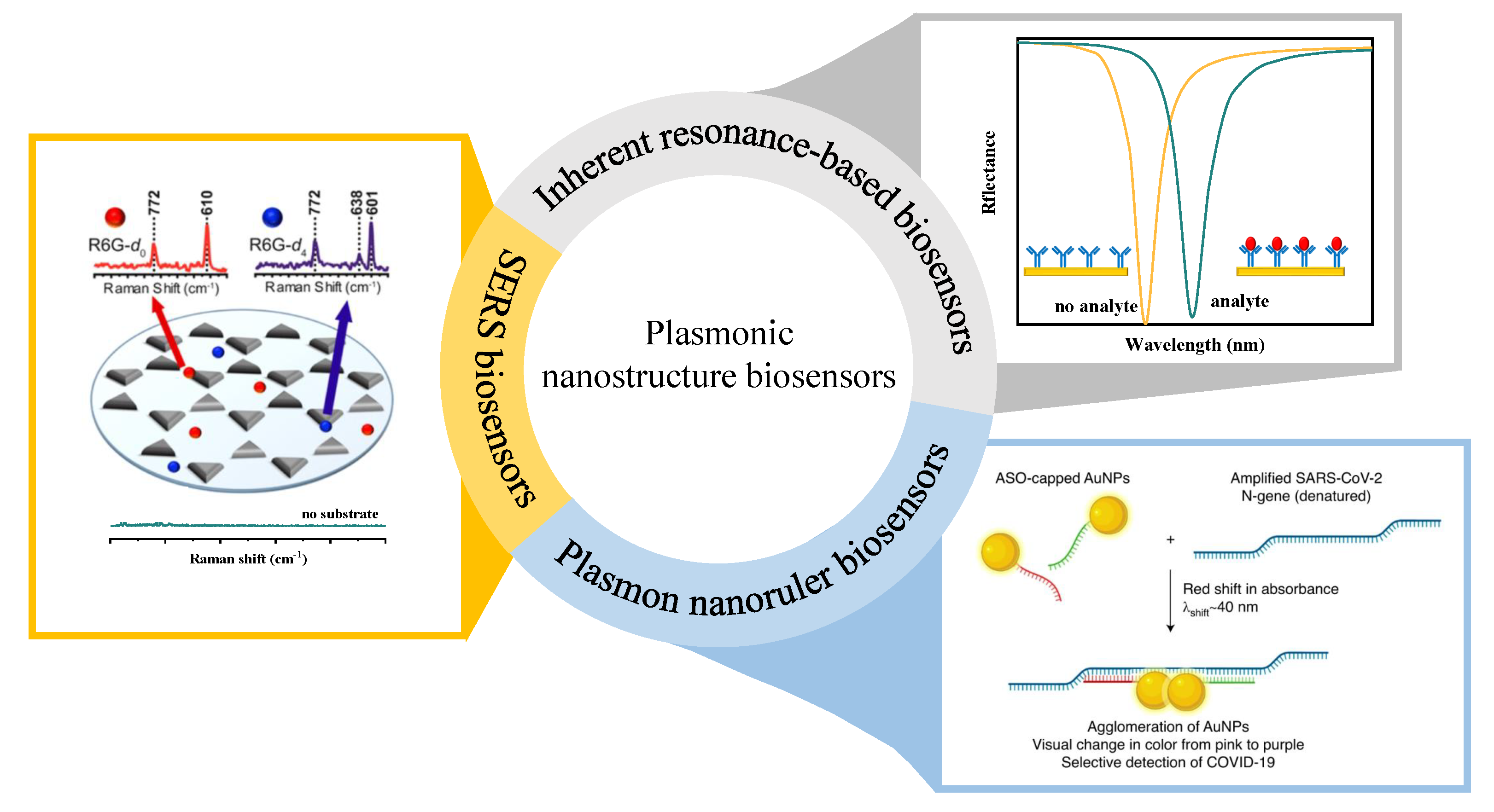
4. Inherent Resonance-Based Biosensing
4.1. Localized Plasmonic Eigenmode Biosensors
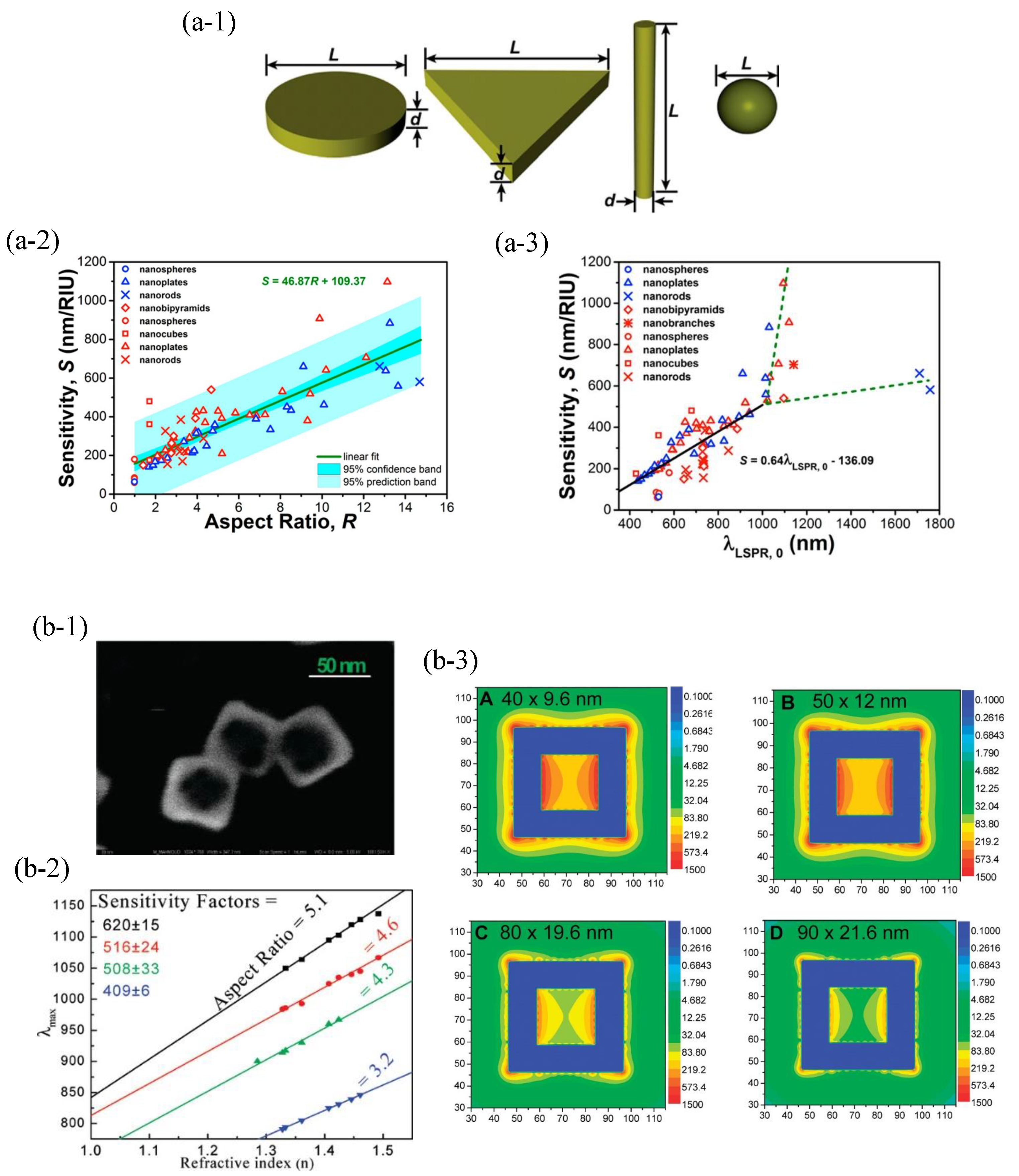
4.1.1. Metal Nanoparticle-Based LSPR Biosensors
4.1.2. Fano Resonance-Based Biosensors
4.2. Propagating Plasmonic Eigenwaves Biosensors
4.2.1. Prism-Coupled Mechanism
4.2.2. Grating-Coupled Mechanism
4.3. Coupled Propagating-Localized Plasmonic Biosensors
5. Plasmon Nanorulers Biosensing
5.1. 2D Plasmon Nanoruler Biosensors
5.2. 3D Plasmon Nanoruler Sensors
6. SERS Biosensing
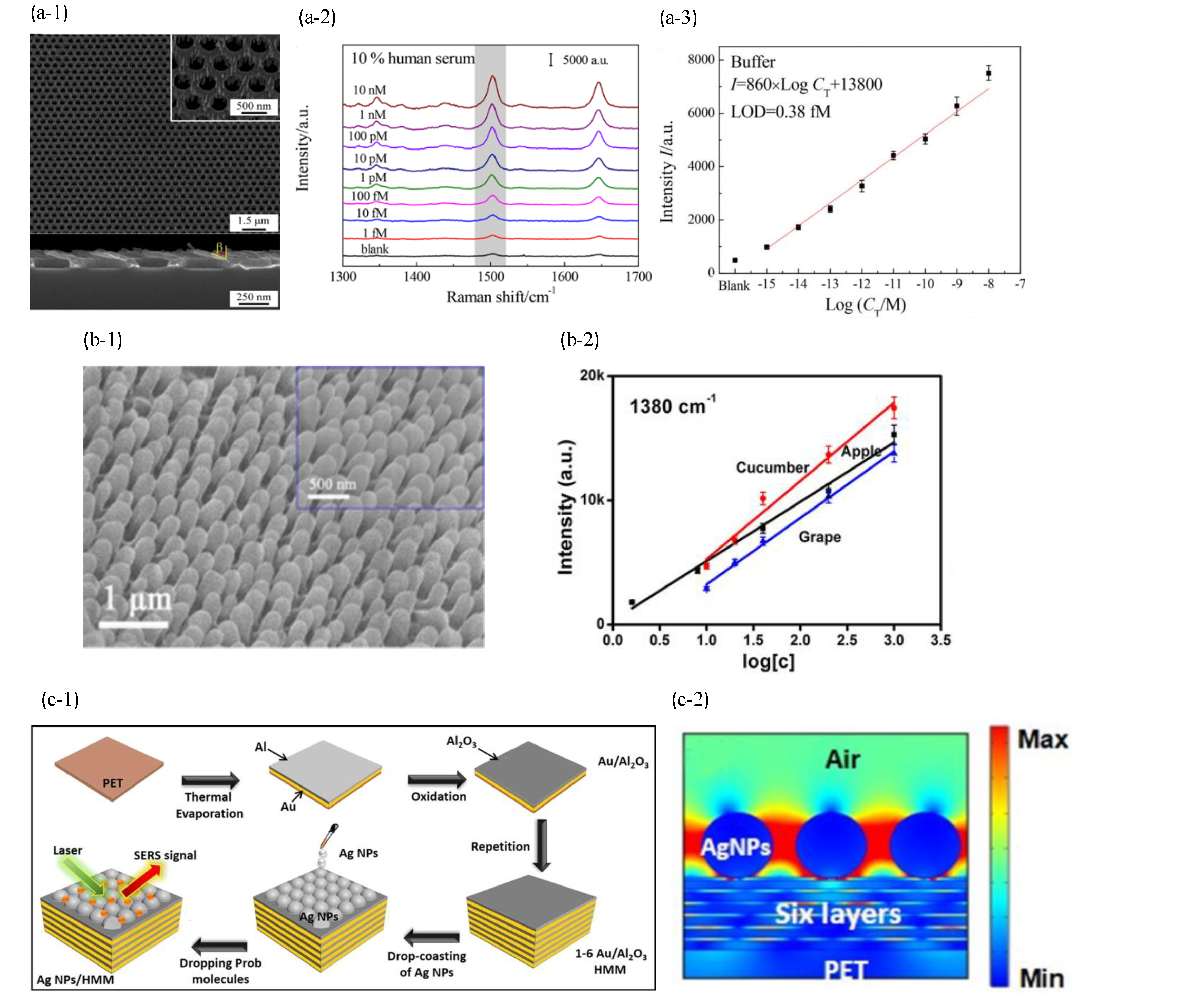
6.1. Metal Nanoparticle-Based SERS Biosensors
6.2. Plasmonic Template-Based SERS Biosensors
7. Advanced Application
7.1. Metal Nanoparticles Based Biosensors
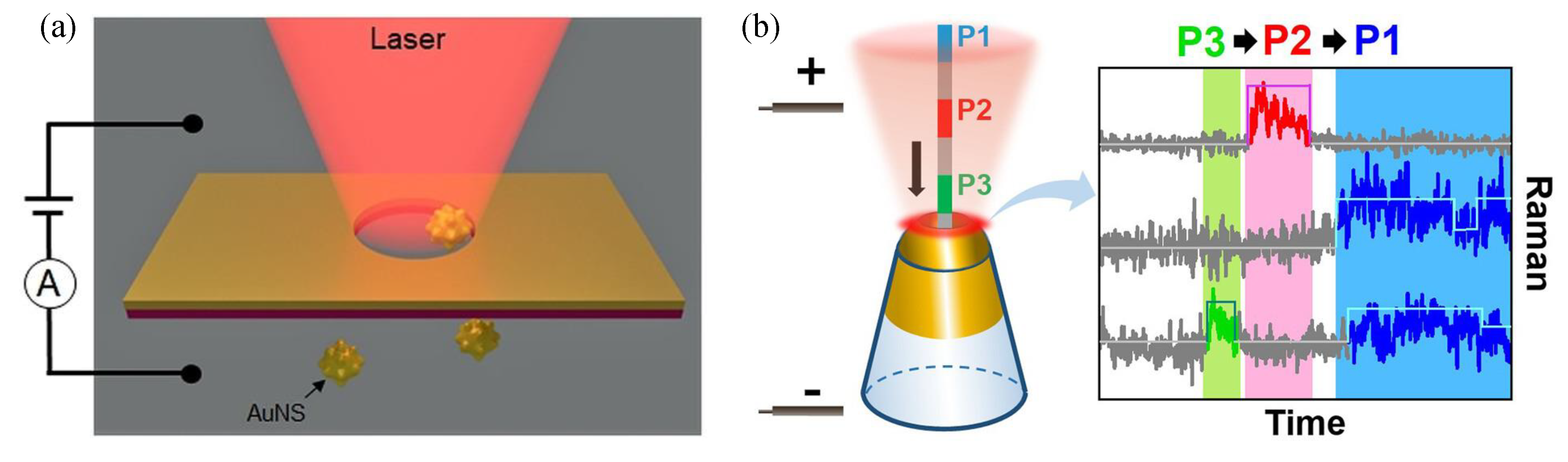
7.2. Nanopores Based Biosensors
8. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nanostructure | FWHM | Sensitivity | FOM | (Detected Analytes) |
|---|---|---|---|---|
| capped gold nanoslits array [43] | 3.68 nm | 926 nm/RIU | 252 | - (BSA/antiBSA) |
| capped gold nanoslits array [42] | - | - | - | g/mL (LMP1) |
| optical disc-based metasurfaces [40] | ∼10 nm | 1475 nm/RIU | 147 | 25 μg/mL (protein G) copies/μL (gamma ray SARS-CoV-2) |
| array of narrow slit and nanohole pairs [41] | ∼13 nm | 1200 nm/RIU | 92 | - |
| Au grating [38] | 10 nm | - | - | - (IgG) |
| metal-insulator-metal [37] | 0.09 deg | ∼86.76 deg/RIU | 964 ± 150 | 30 nM (Bovine Serum Albumin)) |
| Split-trench [39] | 10 nm | 687 nm/RIU | - | 10 pM (bovine serum albumin) |
| Nanocrosses [44] | 200 nm | 1000 nm/RIU | 5 | - |
| Nanocrosses [45] | ∼9 nm | 1015 nm/RIU | 108 | 200 pM (cytochrome c) 15 ng/mL (alpha-fetoprotein) |
| Nanostructure | Resonant Wavelength (nm) | Sensitivity | (Detected Analytes) |
|---|---|---|---|
| gold film [217] | - | 31,400 nm/RIU | - |
| gold or silver film/ [47] | 650 | 68 deg/RIU | 57.2 nM (CT) |
| gold film/Si/HMM array [46] | 1155 | 43,000 nm/RIU | - |
| gold film/gold nanoparticles decorated [50] | - | - | 0.5 fM (miRNA) |
| gold film/Si/ [124] | 1024 | deg/RIU | - |
| gold film/graphene [48] | - | - | 285 nM (kanamycin residues) |
| gold film/(GO(+)/GO(-) pair) [128] | - | 150.38 ± 0.314 deg/RIU | - |
| nanorods array [52] | 1200 | 32,000 nm/RIU | 300 nM (biotin) |
| nanaorod HMMs [9] | 1200 | 41,600 nm/RIU | 2.4 nM (streptavidin) |
| platinum-di-selenide/gold film [218] | - | 140.35 deg/RIU | 1.95 nM (COVID-19) |
| gold film [219] | - | - | 0.5 pg/mL (Ebola virus) |
| /Ag//graphene [220] | 633 | 194 deg/RIU | 0.0001 nM (COVID-19 SARS-CoV-2) |
| gold film [221] | - | mV/RIU | 0.08 pg/mL (COVID-19 spike antigen detection) |
| gold film/Dithiobis (Succinimidyl Undecanoate)/NH2rGO- PAMAM [222] | - | 0.2576 deg/pM | 0.08 pM (dengue virus type 2 E-proteins) |
| ZnO/gold film [223] | ∼640 | 2825 nm/RIU | 2% (alcohol) |
| //silver film [224] | 633 | 194 deg/RIU | - |
| Nanostructure | Resonant Wavelength (nm) | Sensitivity | (Detected Analytes) |
|---|---|---|---|
| Au-covered gratings [58] | 1060 | 900 nm/RIU | - |
| crossed surface relief gratings [59] | - | 647.8 nm/RIU | 8.3 nM (streptavidin) |
| Au grating/(Au/ multilayer HMM) [53] | 1250 | 30,000 nm/RIU | 10 pM (biotin) |
| Au grating/(Au/ multilayer HMM) [54] | 1250 | 7000 deg/RIU | 1 fM (cowpea mosaic virus) |
| Nanostructure | Sensitivity (nm/nm) | (Detected Analytes) |
|---|---|---|
| nanosphere dimer [65] | - | 10 pM (target DNA) |
| nanosphere solution [78] | - | 10 copies/μL (coronavirus) |
| nanoparticles solution [165] | - | 50 pM (DNA) |
| silver film/space layer/microring resonator [72] | 14.8 | - |
| Au nanohole/space layer/gold film [73] | 61 | 11.9 pg/mL (procalcitonin) |
| Nanostructure | Excitation Wavelength (nm) | Wavenumber (/cm) | (Detected Analytes) | |
|---|---|---|---|---|
| Au-decorated Ag nanorod arrays [83] | 785 | - | - | 0.1 fM (R6G) |
| Au nanostars [84] | 532 | 1374.9 | 1.51 mg/L (CV) | |
| AgNPs-modified graphene nanoribbons [85] | 532 | 1576 | - | |
| Fe3O4@Au magnetic nanoparticles [86] | 785 | 1331 | 0.01 ng/mL (CRP) | |
| Ag NR-NH array [87] | 633 | 1500 | 0.7 fM (DNA) | |
| Spiky Au@Ag core-shell nanoparticles [88] | 785 | 559 | 70 nM (Thiram) | |
| Ag triangular nanopyramids [89] | 532 | 610 | - | |
| AgNPs based on HMMs [90] | 532 | 710 | 1 pM (R6G,CV) | |
| Hexagonal gold nanoparticles array [91] | 785 | 736 | 0.8 pM (IL-6) |
References
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Akimov, A.; Mukherjee, A.; Yu, C.; Chang, D.; Zibrov, A.; Hemmer, P.; Park, H.; Lukin, M. Generation of single optical plasmons in metallic nanowires coupled to quantum dots. Nature 2007, 450, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ferry, V.E.; Sweatlock, L.A.; Pacifici, D.; Atwater, H.A. Plasmonic nanostructure design for efficient light coupling into solar cells. Nano Lett. 2008, 8, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.; Hohenau, A.; Ditlbacher, H.; Galler, N.; Reil, F.; Aussenegg, F.; Leitner, A.; List, E.; Krenn, J. Organic plasmon-emitting diode. Nat. Photonics 2008, 2, 684–687. [Google Scholar] [CrossRef]
- DiChristina, M.; Meyerson, B.S. The Top 10 Emerging Technologies of 2018. Available online: https://www.scientificamerican.com/article/the-top-10-emerging-technologies-of-2018/ (accessed on 14 September 2018).
- Meja-Salazar, J.; Oliveira, O.N., Jr. Plasmonic biosensing: Focus review. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Špačková, B.; Wrobel, P.; Bocková, M.; Homola, J. Optical biosensors based on plasmonic nanostructures: A review. Proc. IEEE 2016, 104, 2380–2408. [Google Scholar] [CrossRef]
- Sugumaran, S.; Jamlos, M.F.; Ahmad, M.N.; Bellan, C.S.; Schreurs, D. Nanostructured materials with plasmonic nanobiosensors for early cancer detection: A past and future prospect. Biosens. Bioelectron. 2018, 100, 361–373. [Google Scholar] [CrossRef]
- Yan, R.; Wang, T.; Yue, X.; Wang, H.; Zhang, Y.H.; Xu, P.; Wang, L.; Wang, Y.; Zhang, J. Highly sensitive plasmonic nanorod hyperbolic metamaterial biosensor. Photonics Res. 2022, 10, 84–95. [Google Scholar] [CrossRef]
- Gao, M.; Yang, W.; Wang, Z.; Lin, S.; Zhu, J.; Yang, Z. Plasmonic resonance-linewidth shrinkage to boost biosensing. Photonics Res. 2020, 8, 1226–1235. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Montemurro, D.; Eramo, C.; Di Ronza, S.; Perri, C.; D’agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. Biochemical sensing exploiting plasmonic sensors based on gold nanogratings and polymer optical fibers. Photonics Res. 2021, 9, 1397–1408. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Trinh, K.T.L.; Lee, J.H.; Yoon, W.J.; Ju, H. Reproducible Enhancement of Fluorescence by Bimetal Mediated Surface Plasmon Coupled Emission for Highly Sensitive Quantitative Diagnosis of Double-Stranded DNA. Small 2018, 14, 1801385. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Ali, M.M.; Su, H.M.; Filipe, C.D.; Didar, T.F. Sentinel wraps: Real-time monitoring of food contamination by printing DNAzyme probes on food packaging. ACS Nano 2018, 12, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Lin, J.S.; Tian, X.D.; Li, G.; Zhang, F.L.; Wang, Y.; Li, J.F. Advanced plasmonic technologies for multi-scale biomedical imaging. Chem. Soc. Rev. 2022, 51, 9445–9468. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Reinhard, B.M.; Liphardt, J.; Alivisatos, A.P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 2005, 23, 741–745. [Google Scholar] [CrossRef]
- Liu, G.L.; Yin, Y.; Kunchakarra, S.; Mukherjee, B.; Gerion, D.; Jett, S.D.; Bear, D.G.; Gray, J.W.; Alivisatos, A.P.; Lee, L.P.; et al. A nanoplasmonic molecular ruler for measuring nuclease activity and DNA footprinting. Nat. Nanotechnol. 2006, 1, 47–52. [Google Scholar] [CrossRef]
- Stryer, L. Fluorescence energy transfer as a spectroscopic ruler. Annu. Rev. Biochem. 1978, 47, 819–846. [Google Scholar] [CrossRef]
- Fan, J.A.; Wu, C.; Bao, K.; Bao, J.; Bardhan, R.; Halas, N.J.; Manoharan, V.N.; Nordlander, P.; Shvets, G.; Capasso, F. Self-assembled plasmonic nanoparticle clusters. Science 2010, 328, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.; Saliba, M.; Vogelgesang, R.; Giessen, H.; Alivisatos, A.P.; Liu, N. Transition from isolated to collective modes in plasmonic oligomers. Nano Lett. 2010, 10, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, J.B.; Sobhani, H.; Fan, J.A.; Kundu, J.; Capasso, F.; Nordlander, P.; Halas, N.J. Fano resonances in plasmonic nanoclusters: Geometrical and chemical tunability. Nano Lett. 2010, 10, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Payton, J.L.; Morton, S.M.; Moore, J.E.; Jensen, L. A hybrid atomistic electrodynamics–quantum mechanical approach for simulating surface-enhanced Raman scattering. Accounts Chem. Res. 2014, 47, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhu, T.; Liu, Z. Approaching the electromagnetic mechanism of surface-enhanced Raman scattering: From self-assembled arrays to individual gold nanoparticles. Chem. Soc. Rev. 2011, 40, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Xu, T.; Geng, Z. Strategies to improve performances of LSPR biosensing: Structure, materials, and interface modification. Biosens. Bioelectron. 2021, 174, 112850. [Google Scholar] [CrossRef]
- Khan, A.U.; Zhao, S.; Liu, G. Key parameter controlling the sensitivity of plasmonic metal nanoparticles: Aspect ratio. J. Phys. Chem. C 2016, 120, 19353–19364. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Increased sensitivity of surface plasmon resonance of gold nanoshells compared to that of gold solid colloids in response to environmental changes. Anal. Chem. 2002, 74, 5297–5305. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; El-Sayed, M.A. Gold nanoframes: Very high surface plasmon fields and excellent near-infrared sensors. J. Am. Chem. Soc. 2010, 132, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Charles, D.E.; Ledwith, D.M.; Aherne, D.; Cunningham, S.; Voisin, M.; Blau, W.J.; Gun’ko, Y.K.; Kelly, J.M.; Brennan-Fournet, M.E. Wash-free highly sensitive detection of C-reactive protein using gold derivatised triangular silver nanoplates. RSC Adv. 2014, 4, 29022–29031. [Google Scholar] [CrossRef]
- Tian, L.; Liu, K.K.; Morrissey, J.J.; Gandra, N.; Kharasch, E.D.; Singamaneni, S. Gold nanocages with built-in artificial antibodies for label-free plasmonic biosensing. J. Mater. Chem. B 2014, 2, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lin, Y.; Yan, J.; Di, J. Synthesis of hollow gold nanoparticles on the surface of indium tin oxide glass and their application for plasmonic biosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; El-Sayed, M.A. Substrate effect on the plasmonic sensing ability of hollow nanoparticles of different shapes. J. Phys. Chem. B 2013, 117, 4468–4477. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhang, Y.; Liang, X.; Liu, C.; Wang, C.; Kang, T.; Lu, H.; Zhang, L.; Zhou, P.; Wang, X.; et al. Ultrahigh figure-of-merit in metal–insulator–metal magnetoplasmonic sensors using low loss magneto-optical oxide thin films. ACS Photonics 2017, 4, 1403–1412. [Google Scholar] [CrossRef]
- Xiao, B.; Kogo, G.; Rutherford, G.N.; Bahoura, M. Plasmonic pixel biosensor based on grazing angle illumination and computational imaging. IEEE Sens. J. 2019, 19, 7313–7318. [Google Scholar] [CrossRef]
- Yoo, D.; Barik, A.; de León-Pérez, F.; Mohr, D.A.; Pelton, M.; Martín-Moreno, L.; Oh, S.H. Plasmonic split-trench resonator for trapping and sensing. ACS Nano 2021, 15, 6669–6677. [Google Scholar] [CrossRef]
- Ahmed, R.; Guimarães, C.F.; Wang, J.; Soto, F.; Karim, A.H.; Zhang, Z.; Reis, R.L.; Akin, D.; Paulmurugan, R.; Demirci, U. Large-Scale functionalized metasurface-based SARS-CoV-2 detection and quantification. ACS Nano 2022, 16, 15946–15958. [Google Scholar] [CrossRef]
- Huang, W.X.; Guo, J.J.; Wang, M.S.; Zhao, G.R. Sensor based on Fano resonances of plane metamaterial with narrow slits. Phys. Lett. A 2017, 381, 909–912. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Chang, R.; Huang, Y.Y.; Juan, P.H.; Tahara, H.; Lee, K.Y.; Tsai, M.H.; Wei, P.K.; Sheen, H.J.; Fan, Y.J.; et al. Continuous polymerase chain reaction microfluidics integrated with a gold-capped nanoslit sensing chip for Epstein-Barr virus detection. Biosens. Bioelectron. 2022, 195, 113672. [Google Scholar] [CrossRef]
- Lee, K.L.; Huang, J.B.; Chang, J.W.; Wu, S.H.; Wei, P.K. Ultrasensitive biosensors using enhanced Fano resonances in capped gold nanoslit arrays. Sci. Rep. 2015, 5, 8547. [Google Scholar] [CrossRef] [PubMed]
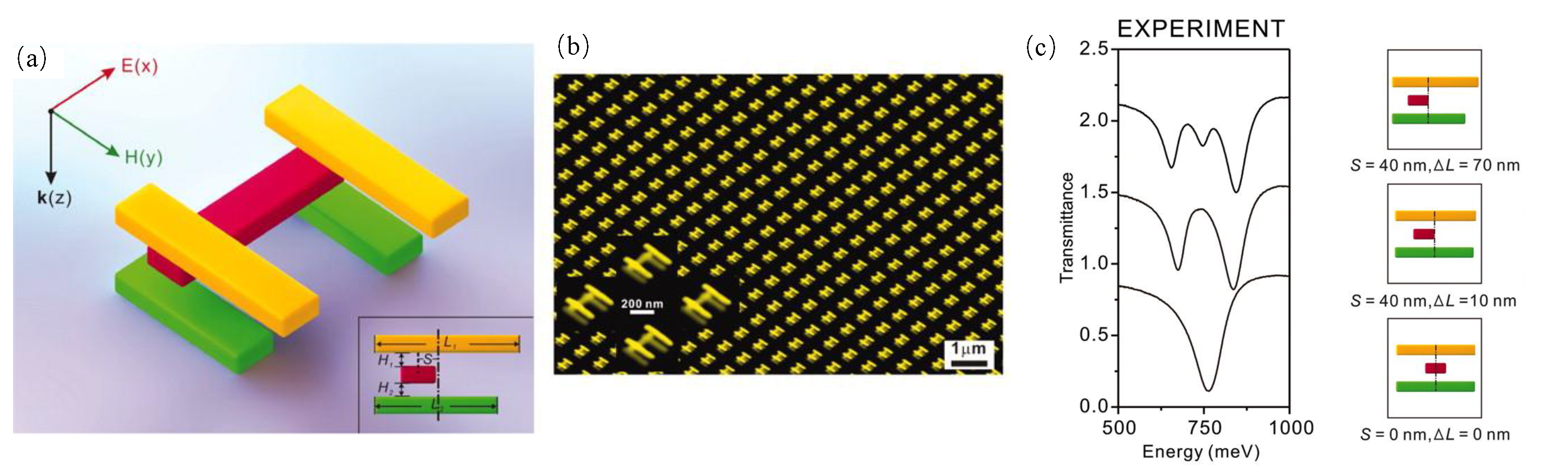
- Verellen, N.; Van Dorpe, P.; Huang, C.; Lodewijks, K.; Vandenbosch, G.A.; Lagae, L.; Moshchalkov, V.V. Plasmon line shaping using nanocrosses for high sensitivity localized surface plasmon resonance sensing. Nano Lett. 2011, 11, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.K.; Wang, X.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Yan, R.; Yue, X.; Wang, L.; Wang, Y.; Zhang, J.; Wang, J. Coupling plasmon-waveguide resonance and multiple plasma modes in hyperbolic metamaterials for high-performance sensing. Nanotechnology 2022, 33, 465203. [Google Scholar] [CrossRef]
- Abbas, A.; Linman, M.J.; Cheng, Q. Sensitivity comparison of surface plasmon resonance and plasmon-waveguide resonance biosensors. Sens. Actuators B Chem. 2011, 156, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Écija-Arenas, Á.; Kirchner, E.M.; Hirsch, T.; Fernández-Romero, J.M. Development of an aptamer-based SPR-biosensor for the determination of kanamycin residues in foods. Anal. Chim. Acta 2021, 1169, 338631. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Wang, Y.H.; Chiu, N.F. Exploring graphene and MoS2 chips based surface plasmon resonance biosensors for diagnostic applications. Front. Chem. 2020, 8, 728. [Google Scholar] [CrossRef]
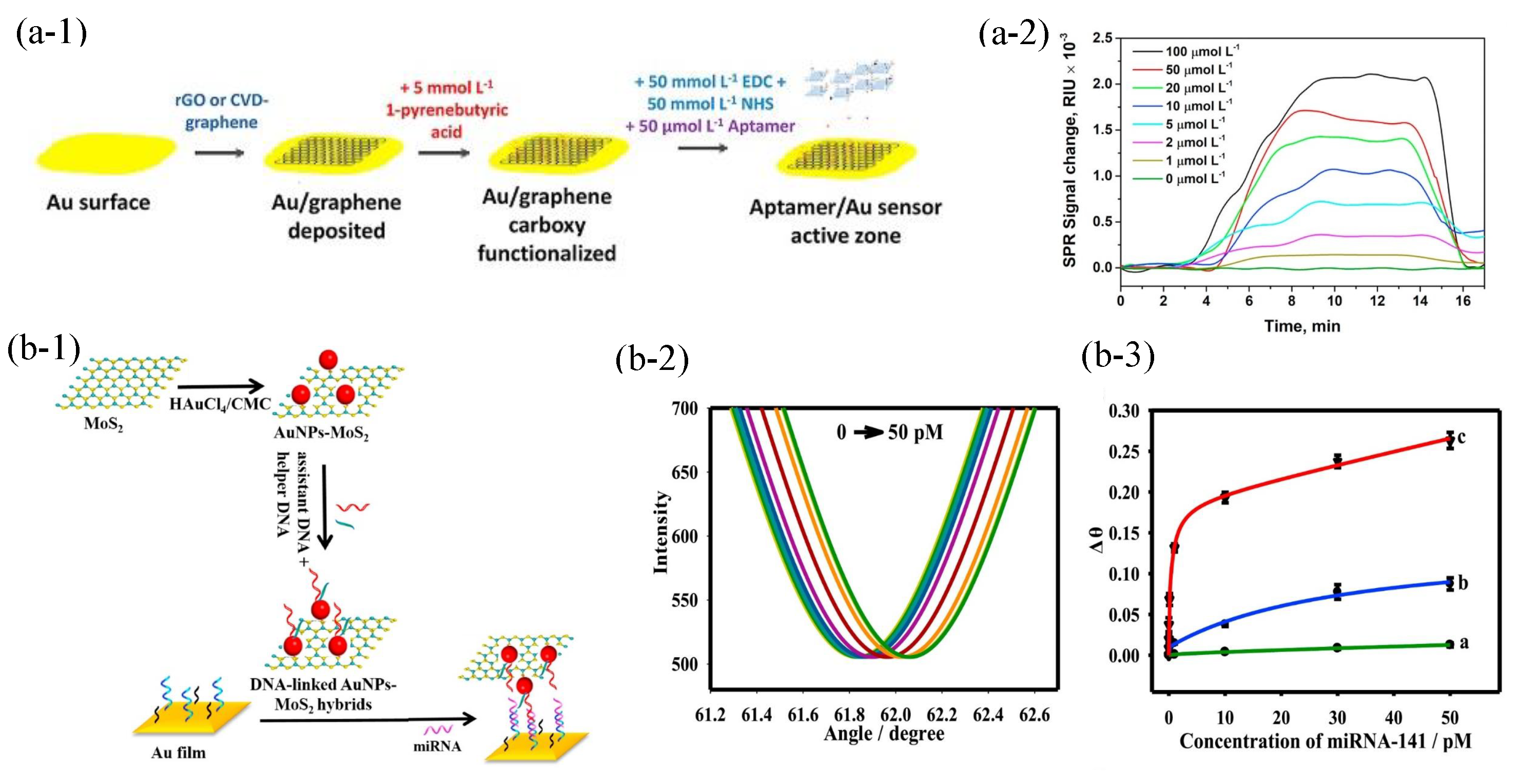
- Nie, W.; Wang, Q.; Yang, X.; Zhang, H.; Li, Z.; Gao, L.; Zheng, Y.; Liu, X.; Wang, K. High sensitivity surface plasmon resonance biosensor for detection of microRNA based on gold nanoparticles-decorated molybdenum sulfide. Anal. Chim. Acta 2017, 993, 55–62. [Google Scholar] [CrossRef]
- Huo, P.; Zhang, S.; Liang, Y.; Lu, Y.; Xu, T. Hyperbolic metamaterials and metasurfaces: Fundamentals and applications. Adv. Opt. Mater. 2019, 7, 1801616. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Wen, A.M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; Steinmetz, N.F.; Strangi, G. Enhancing the angular sensitivity of plasmonic sensors using hyperbolic metamaterials. Adv. Opt. Mater. 2016, 4, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zeng, S.; Xu, Z.; Ouyang, Q.; Zhang, D.H.; Chong, P.H.J.; Coquet, P.; He, S.; Yong, K.T. Multifunctional hyperbolic nanogroove metasurface for submolecular detection. Small 2017, 13, 1700600. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Mahalakshmi, P.; Han, S.; Mani Rajan, M.S.; Choudhury, P.K.; Singh, R. Brewster mode-enhanced sensing with hyperbolic metamaterial. Adv. Opt. Mater. 2019, 7, 1900680. [Google Scholar] [CrossRef]
- Palermo, G.; Sreekanth, K.V.; Maccaferri, N.; Lio, G.E.; Nicoletta, G.; De Angelis, F.; Hinczewski, M.; Strangi, G. Hyperbolic dispersion metasurfaces for molecular biosensing. Nanophotonics 2020, 10, 295–314. [Google Scholar] [CrossRef]
- Choi, B.; Dou, X.; Fang, Y.; Phillips, B.M.; Jiang, P. Outstanding surface plasmon resonance performance enabled by templated oxide gratings. Phys. Chem. Chem. Phys. 2016, 18, 26078–26087. [Google Scholar] [CrossRef]
- Nair, S.; Escobedo, C.; Sabat, R.G. Crossed surface relief gratings as nanoplasmonic biosensors. ACS Sens. 2017, 2, 379–385. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; ElKabbash, M.; Alapan, Y.; Ilker, E.I.; Hinczewski, M.; Gurkan, U.A.; Strangi, G. Hyperbolic metamaterials-based plasmonic biosensor for fluid biopsy with single molecule sensitivity. EPJ Appl. Metamat. 2017, 4, 4. [Google Scholar] [CrossRef]
- Špringer, T.; Chadtová Song, X.; Ermini, M.L.; Lamačová, J.; Homola, J. Functional gold nanoparticles for optical affinity biosensing. Anal. Bioanal. Chem. 2017, 409, 4087–4097. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Ma, P.; Zhang, D.; Li, S.; Wang, X.; Song, D. Gold nanostar-enhanced surface plasmon resonance biosensor based on carboxyl-functionalized graphene oxide. Anal. Chim. Acta 2016, 913, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Zhong, S.; Zhang, J.; Yan, R.; Xu, P.; Zhang, Y.h.; Yue, X.; Wang, L.; Wang, Y.; et al. Sensitivity investigation of a biosensor with resonant coupling of propagating surface plasmons to localized surface plasmons in the near infrared region. Nanoscale 2023, 15, 10826–10833. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.I.; Chen, Y.; Ginger, D.S. Plasmonic nanoparticle dimers for optical sensing of DNA in complex media. J. Am. Chem. Soc. 2010, 132, 9600–9601. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J. Am. Chem. Soc. 2003, 125, 6642–6643. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, M.S.; Mirkin, C.A. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 2006, 1, 246–252. [Google Scholar] [CrossRef]
- Famulok, M.; Hartig, J.S.; Mayer, G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem. Rev. 2007, 107, 3715–3743. [Google Scholar] [CrossRef]
- Ye, W.; Gotz, M.; Celiksoy, S.; Tuting, L.; Ratzke, C.; Prasad, J.; Ricken, J.; Wegner, S.V.; Ahijado-Guzman, R.; Hugel, T.; et al. Conformational dynamics of a single protein monitored for 24 h at video rate. Nano Lett. 2018, 18, 6633–6637. [Google Scholar] [CrossRef]
- Du, J.; Wang, J. Hybrid plasmonic microring nano-ruler. Sci. Rep. 2018, 8, 9219. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Zhu, S.; Ye, S.; Sun, W.; Yue, Y.; Tang, X.; Shi, J.; Xu, X.; Zhang, J.; Yang, B. Ultrahigh-Sensitivity Sandwiched Plasmon Ruler for Label-Free Clinical Diagnosis. Adv. Mater. 2020, 32, 1905927. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hentschel, M.; Weiss, T.; Alivisatos, A.P.; Giessen, H. Three-dimensional plasmon rulers. Science 2011, 332, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, B.M.; Siu, M.; Agarwal, H.; Alivisatos, A.P.; Liphardt, J. Calibration of dynamic molecular rulers based on plasmon coupling between gold nanoparticles. Nano Lett. 2005, 5, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Alafeef, M.; Moitra, P.; Dighe, K.; Pan, D. RNA-extraction-free nano-amplified colorimetric test for point-of-care clinical diagnosis of COVID-19. Nat. Protoc. 2021, 16, 3141–3162. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Tan, E.; Wong, J.; Nguyen, D.; Zhang, Y.; Erwin, B.; Van Ness, L.K.; Baker, S.M.; Galas, D.J.; Niemz, A. Isothermal DNA amplification coupled with DNA nanosphere-based colorimetric detection. Anal. Chem. 2005, 77, 7984–7992. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal amplification of nucleic acids: The race for the next “gold standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Xiao, S.; Qin, M.; Duan, J.; Wu, F.; Liu, T. Polarization-controlled dynamically switchable high-harmonic generation from all-dielectric metasurfaces governed by dual bound states in the continuum. Phys. Rev. B 2022, 105, 195440. [Google Scholar] [CrossRef]
- Dan, Y.; Zhong, C.; Zhu, H.; Wang, J. Highly ordered Au-decorated Ag nanorod arrays as an ultrasensitive and reusable substrate for surface enhanced Raman scattering. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 560, 360–365. [Google Scholar] [CrossRef]
- Bibikova, O.; Haas, J.; López-Lorente, A.I.; Popov, A.; Kinnunen, M.; Meglinski, I.; Mizaikoff, B. Towards enhanced optical sensor performance: SEIRA and SERS with plasmonic nanostars. Analyst 2017, 142, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Nong, J.; Tang, L.; Lan, G.; Luo, P.; Li, Z.; Huang, D.; Shen, J.; Wei, W. Combined Visible Plasmons of Ag Nanoparticles and Infrared Plasmons of Graphene Nanoribbons for High-Performance Surface-Enhanced Raman and Infrared Spectroscopies. Small 2021, 17, 2004640. [Google Scholar] [CrossRef]
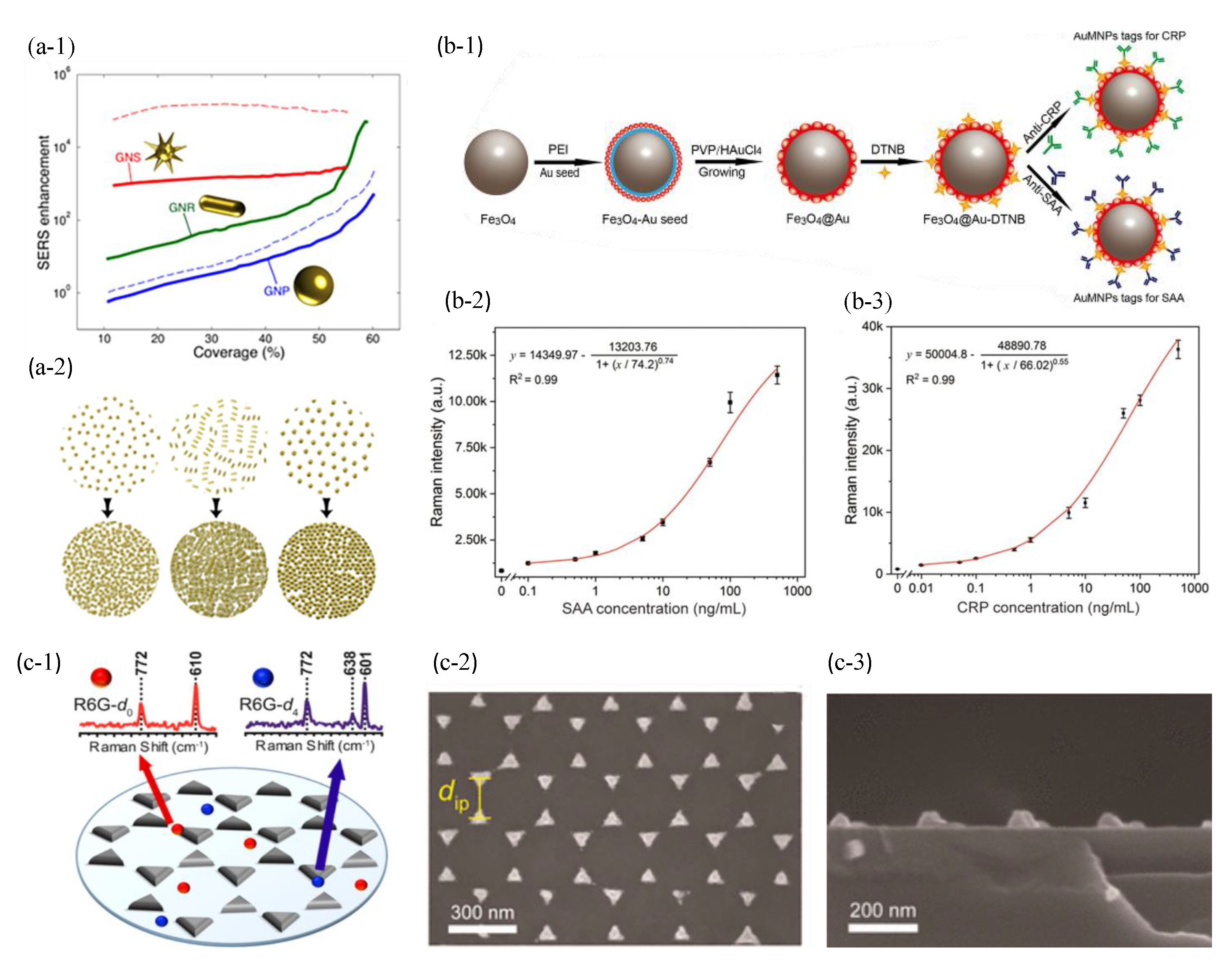
- Liu, X.; Yang, X.; Li, K.; Liu, H.; Xiao, R.; Wang, W.; Wang, C.; Wang, S. Fe3O4@ Au SERS tags-based lateral flow assay for simultaneous detection of serum amyloid A and C-reactive protein in unprocessed blood sample. Sens. Actuators B Chem. 2020, 320, 128350. [Google Scholar] [CrossRef]
- Song, C.; Jiang, X.; Yang, Y.; Zhang, J.; Larson, S.; Zhao, Y.; Wang, L. High-sensitive assay of nucleic acid using tetrahedral DNA probes and DNA concatamers with a surface-enhanced Raman scattering/surface plasmon resonance dual-mode biosensor based on a silver nanorod-covered silver nanohole array. ACS Appl. Mater. Interfaces 2020, 12, 31242–31254. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Meng, G.; Hu, X.; Pan, Q.; Huo, D.; Zhou, H.; Ke, Y.; Wu, N. Plasmon-tunable Au@ Ag core-shell spiky nanoparticles for surface-enhanced Raman scattering. Nano Res. 2019, 12, 449–455. [Google Scholar] [CrossRef]
- Zrimsek, A.B.; Henry, A.I.; Van Duyne, R.P. Single molecule surface-enhanced Raman spectroscopy without nanogaps. J. Phys. Chem. Lett. 2013, 4, 3206–3210. [Google Scholar] [CrossRef]
- Shafi, M.; Liu, R.; Zha, Z.; Li, C.; Du, X.; Wali, S.; Jiang, S.; Man, B.; Liu, M. Highly efficient SERS substrates with different Ag interparticle nanogaps based on hyperbolic metamaterials. Appl. Surf. Sci. 2021, 555, 149729. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.s.; Huang, Q. Aptamer-functionalized Au nanoparticles array as the effective SERS biosensor for label-free detection of interleukin-6 in serum. Sens. Actuators B Chem. 2021, 334, 129607. [Google Scholar] [CrossRef]
- Chang, Y.C.; Dvoynenko, M.M.; Ke, H.; Hsiao, H.H.; Wang, Y.L.; Wang, J.K. Double Resonance SERS Substrates: Ag Nanoparticles on Grating. J. Phys. Chem. C 2021, 125, 27267–27274. [Google Scholar] [CrossRef]
- Hsiao, H.H.; Ke, H.; Dvoynenko, M.M.; Wang, J.K. Multipolar resonances of Ag nanoparticle arrays in anodic aluminum oxide nanochannels for enhanced hot spot intensity and signal-to-background ratio in surface-enhanced raman scattering. ACS Appl. Nano Mater. 2020, 3, 4477–4485. [Google Scholar] [CrossRef]
- Pandey, P.; Seo, M.K.; Shin, K.H.; Lee, Y.W.; Sohn, J.I. Hierarchically Assembled Plasmonic Metal-Dielectric-Metal Hybrid Nano-Architectures for High-Sensitivity SERS Detection. Nanomaterials 2022, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, C.; Bochterle, J.; Toma, A.; Huck, C.; Neubrech, F.; Messina, E.; Fazio, B.; Marago, O.M.; Di Fabrizio, E.; Lamy de La Chapelle, M.; et al. Optical nanoantennas for multiband surface-enhanced infrared and Raman spectroscopy. ACS Nano 2013, 7, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.; Duenas, J.; Boyle, M.; Doherty, M.; Bell, S.; Kern, A.; Martin, O.; Teh, A.S.; Teo, K.; Milne, W. Combined antenna and localized plasmon resonance in Raman scattering from random arrays of silver-coated, vertically aligned multiwalled carbon nanotubes. Nano Lett. 2011, 11, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Schasfoort, R. Surface plasmon resonance instruments. In Handbook of Surface Plasmon Resonance; Royal Society of Chemistry: London, UK, 2017; pp. 60–105. [Google Scholar]
- Pitarke, J.; Silkin, V.; Chulkov, E.; Echenique, P. Theory of surface plasmons and surface-plasmon polaritons. Rep. Prog. Phys. 2006, 70, 1. [Google Scholar] [CrossRef]
- Zayats, A.V.; Smolyaninov, I.I.; Maradudin, A.A. Nano-optics of surface plasmon polaritons. Phys. Rep. 2005, 408, 131–314. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Plasma resonance emission in solid bodies(Plasma resonance light emission mechanisms in solid bodies, discussing production by electron and light irradiation of body surface). Z. Naturforschung Ausg. A 1968, 23, 615–617. [Google Scholar] [CrossRef]
- Wang, H.; Brandl, D.W.; Le, F.; Nordlander, P.; Halas, N.J. Nanorice: A hybrid plasmonic nanostructure. Nano Lett. 2006, 6, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.J.; Chang, S.H.; Schatz, G.C.; Van Duyne, R.P.; Wiley, B.J.; Xia, Y. Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett. 2005, 5, 2034–2038. [Google Scholar] [CrossRef]
- Bukasov, R.; Shumaker-Parry, J.S. Highly tunable infrared extinction properties of gold nanocrescents. Nano Lett. 2007, 7, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Kong, D.; Ebendorff-Heidepriem, H. Flexible plasmonic tapes with nanohole and nanoparticle arrays for refractometric and strain sensing. ACS Appl. Nano Mater. 2020, 3, 8242–8246. [Google Scholar] [CrossRef]
- Otte, M.A.; Sepulveda, B.; Ni, W.; Juste, J.P.; Liz-Marzán, L.M.; Lechuga, L.M. Identification of the optimal spectral region for plasmonic and nanoplasmonic sensing. ACS Nano 2010, 4, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, E. Plasmonics: Merging photonics and electronics at nanoscale dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Gramotnev, D.K.; Bozhevolnyi, S.I. Plasmonics beyond the diffraction limit. Nat. Photonics 2010, 4, 83–91. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Yan, R.; Wang, H.; Yue, X.; Wang, L.; Wang, Y.; Yuan, X.; Wang, J. Design of self-coupled plasmonic hyperbolic metamaterials refractive index sensor based on intensity shift. Phys. Scr. 2023. [Google Scholar] [CrossRef]
- Haes, A.J.; Zou, S.; Schatz, G.C.; Van Duyne, R.P. A nanoscale optical biosensor: The long range distance dependence of the localized surface plasmon resonance of noble metal nanoparticles. J. Phys. Chem. B 2004, 108, 109–116. [Google Scholar] [CrossRef]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, S. On the Origin of the Plasmonic Properties of Gold Nanoparticles. Bull. Korean Chem. Soc. 2021, 42, 1058–1065. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Ryu, K.R.; Ha, J.W. Enhanced detection sensitivity of the chemisorption of pyridine and biotinylated proteins at localized surface plasmon resonance inflection points in single gold nanorods. Analyst 2021, 146, 3543–3548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.Y.; Chang, W.S.; Link, S. Optical characterization of single plasmonic nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized gold nanoparticles: Synthesis, properties and biomedical applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- Gao, P.F.; Li, Y.F.; Huang, C.Z. Localized surface plasmon resonance scattering imaging and spectroscopy for real-time reaction monitoring. Appl. Spectrosc. Rev. 2019, 54, 237–249. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, P.; Zhou, X.; Akimov, Y.; Png, C.E.; Ang, L.K.; Knoll, W.; Wu, L. Optical refractive index sensors with plasmonic and photonic structures: Promising and inconvenient truth. Adv. Opt. Mater. 2019, 7, 1801433. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Yan, R.; Yue, X.; Wang, L.; Wang, H.; Zhang, J.; Yuan, X.; Zeng, J.; Wang, J. A Tunable Strong Electric Field Ultra-Narrow-Band Fano Resonance Hybrid Metamaterial Sensor Based on LSPR. IEEE Sens. J. 2023, 23, 14662–14669. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zeng, S.; Jiang, L.; Qu, J.; Dinh, X.Q.; Qian, J.; He, S.; Coquet, P.; Yong, K.T. Two-dimensional transition metal dichalcogenide enhanced phase-sensitive plasmonic biosensors: Theoretical insight. J. Phys. Chem. C 2017, 121, 6282–6289. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zeng, S.; Jiang, L.; Hong, L.; Xu, G.; Dinh, X.Q.; Qian, J.; He, S.; Qu, J.; Coquet, P.; et al. Sensitivity enhancement of transition metal dichalcogenides/silicon nanostructure-based surface plasmon resonance biosensor. Sci. Rep. 2016, 6, 28190. [Google Scholar] [CrossRef]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Karpan, V.v.; van den Brink, J.; Kelly, P.J. Doping graphene with metal contacts. Phys. Rev. Lett. 2008, 101, 026803. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Rani, A.; Lee, J.E.; Kim, J.E.; Kim, Y.; Yang, H.; Kim, S.O.; Kim, D.; Kim, D.H. Systematic study on the sensitivity enhancement in graphene plasmonic sensors based on layer-by-layer self-assembled graphene oxide multilayers and their reduced analogues. ACS Appl. Mater. Interfaces 2015, 7, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.X.; Zhai, X.; Wang, L.L.; Wen, S.C. Plasmonically induced transparency in double-layered graphene nanoribbons. Photonics Res. 2018, 6, 692–702. [Google Scholar] [CrossRef]
- Xia, S.; Zhai, X.; Wang, L.; Xiang, Y.; Wen, S. Plasmonically induced transparency in phase-coupled graphene nanoribbons. Phys. Rev. B 2022, 106, 075401. [Google Scholar] [CrossRef]
- Xia, S.X.; Zhang, D.; Zhai, X.; Wang, L.L.; Wen, S.C. Phase-controlled topological plasmons in 1D graphene nanoribbon array. Appl. Phys. Lett. 2023, 123, 101102. [Google Scholar] [CrossRef]
- Xia, S.X.; Zhang, D.; Zheng, Z.; Zhai, X.; Li, H.; Liu, J.Q.; Wang, L.L.; Wen, S.C. Topological plasmons in stacked graphene nanoribbons. Opt. Lett. 2023, 48, 644–647. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, T.; Liu, T.; Zhou, C.; Jiang, X.; Zhang, J. Active metamaterials and metadevices: A review. J. Phys. D Appl. Phys. 2020, 53, 503002. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-dimensional nanomaterials: Fascinating materials in biomedical field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Krasavin, A.V.; Liu, L.; Jiang, Y.; Li, Z.; Guo, X.; Tong, L.; Zayats, A.V. Molecular plasmonics with metamaterials. Chem. Rev. 2022, 122, 15031–15081. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Koudela, I.; Yee, S.S. Surface plasmon resonance sensors based on diffraction gratings and prism couplers: Sensitivity comparison. Sens. Actuators B Chem. 1999, 54, 16–24. [Google Scholar] [CrossRef]
- Vukusic, P.; Bryan-Brown, G.; Sambles, J. Surface plasmon resonance on gratings as a novel means for gas sensing. Sens. Actuators B Chem. 1992, 8, 155–160. [Google Scholar] [CrossRef]
- Jory, M.; Vukusic, P.; Sambles, J. Development of a prototype gas sensor using surface plasmon resonance on gratings. Sens. Actuators B Chem. 1994, 17, 203–209. [Google Scholar] [CrossRef]
- Adam, P.; Dostálek, J.; Homola, J. Multiple surface plasmon spectroscopy for study of biomolecular systems. Sensors Actuators B Chem. 2006, 113, 774–781. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Ferreira, J.; Sabat, R.G.; Rochon, P.; Santos, M.J.L.; Girotto, E.M. SPR based biosensor using surface relief grating in transmission mode. Sens. Actuators B Chem. 2012, 174, 270–273. [Google Scholar] [CrossRef]
- Dou, X.; Chung, P.Y.; Jiang, P.; Dai, J. Surface plasmon resonance and surface-enhanced Raman scattering sensing enabled by digital versatile discs. Appl. Phys. Lett. 2012, 100, 041116. [Google Scholar] [CrossRef]
- Dou, X.; Phillips, B.M.; Chung, P.Y.; Jiang, P. High surface plasmon resonance sensitivity enabled by optical disks. Opt. Lett. 2012, 37, 3681–3683. [Google Scholar] [CrossRef]
- Chien, F.C.; Lin, C.Y.; Yih, J.N.; Lee, K.L.; Chang, C.W.; Wei, P.K.; Sun, C.C.; Chen, S.J. Coupled waveguide–surface plasmon resonance biosensor with subwavelength grating. Biosens. Bioelectron. 2007, 22, 2737–2742. [Google Scholar] [CrossRef]
- Lodewijks, K.; Ryken, J.; Van Roy, W.; Borghs, G.; Lagae, L.; Van Dorpe, P. Tuning the Fano resonance between localized and propagating surface plasmon resonances for refractive index sensing applications. Plasmonics 2013, 8, 1379–1385. [Google Scholar] [CrossRef]
- Cetin, A.; Yanik, A.A.; Yilmaz, C.; Somu, S.; Busnaina, A.; Altug, H. Monopole antenna arrays for optical trapping, spectroscopy, and sensing. Appl. Phys. Lett. 2011, 98, 111110. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Wong, T.I.; Bauch, M.; Zhang, Q.; Zhang, J.; Liu, X.; Zhou, X.; Bai, P.; Dostalek, J.; et al. Directional fluorescence emission co-enhanced by localized and propagating surface plasmons for biosensing. Nanoscale 2016, 8, 8008–8016. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Meng, L.; Hu, J.; Yang, Z. Fano interference between higher localized and propagating surface plasmon modes in nanovoid arrays. Plasmonics 2015, 10, 71–76. [Google Scholar] [CrossRef]
- Live, L.S.; Dhawan, A.; Gibson, K.F.; Poirier-Richard, H.P.; Graham, D.; Canva, M.; Vo-Dinh, T.; Masson, J.F. Angle-dependent resonance of localized and propagating surface plasmons in microhole arrays for enhanced biosensing. Anal. Bioanal. Chem. 2012, 404, 2859–2868. [Google Scholar] [CrossRef]
- Kelf, T.; Sugawara, Y.; Cole, R.; Baumberg, J.; Abdelsalam, M.; Cintra, S.; Mahajan, S.; Russell, A.; Bartlett, P. Localized and delocalized plasmons in metallic nanovoids. Phys. Rev. B 2006, 74, 245415. [Google Scholar] [CrossRef]
- Chang, S.H.; Gray, S.K.; Schatz, G.C. Surface plasmon generation and light transmission by isolated nanoholes and arrays of nanoholes in thin metal films. Opt. Express 2005, 13, 3150–3165. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Najiminaini, M.; Davieau, K.; Kaminska, B.; Singh, M.R.; Carson, J.J.; Lagugné-Labarthet, F. Tunable 3D plasmonic cavity nanosensors for surface-enhanced Raman spectroscopy with sub-femtomolar limit of detection. Acs Photonics 2015, 2, 752–759. [Google Scholar] [CrossRef]
- Schwind, M.; Kasemo, B.; Zoric, I. Localized and propagating plasmons in metal films with nanoholes. Nano Lett. 2013, 13, 1743–1750. [Google Scholar] [CrossRef]
- Couture, M.; Live, L.S.; Dhawan, A.; Masson, J.F. EOT or Kretschmann configuration? Comparative study of the plasmonic modes in gold nanohole arrays. Analyst 2012, 137, 4162–4170. [Google Scholar] [CrossRef]
- Hong, X.; Hall, E.A. Contribution of gold nanoparticles to the signal amplification in surface plasmon resonance. Analyst 2012, 137, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
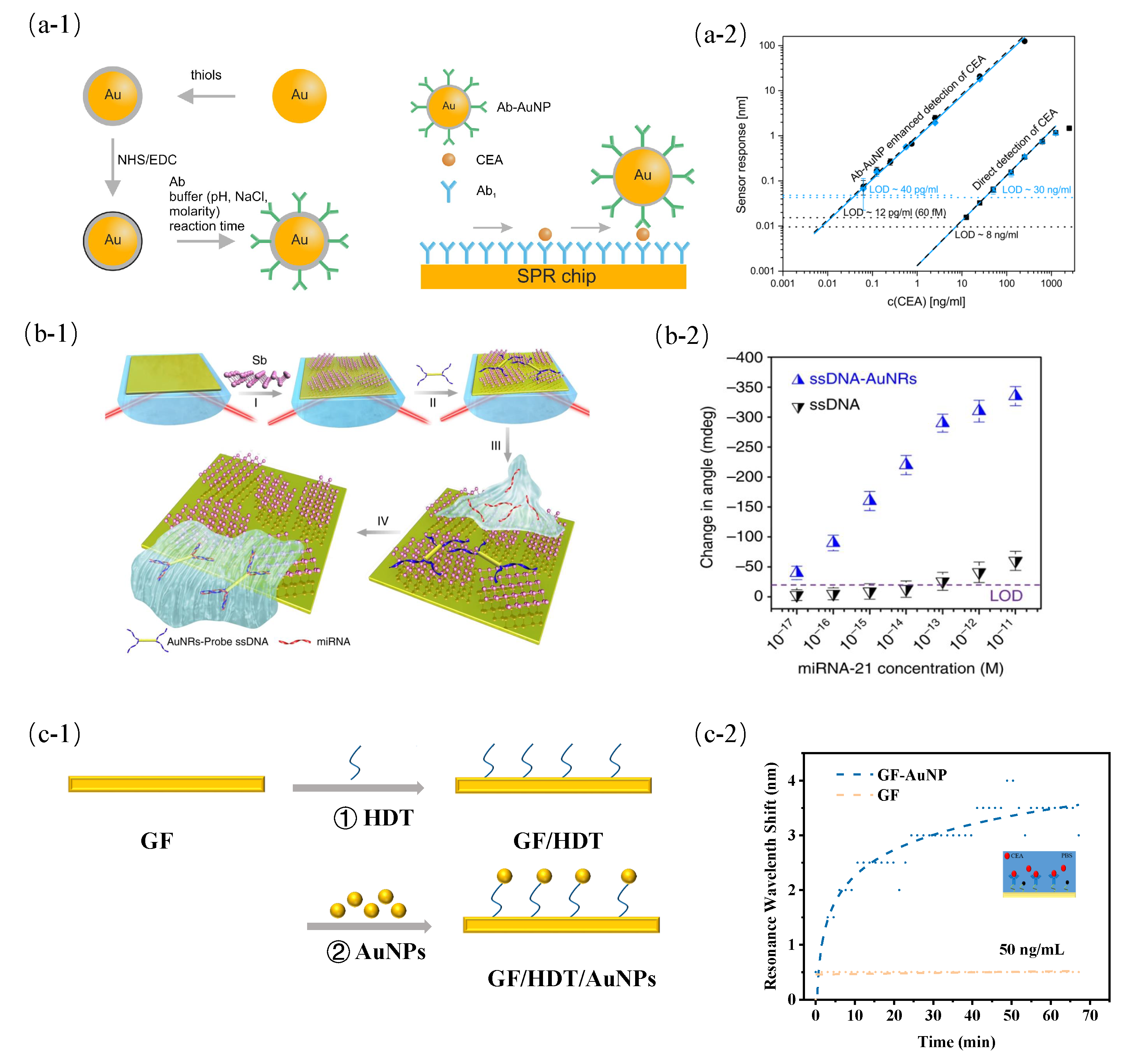
- Špringer, T.; Homola, J. Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma. Anal. Bioanal. Chem. 2012, 404, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feng, F.; Chen, Z.Z.; Bai, Y.F.; Guo, F.F.; Wu, F.Y.; Zhou, G. Sensitive detection of carcinoembryonic antigen using surface plasmon resonance biosensor with gold nanoparticles signal amplification. Talanta 2015, 140, 143–149. [Google Scholar] [CrossRef]
- Yang, C.T.; Wu, L.; Bai, P.; Thierry, B. Investigation of plasmonic signal enhancement based on long range surface plasmon resonance with gold nanoparticle tags. J. Mater. Chem. C 2016, 4, 9897–9904. [Google Scholar] [CrossRef]
- Reinhard, B.M.; Sheikholeslami, S.; Mastroianni, A.; Alivisatos, A.P.; Liphardt, J. Use of plasmon coupling to reveal the dynamics of DNA bending and cleavage by single EcoRV restriction enzymes. Proc. Natl. Acad. Sci. USA 2007, 104, 2667–2672. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Kretschmer, F.; Muehlig, S.; Hoeppener, S.; Winter, A.; Hager, M.D.; Rockstuhl, C.; Pertsch, T.; Schubert, U.S. Survey of plasmonic nanoparticles: From synthesis to application. Part. Part. Syst. Charact. 2014, 31, 721–744. [Google Scholar] [CrossRef]
- Nordlander, P.; Oubre, C.; Prodan, E.; Li, K.; Stockman, M. Plasmon hybridization in nanoparticle dimers. Nano Lett. 2004, 4, 899–903. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Zagorovsky, K.; Chan, W.C. A plasmonic DNAzyme strategy for point-of-care genetic detection of infectious pathogens. Angew. Chem. Int. Ed. 2013, 52, 3168–3171. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the detection of analytes at ultralow concentrations with the naked eye. Nat. Protoc. 2013, 8, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Wang, H.; Reinhard, B.M. Insights from a nanoparticle minuet: Two-dimensional membrane profiling through silver plasmon ruler tracking. Nano Lett. 2010, 10, 230–238. [Google Scholar] [CrossRef]
- Lee, S.E.; Chen, Q.; Bhat, R.; Petkiewicz, S.; Smith, J.M.; Ferry, V.E.; Correia, A.L.; Alivisatos, A.P.; Bissell, M.J. Reversible aptamer-Au plasmon rulers for secreted single molecules. Nano Lett. 2015, 15, 4564–4570. [Google Scholar] [CrossRef]
- Chen, J.I.; Durkee, H.; Traxler, B.; Ginger, D.S. Optical detection of protein in complex media with plasmonic nanoparticle dimers. Small 2011, 7, 1993–1997. [Google Scholar] [CrossRef]
- Smekal, A. Zur quantentheorie der dispersion. Naturwissenschaften 1923, 11, 873–875. [Google Scholar] [CrossRef]
- Almehmadi, L.M.; Curley, S.M.; Tokranova, N.A.; Tenenbaum, S.A.; Lednev, I.K. Surface enhanced Raman spectroscopy for single molecule protein detection. Sci. Rep. 2019, 9, 12356. [Google Scholar] [CrossRef]
- Wang, H.N.; Register, J.K.; Fales, A.M.; Gandra, N.; Cho, E.H.; Boico, A.; Palmer, G.M.; Klitzman, B.; Vo-Dinh, T. Surface-enhanced Raman scattering nanosensors for in vivo detection of nucleic acid targets in a large animal model. Nano Res. 2018, 11, 4005–4016. [Google Scholar] [CrossRef]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Solis, D.M.; Taboada, J.M.; Obelleiro, F.; Liz-Marzan, L.M.; Garcia de Abajo, F.J. Optimization of nanoparticle-based SERS substrates through large-scale realistic simulations. ACS Photonics 2017, 4, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Shell thickness-dependent Au@ Ag nanoparticles aggregates for high-performance SERS applications. Talanta 2019, 195, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Michaels, A.M.; Jiang, N.; Brus, L. Ag nanocrystal junctions as the site for surface-enhanced Raman scattering of single rhodamine 6G molecules. J. Phys. Chem. B 2000, 104, 11965–11971. [Google Scholar] [CrossRef]
- Atay, T.; Song, J.H.; Nurmikko, A.V. Strongly interacting plasmon nanoparticle pairs: From dipole-dipole interaction to conductively coupled regime. Nano Lett. 2004, 4, 1627–1631. [Google Scholar] [CrossRef]
- Gunnarsson, L.; Rindzevicius, T.; Prikulis, J.; Kasemo, B.; Käll, M.; Zou, S.; Schatz, G.C. Confined plasmons in nanofabricated single silver particle pairs: Experimental observations of strong interparticle interactions. J. Phys. Chem. B 2005, 109, 1079–1087. [Google Scholar] [CrossRef]
- Marhaba, S.; Bachelier, G.; Bonnet, C.; Broyer, M.; Cottancin, E.; Grillet, N.; Lermé, J.; Vialle, J.L.; Pellarin, M. Surface plasmon resonance of single gold nanodimers near the conductive contact limit. J. Phys. Chem. C 2009, 113, 4349–4356. [Google Scholar] [CrossRef]
- McMahon, J.M.; Li, S.; Ausman, L.K.; Schatz, G.C. Modeling the effect of small gaps in surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2012, 116, 1627–1637. [Google Scholar] [CrossRef]
- Wang, P.; Wu, L.; Lu, Z.; Li, Q.; Yin, W.; Ding, F.; Han, H. Gecko-inspired nanotentacle surface-enhanced Raman spectroscopy substrate for sampling and reliable detection of pesticide residues in fruits and vegetables. Anal. Chem. 2017, 89, 2424–2431. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Jain, U. Single molecule detection; from microscopy to sensors. Int. J. Biol. Macromol. 2022, 209, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G. ABC Spotlight on single-molecule detection. Anal. Bioanal. Chem. 2020, 412, 7043–7045. [Google Scholar] [CrossRef] [PubMed]
- Melnychuk, N.; Egloff, S.; Runser, A.; Reisch, A.; Klymchenko, A.S. Light-Harvesting Nanoparticle Probes for FRET-Based Detection of Oligonucleotides with Single-Molecule Sensitivity. Angew. Chem. Int. Ed. 2020, 59, 6811–6818. [Google Scholar] [CrossRef] [PubMed]
- Akkilic, N.; Geschwindner, S.; Höök, F. Single-molecule biosensors: Recent advances and applications. Biosens. Bioelectron. 2020, 151, 111944. [Google Scholar] [CrossRef]
- Guo, H.; Qian, K.; Cai, A.; Tang, J.; Liu, J. Ordered gold nanoparticle arrays on the tip of silver wrinkled structures for single molecule detection. Sens. Actuators B Chem. 2019, 300, 126846. [Google Scholar] [CrossRef]
- Zong, C.; Premasiri, R.; Lin, H.; Huang, Y.; Zhang, C.; Yang, C.; Ren, B.; Ziegler, L.D.; Cheng, J.X. Plasmon-enhanced stimulated Raman scattering microscopy with single-molecule detection sensitivity. Nat. Commun. 2019, 10, 5318. [Google Scholar] [CrossRef]
- Jaculbia, R.B.; Imada, H.; Miwa, K.; Iwasa, T.; Takenaka, M.; Yang, B.; Kazuma, E.; Hayazawa, N.; Taketsugu, T.; Kim, Y. Single-molecule resonance Raman effect in a plasmonic nanocavity. Nat. Nanotechnol. 2020, 15, 105–110. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2023, 35, 2107986. [Google Scholar] [CrossRef]
- Kar, A.; Praneeth, N.; Khatua, S.; Datta, B. Use of Single-Molecule Plasmon-Enhanced Fluorescence to Investigate Ligand Binding to G-Quadruplex DNA. J. Phys. Chem. Lett. 2023, 14, 6321–6327. [Google Scholar] [CrossRef]
- Li, C.Y.; Duan, S.; Yi, J.; Wang, C.; Radjenovic, P.M.; Tian, Z.Q.; Li, J.F. Real-time detection of single-molecule reaction by plasmon-enhanced spectroscopy. Sci. Adv. 2020, 6, eaba6012. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-nanopore biosensors for superior single-molecule detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Maccaferri, N.; Krahne, R.; Wang, K.; Garoli, D. Enhanced optical spectroscopy for multiplexed DNA and protein-sequencing with plasmonic nanopores: Challenges and prospects. Anal. Chem. 2022, 94, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Verschueren, D.V.; Dekker, C. Active delivery of single DNA molecules into a plasmonic nanopore for label-free optical sensing. Nano Lett. 2018, 18, 8003–8010. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Ahmed, S.A.; Jiang, Q.C.; Shen, Q.; Zhan, K.; Wang, K. Gold Nanotriangle-Assembled Nanoporous Structures for Electric Field-Assisted Surface-Enhanced Raman Scattering Detection of Adenosine Triphosphate. ACS Sens. 2023, 8, 1280–1286. [Google Scholar] [CrossRef]
- Verschueren, D.V.; Pud, S.; Shi, X.; De Angelis, L.; Kuipers, L.; Dekker, C. Label-free optical detection of DNA translocations through plasmonic nanopores. ACS Nano 2019, 13, 61–70. [Google Scholar] [CrossRef]
- Zhou, J.; Lan, Q.; Li, W.; Ji, L.N.; Wang, K.; Xia, X.H. Single Molecule Protein Segments Sequencing by a Plasmonic Nanopore. Nano Lett. 2023, 23, 2800–2807. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Kerman, S.; Neutens, P.; Willems, K.; Cornelissen, S.; Lagae, L.; Stakenborg, T.; Van Dorpe, P. High spatial resolution nanoslit SERS for single-molecule nucleobase sensing. Nat. Commun. 2018, 9, 1733. [Google Scholar] [CrossRef]
- Huang, J.A.; Mousavi, M.Z.; Giovannini, G.; Zhao, Y.; Hubarevich, A.; Soler, M.A.; Rocchia, W.; Garoli, D.; De Angelis, F. Multiplexed discrimination of single amino acid residues in polypeptides in a single SERS hot spot. Angew. Chem. Int. Ed. 2020, 59, 11423–11431. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Lan, Q.; Ding, X.L.; Pan, X.T.; Ahmed, S.A.; Ji, L.N.; Wang, K.; Xia, X.H. Single-Molecule Electrical and Spectroscopic Profiling Protein Allostery Using a Gold Plasmonic Nanopore. Nano Lett. 2023, 23, 2586–2592. [Google Scholar] [CrossRef]
- Shi, W.; Friedman, A.K.; Baker, L.A. Nanopore sensing. Anal. Chem. 2017, 89, 157–188. [Google Scholar] [CrossRef]
- Assad, O.N.; Gilboa, T.; Spitzberg, J.; Juhasz, M.; Weinhold, E.; Meller, A. Light-enhancing plasmonic-nanopore biosensor for superior single-molecule detection. Adv. Mater 2017, 29, 1605442. [Google Scholar] [CrossRef] [PubMed]
- Zambrana-Puyalto, X.; Ponzellini, P.; Maccaferri, N.; Garoli, D. Förster-resonance energy transfer between diffusing molecules and a functionalized plasmonic nanopore. Phys. Rev. Appl. 2020, 14, 054065. [Google Scholar] [CrossRef]
- Klughammer, N.; Dekker, C. Palladium zero-mode waveguides for optical single-molecule detection with nanopores. Nanotechnology 2021, 32, 18LT01. [Google Scholar] [CrossRef] [PubMed]
- Barulin, A.; Claude, J.B.; Patra, S.; Bonod, N.; Wenger, J. Deep ultraviolet plasmonic enhancement of single protein autofluorescence in zero-mode waveguides. Nano Lett. 2019, 19, 7434–7442. [Google Scholar] [CrossRef]
- Pang, Y.; Gordon, R. Optical trapping of a single protein. Nano Lett. 2012, 12, 402–406. [Google Scholar] [CrossRef]
- Belkin, M.; Chao, S.H.; Jonsson, M.P.; Dekker, C.; Aksimentiev, A. Plasmonic nanopores for trapping, controlling displacement, and sequencing of DNA. ACS Nano 2015, 9, 10598–10611. [Google Scholar] [CrossRef]
- Rogez, B.; Marmri, Z.; Thibaudau, F.; Baffou, G. Thermoplasmonics of metal layers and nanoholes. APL Photonics 2021, 6, 101101. [Google Scholar] [CrossRef]
- Lu, J.; Yang, H.; Zhou, L.; Yang, Y.; Luo, S.; Li, Q.; Qiu, M. Light-induced pulling and pushing by the synergic effect of optical force and photophoretic force. Phys. Rev. Lett. 2017, 118, 043601. [Google Scholar] [CrossRef]
- Lin, L.; Wang, M.; Peng, X.; Lissek, E.N.; Mao, Z.; Scarabelli, L.; Adkins, E.; Coskun, S.; Unalan, H.E.; Korgel, B.A.; et al. Opto-thermoelectric nanotweezers. Nat. Photonics 2018, 12, 195–201. [Google Scholar] [CrossRef]
- Hong, C.; Yang, S.; Ndukaife, J.C. Stand-off trapping and manipulation of sub-10 nm objects and biomolecules using opto-thermo-electrohydrodynamic tweezers. Nat. Nanotechnol. 2020, 15, 908–913. [Google Scholar] [CrossRef]
- Zhao, Y.; Iarossi, M.; De Fazio, A.F.; Huang, J.A.; De Angelis, F. Label-free optical analysis of biomolecules in solid-state nanopores: Toward single-molecule protein sequencing. ACS Photonics 2022, 9, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Shalabney, A.; Abdulhalim, I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photonics Rev. 2011, 5, 571–606. [Google Scholar] [CrossRef]
- Shalabney, A.; Abdulhalim, I. Electromagnetic fields distribution in multilayer thin film structures and the origin of sensitivity enhancement in surface plasmon resonance sensors. Sens. Actuators A Phys. 2010, 159, 24–32. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; He, Y.; Ma, H. Resolution enhancement of surface plasmon resonance sensors with spectral interrogation: Resonant wavelength considerations. Appl. Opt. 2016, 55, 884–891. [Google Scholar] [CrossRef]
- Akib, T.B.A.; Mou, S.F.; Rahman, M.M.; Rana, M.M.; Islam, M.R.; Mehedi, I.M.; Mahmud, M.P.; Kouzani, A.Z. Design and numerical analysis of a graphene-coated SPR biosensor for rapid detection of the novel coronavirus. Sensors 2021, 21, 3491. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Kumar, J.S.; Singh, V.V.; Biswas, U.; Sarkar, S.S.; Alam, S.I.; Dash, P.K.; Boopathi, M.; Ganesan, K.; Jain, R. Surface plasmon resonance sensing of Ebola virus: A biological threat. Anal. Bioanal. Chem. 2020, 412, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Moznuzzaman, M.; Khan, I.; Islam, M.R. Nano-layered surface plasmon resonance-based highly sensitive biosensor for virus detection: A theoretical approach to detect SARS-CoV-2. AIP Adv. 2021, 11, 065023. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, X.; Wang, Y.; Li, M.; Zhou, K.; Zhang, L.; Tan, Y. Surface plasmon resonance biosensor with laser heterodyne feedback for highly-sensitive and rapid detection of COVID-19 spike antigen. Biosens. Bioelectron. 2022, 206, 114163. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Mustapha Kamil, Y.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Zhu, P.; Zhao, W.; Liu, T.; Wang, Q.; Zhao, T. Alcohol sensor based on surface plasmon resonance of ZnO nanoflowers/Au structure. Materials 2021, 15, 189. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rana, M.M.; Rahman, M.S.; Anower, M.; Mollah, M.A.; Paul, A.K. Sensitivity enhancement of SPR biosensors employing heterostructure of PtSe2 and 2D materials. Opt. Mater. 2020, 107, 110123. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, T.; Yuan, X.; Wang, Y.; Yue, X.; Wang, L.; Zhang, J.; Wang, J. Plasmonic Nanostructure Biosensors: A Review. Sensors 2023, 23, 8156. https://doi.org/10.3390/s23198156
Wang H, Wang T, Yuan X, Wang Y, Yue X, Wang L, Zhang J, Wang J. Plasmonic Nanostructure Biosensors: A Review. Sensors. 2023; 23(19):8156. https://doi.org/10.3390/s23198156
Chicago/Turabian StyleWang, Huimin, Tao Wang, Xuyang Yuan, Yuandong Wang, Xinzhao Yue, Lu Wang, Jinyan Zhang, and Jian Wang. 2023. "Plasmonic Nanostructure Biosensors: A Review" Sensors 23, no. 19: 8156. https://doi.org/10.3390/s23198156





