Effect of Ambient Environment on Laser Reduction of Graphene Oxide for Applications in Electrochemical Sensing
Abstract
:1. Introduction
2. Materials and Methods
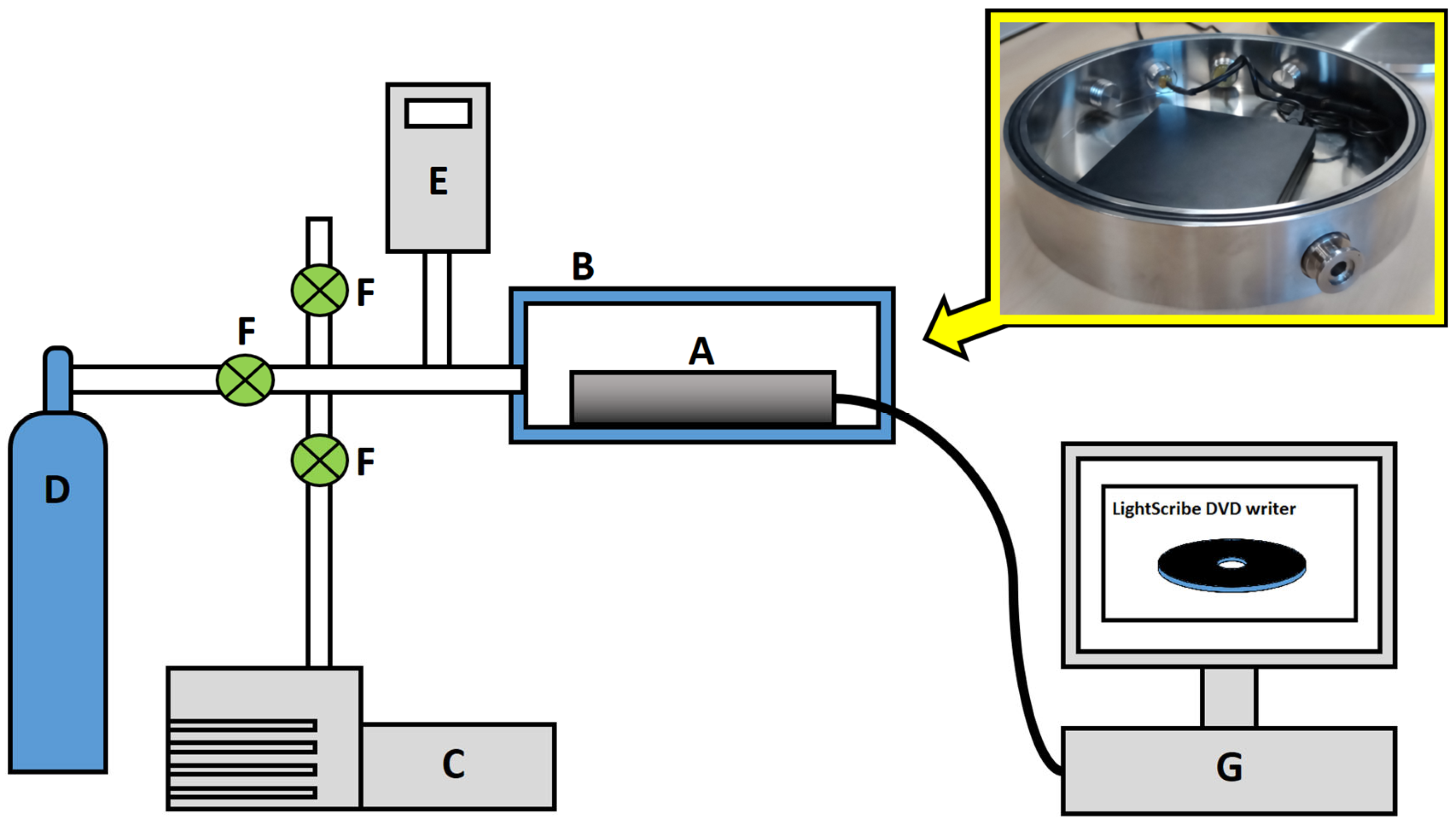
2.1. Hardware
- A LightScribe DVD writer connected to a PC. The LightScribe is a direct disc-labelling system that burns a user-defined image on the nondata side of a LightScribe DVD [51];
- A custom-made, stainless-steel environmental chamber with electrical feedthroughs incorporated into the chamber wall allowing for the operation of the enclosed LightScribe system;
- A gas line consisting of a vacuum pump, a pressure sensor and any required gas cylinders.
2.2. Fabrication Process
2.3. Characterisation
2.3.1. SEM
2.3.2. Raman Spectroscopy
2.3.3. X-ray Photoelectron Spectroscopy
2.3.4. Cyclic Voltammetry
3. Results and Discussion
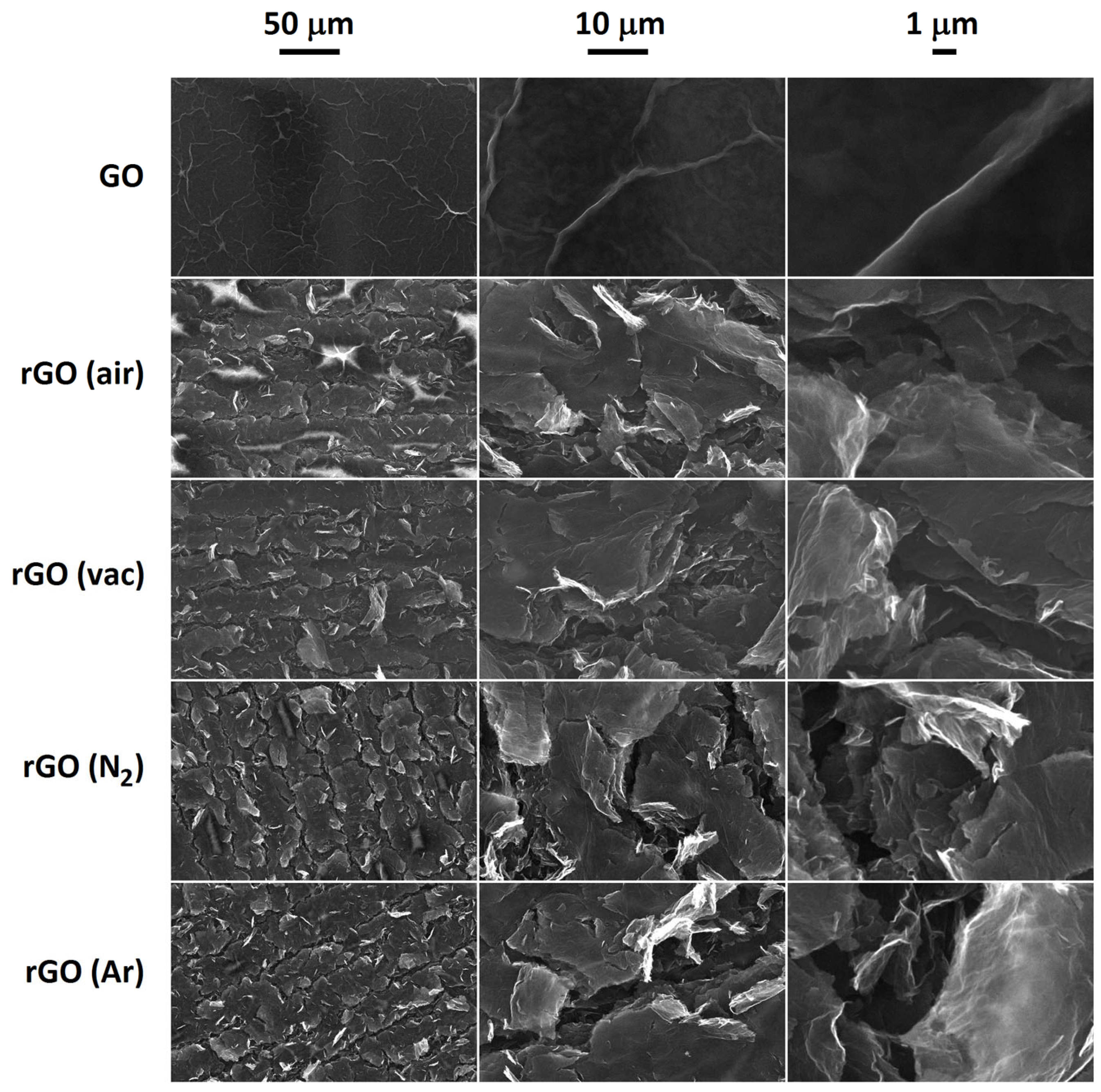
3.1. SEM
3.2. Raman
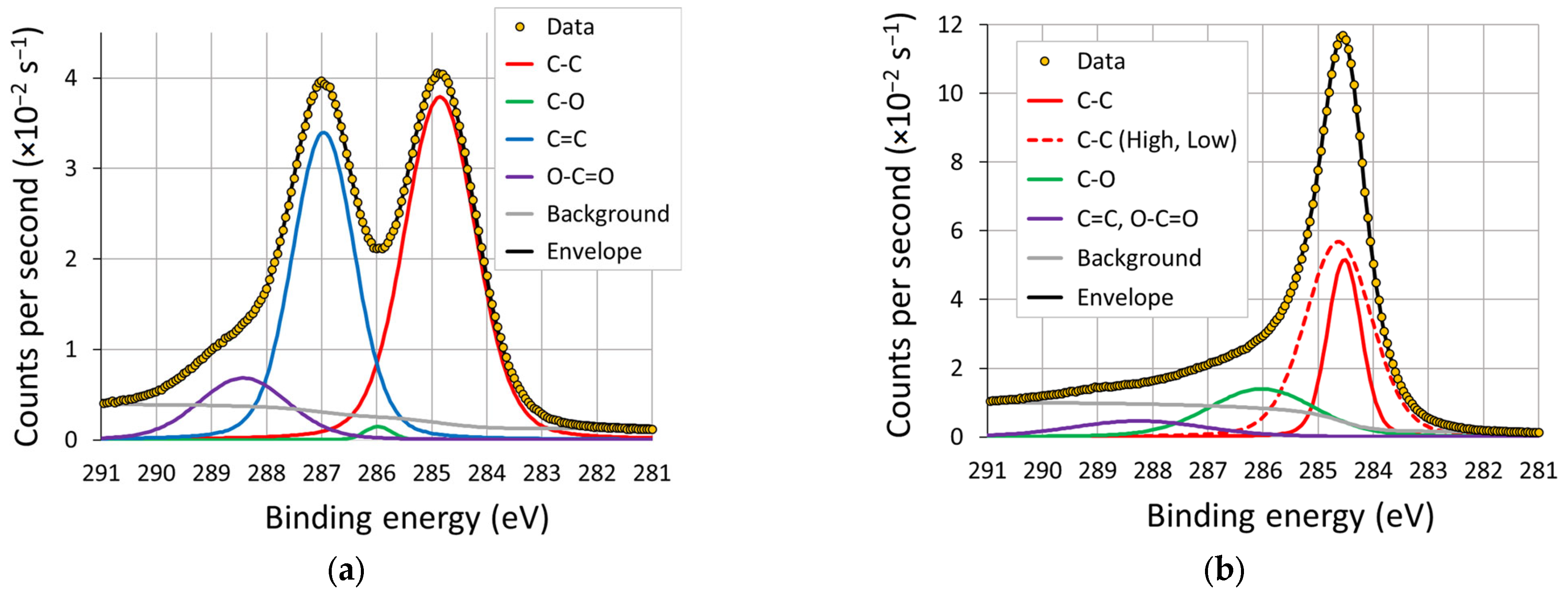
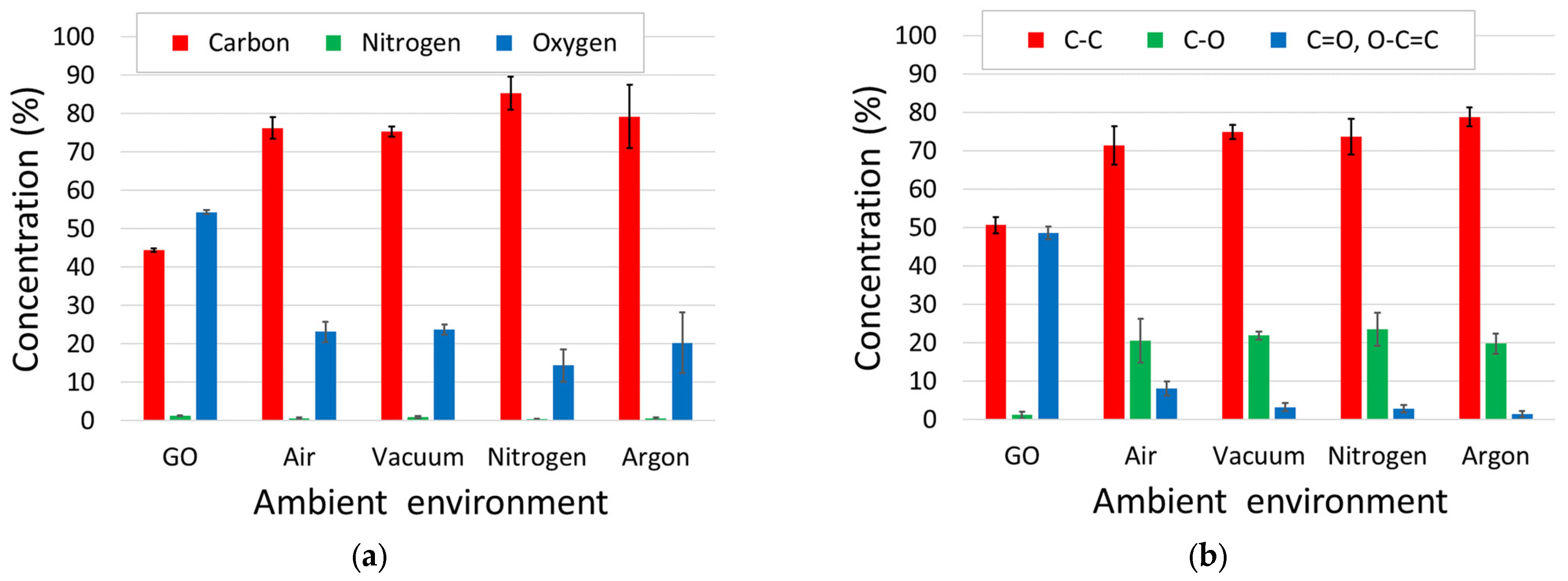
3.3. XPS
3.4. Cyclic Voltammetry
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Binding Energies
| Survey Scan [57] | Carbon Peak [61] | ||
|---|---|---|---|
| Regions | Binding Energy (eV) | Regions | Binding Energy (eV) |
| Au (4f7/2, 4f5/2) | 84.0, 87.7 | C-C low | 283.4–284.0 |
| C (sp2, sp3) | 284.1, 284.8 | C-C, C-H | 284.2–284.6 |
| N | 400 | C-C high | 284.8–285.4 |
| O | 533 | C-O | 285.9–286.6 |
| C=O | 286.7–287.5 | ||
| O-C=O | 288.3–288.9 | ||
Appendix B. Theory of Cyclic Voltammetry
- k0 > 500 µms−1. The system is regarded as a reversible (or Nerstian) system in which the electron transfer rate is fast enough to sustain the concentrations of the oxidation and reduction electroactive species;
- k0 < 0.1 µms−1. The system is regarded as an irreversible system in which the electron transfer rate is slower than the diffusion constant of the analyte, and so the electrochemically generated species diffuse from the surface of the electrode before a reverse electron transfer reaction can occur;
- 0.1 µms−1 < k0 < 500 µms−1. The system is regarded as a quasi-reversible process in which both the electron transfer reaction and the mass transfer process determine the overall rate of the electrode process.
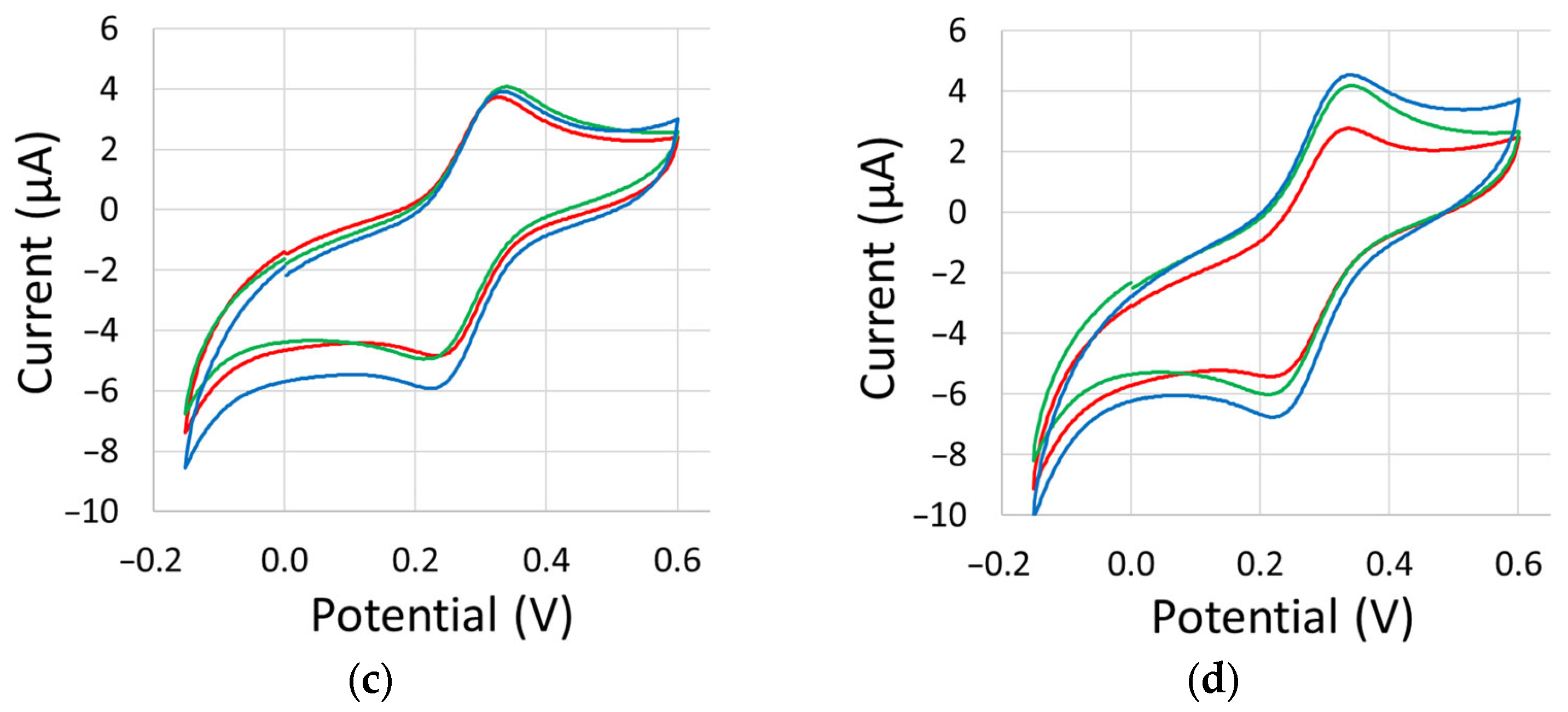
Appendix C. Cyclic Voltammetry Scans

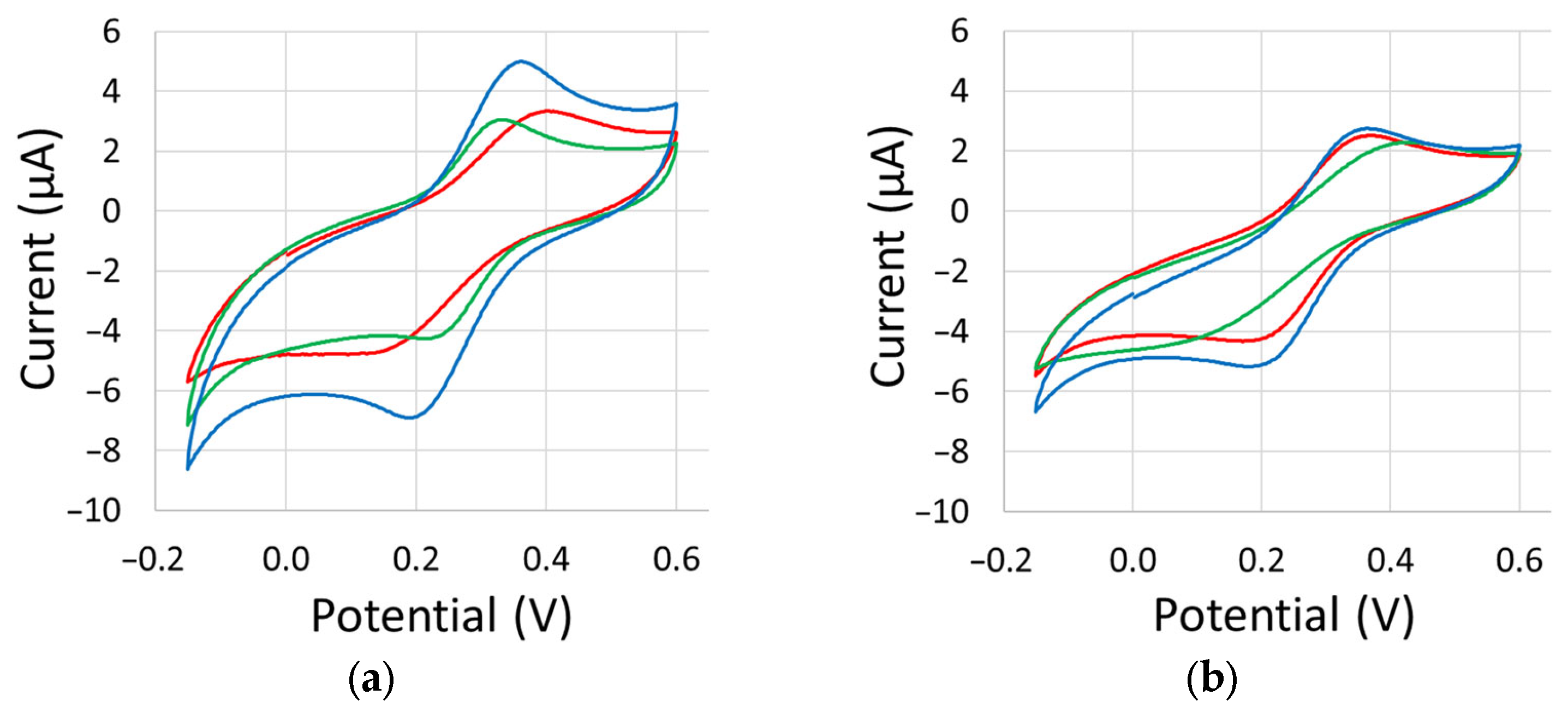
References
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Hendel, S.J.; Young, E.R. Introduction to electrochemistry and the use of electrochemistry to synthesize and evaluate catalysts for water oxidation and reduction. J. Chem. Educ. 2016, 93, 1951–1956. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A.; Gros, P. Electrochemical sensors and devices for heavy metals assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.K.; Dhau, J.S.; Gohel, H.; Mishra, Y.K.; Kateb, B.; Kim, N.-Y.; Goswami, D.Y. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio Mater. 2020, 3, 7306–7325. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.C.; Santos, A.; Bueno, P.R. An outlook on electrochemical approaches for molecular diagnostics assays and discussions on the limitations of miniaturized technologies for point-of-care devices. Sens. Actuators Rep. 2022, 4, 100087. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, A.J.J. Electrode Materials in Modern Organic Electrochemistry. Angew. Chem. Int. Ed. 2020, 59, 18866–18884. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fu, C.; Lu, Q. Advanced technology for functionalization of carbon nanotubes. Prog. Nat. Sci. 2009, 19, 801–810. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Kwon, O.-S.; Kim, H.; Ko, H.; Lee, J.; Lee, B.; Jung, C.-H.; Choi, J.-H.; Shin, K. Fabrication and characterization of inkjet-printed carbon nanotube electrode patterns on paper. Carbon 2013, 58, 116–127. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, X. Electrochemical sensors based on carbon nanomaterial used in diagnosing metabolic disease. Front. Chem. 2020, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gao, W.; Emaminejad, S.; Kiriya, D.; Ota, H.; Nyein, H.Y.Y.; Takei, K.; Javey, A. Carbon Nanotubes: Printed Carbon Nanotube Electronics and Sensor Systems. Adv. Mater. 2016, 28, 4397–4414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, F.; Liu, Z.-Q.; Chen, Y.-S.; Luo, Y.-L. Novel electrochemical sensors from poly [N-(ferrocenyl formacyl) pyrrole]@ multi-walled carbon nanotubes nanocomposites for simultaneous determination of ascorbic acid, dopamine and uric acid. Nanotechnology 2020, 31, 085503. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Niu, Y.; Zhang, G.; Xu, Y.; Li, J. Electrochemistry in carbon-based quantum dots. Chem. Asian J. 2020, 15, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Griffiths, K.; Dale, C.; Hedley, J.; Kowal, M.D.; Kaner, R.B.; Keegan, N. Laser-scribed graphene presents an opportunity to print a new generation of disposable electrochemical sensors. Nanoscale 2014, 6, 13613–13622. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Aziz, A.; Khe, C.-S.; Hidayah, N.M.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Skrypnychuk, V.; Boulanger, N.; Nordenström, A.; Talyzin, A. Aqueous activated graphene dispersions for deposition of high-surface area supercapacitor electrodes. J. Phys. Chem. Lett. 2020, 11, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Dideikin, A.T.; Vul, A.Y. Graphene oxide and derivatives: The place in graphene family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the Gap between Reality and Ideal in Chemical Vapor Deposition Growth of Graphene. Chem. Rev. 2018, 118, 9281–9343. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.S.; Zhang, R.; Zhu, J. Graphene synthesis: A Review. Mater. Sci. Pol. 2015, 33, 566–578. [Google Scholar] [CrossRef]
- De Heer, W.A.; Berger, C.; Ruan, M.; Sprinkle, M.; Li, X.; Hu, Y.; Zhang, B.; Hankinson, J.; Conrad, E. Large area and structured epitaxial graphene produced by confinement controlled sublimation of silicon carbide. Proc. Natl. Acad. Sci. USA 2011, 108, 16900–16905. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N. Graphene dispersion and exfoliation in low boiling point solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Feng, X.; Müllen, K. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef] [PubMed]
- Purkait, T.; Singh, G.; Kumar, D.; Singh, M.; Dey, R.S. High-performance flexible supercapacitors based on electrochemically tailored three-dimensional reduced graphene oxide networks. Sci. Rep. 2018, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Namdar, N.; Ghasemi, F.; Sanaee, Z. Plasma-assisted three-dimensional lightscribe graphene as high-performance supercapacitors. Sci. Rep. 2022, 12, 4254. [Google Scholar] [CrossRef] [PubMed]
- Puetz, P.; Behrent, A.; Baeumner, A.J.; Wegener, J. Laser-scribed graphene (LSG) as new electrode material for impedance-based cellular assays. Sens. Actuators B 2020, 321, 128443. [Google Scholar] [CrossRef]
- Sehrawat, P.; Abid, A.; Islam, S.S.; Mauger, A.; Julien, C.M. Nanostructured Graphene Oxide-Based Hybrids as Anodes for Lithium-Ion Batteries. C 2020, 6, 81. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.J.; Vajtai, R.; Zhang, Q.; Wei, B.Q.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef]
- Furio, A.; Landi, G.; Altavilla, C.; Sofia, D.; Iannace, S.; Sorrentino, A.; Neitzert, H.C. Light irradiation tuning of surface wettability, optical, and electric properties of graphene oxide thin films. Nanotechnology 2017, 28, 054003. [Google Scholar] [CrossRef]
- Komarov, I.A.; Struchkov, N.S.; Polikarpova, I.A.; Peretiyagin, V.G.; Buyanov, A.D.; Danilova, E.A.; Denisenko, E.I.; Onoprienko, E.A. Comparison of low cost lasers for graphene oxide thin films reduction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 934, 012040. [Google Scholar] [CrossRef]
- Mortazavi, S.; Mollabashi, M.; Barri, R.; Jones, K.; Xiao, J.Q.; Opila, R.L.; Ismat Shah, S. Modification of graphene oxide film propertiesusing KrF laser irradiation. RSC Adv. 2018, 8, 12808–12814. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, S.; Haylock, B.; Kaur, J.; Tanner, P.; Thiel, D.; Sang, R.; Cole, I.S.; Li, X.; Lobino, M.; et al. Tuning the sub-processes in laser reduction of graphene oxide by adjusting the power and scanning speed of laser. Carbon 2019, 141, 83–91. [Google Scholar] [CrossRef]
- Naik, G.; Krishnaswamy, S. Photoreduction and Thermal Properties of Graphene-Based Flexible Films. Graphene 2017, 6, 27–40. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Barkauskas, J.; Stankevičienė, I.; Niaura, G.; Mažeikienė, R. Reduction of graphite oxide to graphene with laser irradiation. Carbon 2013, 52, 574–582. [Google Scholar] [CrossRef]
- Orekhov, N.D.; Bondareva, J.V.; Potapov, D.O.; Dyakonov, P.V.; Dubinin, O.N.; Tarkhov, M.A.; Diudbin, G.D.; Maslakov, K.I.; Logunov, M.A.; Kvashnin, D.G.; et al. Mechanism of graphene oxide laser reduction at ambient conditions: Experimental and ReaxFF study. Carbon 2022, 191, 546–554. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent Advances in Laser Utilization in the Chemical Modification of Graphene Oxide and Its Applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Ghanam, A.; Lahcen, A.A.; Beduk, T.; Alshareef, H.N.; Amine, A.; Salama, K.N. Laser scribed graphene: A novel platform for highly sensitive detection of electroactive biomolecules. Biosens. Bioelectron. 2020, 168, 112509. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Mohammad, M.A.; Mi, W.-T.; Mi, W.-T.; Yang, Y.; Ren, T.-L. Laser-scribing technology for wafer-scale graphene devices. In Advances in Carbon Nanostructures; Silva, A.M.T., Carabineiro, S.A.C., Eds.; IntechOpen Limited: London, UK, 2016. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman Spectroscopy Investigation of Graphene Oxide Reduction by Laser Scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Watanabe, A.; Qin, G.; Cai, J. Laser Direct Writing on Copper Nanoparticle Film by LightScribe Technique. J. Photopolym. Sci. Technol. 2015, 28, 99–102. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Application Note: The Raman Spectroscopy of Graphene and the Determination of Layer Thickness. Available online: https://assets.thermofisher.com/TFS-Assets/MSD/Application-Notes/raman-spectroscopy-graphene-determination-layer-thickness-an52252.pdf (accessed on 29 July 2023).
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. In Proceedings of the International Conference of Global Network for Innovative Technology and AWAM International Conference in Civil Engineering (IGNITE-AICCE’17), Penang, Malaysia, 8–10 August 2017. [Google Scholar] [CrossRef]
- Jorio, A.; Martins Ferreira, E.H.; Moutinho, M.V.O.; Stavale, F.; Achete, C.A.; Capaz, R.B. Measuring disorder in graphene with the G and D bands. Phys. Status Solidi B 2010, 247, 2980–2982. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy of Atomic Elements. Available online: https://www.thermofisher.com/uk/en/home/materials-science/learning-center/periodic-table.html (accessed on 29 July 2023).
- Priante, F.; Salim, M.; Ottaviano, L.; Perrozzi, F. XPS study of graphene oxide reduction induced by (100) and (111)-oriented Si substrates. Nanotechnology 2018, 29, 075704. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Yamada, Y.; Tanaka, H.; Kubo, S.; Sato, S. Unveiling bonding states and roles of edges in nitrogen-doped graphene nanoribbon by X-ray photoelectron spectroscopy. Carbon 2021, 185, 342–367. [Google Scholar] [CrossRef]
- Smith, M.; Scudiero, L.; Espinal, J.; McEwen, J.-S.; Garcia-Perez, M. Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon 2016, 110, 155–171. [Google Scholar] [CrossRef]
- Sharma, R.; Chadha, N.; Saini, P. Determination of defect density, crystallite size and number of graphene layers in graphene analogues using X-ray diffraction and Raman spectroscopy. Indian J. Pure Appl. Phys. 2017, 55, 625–629. [Google Scholar]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Huh, S.H. Thermal Reduction of Graphene Oxide. In Physics and Applications of Graphene—Experiments; Mikhailov, S., Ed.; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef]
- Valota, A.T.; Kinloch, I.A.; Novoselov, K.S.; Casiraghi, C.; Eckmann, A.; Hill, E.W.; Dryfe, R.A. Electrochemical behavior of monolayer and bilayer graphene. ACS Nano 2011, 5, 8809–8815. [Google Scholar] [CrossRef]
- Plana, D.; Jones, F.G.; Dryfe, R.A. The voltammetric response of bipolar cells: Reversible electron transfer. J. Electroanal. Chem. 2010, 646, 107–113. [Google Scholar] [CrossRef]
- Alba, A.F.; Totoricaguena-Gorriño, J.; Sánchez-Ilárduya, M.B.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S.; Javier del Campo, F. Laser-activated screen-printed carbon electrodes for enhanced dopamine determination in the presence of ascorbic and uric acid. Electrochim. Acta 2021, 399, 139374. [Google Scholar] [CrossRef]
- Randviir, E.; Brownson, D.; Gómez-Mingot, M.; Kampouris, D.; Iniesta, J.; Banks, C. Electrochemistry of Q-Graphene. Nanoscale 2012, 4, 6470–6480. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Aristov, N.; Habekost, A. Cyclic voltammetry-A versatile electrochemical method investigating electron transfer processes. World J. Chem. Educ. 2015, 3, 115–119. [Google Scholar]
- Khalafi, L.; Rafiee, M. Cyclic Voltammetry. In Encyclopedia of Physical Organic Chemistry, 1st ed.; Wang, Z., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 3437–3478. ISBN 978-1-118-46858-6. [Google Scholar]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An Extended Method for the Practical Evaluation of the Standard Rate Constant from Cyclic Voltammetric Data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]



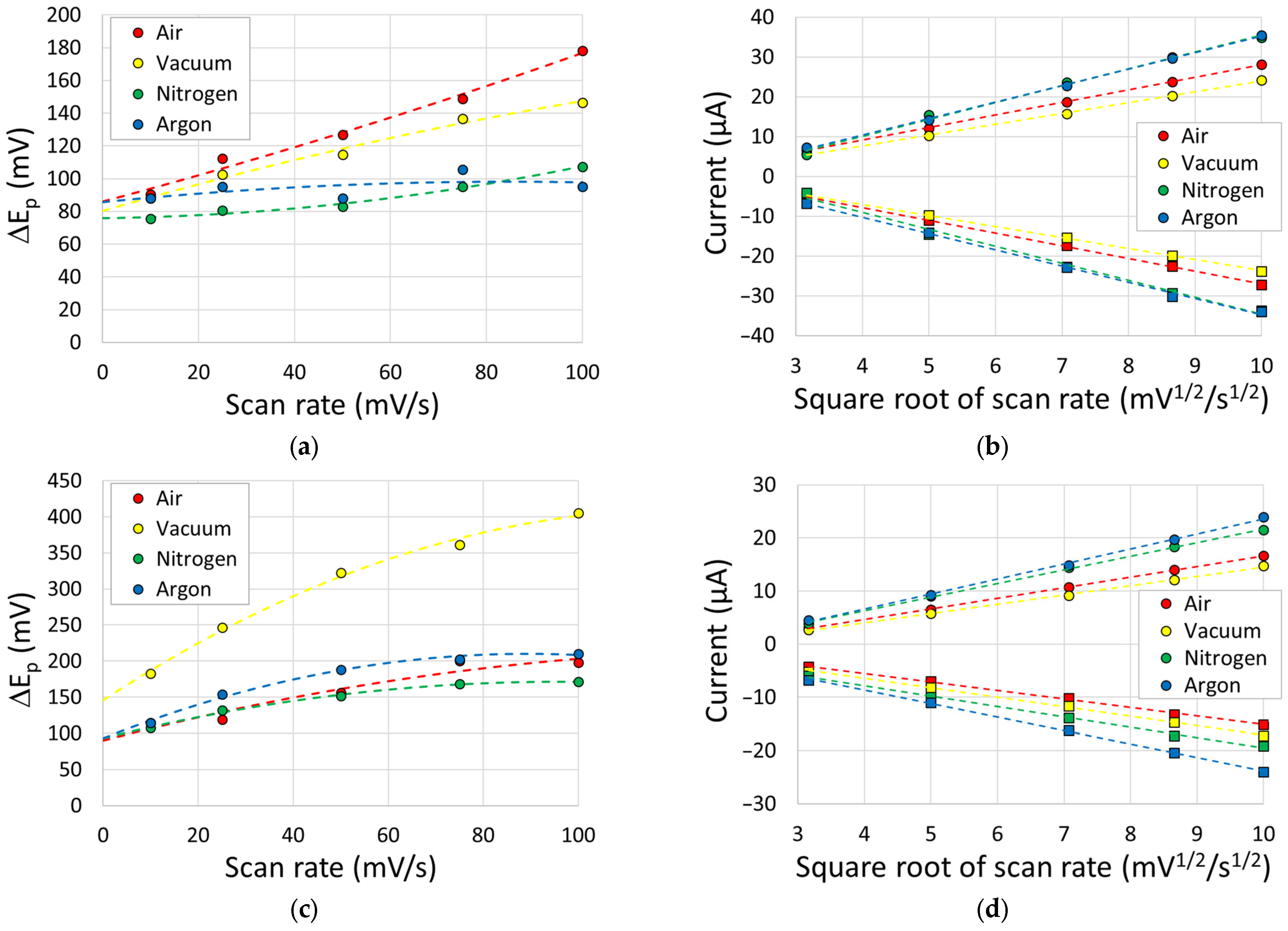
| Atmosphere | Outer Sphere ΔEp (mV) | Inner Sphere ΔEp (mV) | ||
|---|---|---|---|---|
| Average ± SD | Lowest Achieved | Average ± SD | Lowest Achieved | |
| Air | 142 ± 70 | 73 | 153 ± 70 | 88 |
| Vacuum | 125 ± 99 | 63 | 181 ± 78 | 129 |
| Nitrogen | 63 ± 1 | 61 | 83 ± 15 | 66 |
| Argon | 67 ± 3 | 63 | 95 ± 10 | 85 |
| Electrode | k0 (×10−3 cms−1) | Reference | |
|---|---|---|---|
| Average | Best | ||
| Laser-scribed graphene in air | 0.50 | 2.33 | This work |
| Laser-scribed graphene under vacuum | 0.31 | 0.77 | This work |
| Laser-scribed graphene in nitrogen | 2.87 | 10.5 | This work |
| Laser-scribed graphene in argon | 1.80 | 2.57 | This work |
| Edge plane pyrolytic graphite | 2.60 | [17] | |
| Basal plane pyrolytic graphite | 0.33 | [17] | |
| Laser-scribed graphene in air | 23.7 | [17] | |
| Laser-activated graphite screen-printed electrodes | 8 | [67] | |
| Edge plane pyrolytic graphite | 4.66 | [68] | |
| Q-graphene modified-edge plane pyrolytic graphite | 18.6 | [68] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faqihi, A.A.; Keegan, N.; Šiller, L.; Hedley, J. Effect of Ambient Environment on Laser Reduction of Graphene Oxide for Applications in Electrochemical Sensing. Sensors 2023, 23, 8002. https://doi.org/10.3390/s23188002
Faqihi AA, Keegan N, Šiller L, Hedley J. Effect of Ambient Environment on Laser Reduction of Graphene Oxide for Applications in Electrochemical Sensing. Sensors. 2023; 23(18):8002. https://doi.org/10.3390/s23188002
Chicago/Turabian StyleFaqihi, Abdullah A., Neil Keegan, Lidija Šiller, and John Hedley. 2023. "Effect of Ambient Environment on Laser Reduction of Graphene Oxide for Applications in Electrochemical Sensing" Sensors 23, no. 18: 8002. https://doi.org/10.3390/s23188002
APA StyleFaqihi, A. A., Keegan, N., Šiller, L., & Hedley, J. (2023). Effect of Ambient Environment on Laser Reduction of Graphene Oxide for Applications in Electrochemical Sensing. Sensors, 23(18), 8002. https://doi.org/10.3390/s23188002






