A Smartphone-Enabled Imaging Device for Chromotropic Acid-Based Measurement of Nitrate in Soil Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setup of the Imaging Device
2.2. Laboratory Extraction and Device Calibration
2.3. Image Analysis and SMART NP Development
- Step 1: Normalize the RGB values: divide the RGB values by 255 to bring them into the range of 0 to 1.
- Step 2: Calculate the maximum (max) and minimum (min) values among R′, G′, and B′:
- Step 3: Calculate the hue (H) component:
- Step 4: Calculate the saturation (S) component:
- Step 5: Calculate the value (V) component:
2.4. Sample Collection and Device Performance Validation
2.5. Soil NO3− Spatial Variability Mapping Using the Device
3. Results and Discussion
3.1. NO3− Calibration Models
3.2. Device Characteristics
3.3. Real Soil Test Performance
3.4. Spatial Variability Mapping of Soil NO3−
3.5. Practical Utility of the Proposed Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heuvelink, G.B.M. Uncertainty analysis in environmental modelling under a change of spatial scale. In Soil and Water Quality at Different Scales, Developments in Plant and Soil Sciences; Finke, P.A., Bouma, J., Hoosbeek, M.R., Eds.; Springer: Dordrecht, The Netherlands, 1998; Volume 80, pp. 255–264. [Google Scholar]
- Laurent, F.; Ruelland, D. Assessing impacts of alternative land use and agricultural practices on nitrate pollution at the catchment scale. J. Hydrol. 2011, 409, 444–450. [Google Scholar] [CrossRef]
- Birrell, S.J.; Hummel, J.W. Membrane selection and ISFET configuration evaluation for soil nitrate sensing. Trans. ASAE 2000, 43, 197. [Google Scholar] [CrossRef]
- Milham, P.J.; Awad, A.S.; Paull, R.E.; Bull, J.H. Analysis of plants, soils and waters for nitrate by using an ion-selective electrode. Analyst 1970, 95, 751–757. [Google Scholar] [CrossRef]
- Szekely, E. A rapid colorimetric method for analysis of nitrate nitrogen by reduction to nitrite. Commun. Soil Sci. Plant Anal. 1991, 22, 1295–1302. [Google Scholar] [CrossRef]
- Shinn, M.B. Colorimetric method for determination of nitrate. Ind. Eng. Chem. Anal. Ed. 1941, 13, 33–35. [Google Scholar] [CrossRef]
- Rock, L.; Ellert, B.H.; Mayer, B. Tracing sources of soil nitrate using the dual isotopic composition of nitrate in 2 M KCl-extracts. Soil Biol. Biochem. 2011, 43, 2397–2405. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M.A. Ion chromatographic determination of sulfate and nitrate in soils. Soil Sci. Soc. Am. J. 1979, 43, 899–904. [Google Scholar] [CrossRef]
- Swetha, R.K.; Bende, P.; Singh, K.; Gorthi, S.; Biswas, A.; Li, B.; Weindorf, D.C.; Chakraborty, S. Predicting soil texture from smartphone-captured digital images and an application. Geoderma 2020, 376, 114562. [Google Scholar] [CrossRef]
- Gorthi, S.; Swetha, R.K.; Chakraborty, S.; Li, B.; Weindorf, D.C.; Dutta, S.; Banerjee, H.; Das, K.; Majumdar, K. Soil organic matter prediction using smartphone-captured digital images: Use of reflectance image and image perturbation. Biosyst. Eng. 2021, 209, 154–169. [Google Scholar] [CrossRef]
- Hossain, M.R.H.; Kabir, M.A. Machine learning techniques for estimating soil moisture from smartphone captured images. Agriculture 2023, 13, 574. [Google Scholar] [CrossRef]
- Das, P.; Paul, S.; Bhattacharya, S.S.; Nath, P. Smartphone-based spectrometric analyzer for accurate estimation of pH value in soil. IEEE Sens. J. 2020, 21, 2839–2845. [Google Scholar] [CrossRef]
- Sumriddetchkajorn, S.; Chaitavon, K.; Intaravanne, Y. Mobile device-based self-referencing colorimeter for monitoring chlorine concentration in water. Sens. Actuators B Chem. 2013, 182, 592–597. [Google Scholar] [CrossRef]
- Das, P.; Chetry, B.; Paul, S.; Bhattacharya, S.S.; Nath, P. Detection and quantification of phosphate in water and soil using a smartphone. Microchem. J. 2022, 172, 106949. [Google Scholar] [CrossRef]
- Moonrungsee, N.; Pencharee, S.; Jakmunee, J. Colorimetric analyzer based on mobile phone camera for determination of available phosphorus in soil. Talanta 2015, 136, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, V.; Nayak, A.; Dasgupta, S.; Urkude, S.; Dey, S.; Biswas, A.; Li, B.; Weindorf, D.C.; Chakraborty, S. A smartphone-integrated imaging device for measuring nitrate and phosphate in soil and water samples. Microchem. J. 2023, 193, 109042. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Vakh, C. Analytical applications of smartphones for agricultural soil analysis. Anal. Bioanal. Chem. 2023, 415, 3703–3715. [Google Scholar] [CrossRef] [PubMed]
- e Silva, G.M.; Garcia, J.A.; de Alencar Garitta, J.; Cunha, D.G.F.; Finkler, N.R.; Mendiondo, E.M.; Ghiglieno, F. Smartphone-based spectrometry system as a prescreening assessment of copper and iron for real time control of water pollution. J. Environ. Manag. 2022, 323, 116214. [Google Scholar] [CrossRef]
- Gómez-Robledo, L.; López-Ruiz, N.; Melgosa, M.; Palma, A.J.; Capitán-Vallvey, L.F.; Sánchez-Marañón, M. Using the mobile phone as Munsell soil-colour sensor: An experiment under controlled illumination conditions. Comput. Electron. Agric. 2013, 99, 200–208. [Google Scholar] [CrossRef]
- Sims, J.R.; Jackson, G.D. Rapid analysis of soil nitrate with chromotropic acid. Soil Sci. Soc. Am. J. 1971, 35, 603–606. [Google Scholar] [CrossRef]
- Cogan, D.; Fay, C.; Boyle, D.; Osborne, C.; Kent, N.; Cleary, J.; Diamond, D. Development of a low cost microfluidic sensor for the direct determination of nitrate using chromotropic acid in natural waters. Anal. Methods 2015, 7, 5396–5405. [Google Scholar] [CrossRef]
- Nakra, B.C.; Chaudhry, K.K. Instrumentation, Measurement and Analysis; Tata McGraw-Hill Education: New York, NY, USA, 2003. [Google Scholar]
- Ibraheem, N.A.; Hasan, M.M.; Khan, R.Z.; Mishra, P.K. Understanding color models: A review. ARPN J. Sci. Technol. 2012, 2, 265–275. [Google Scholar]
- Woods, R.C.; Gonzalez, R.E. Digital Image Processing, 3rd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Dasgupta, S.; Chakraborty, S.; Weindorf, D.C.; Li, B.; Silva, S.H.G.; Bhattacharyya, K. Influence of auxiliary soil variables to improve PXRF-based soil fertility evaluation in India. Geoderma Reg. 2022, 30, e00557. [Google Scholar] [CrossRef]
- Camden, R.K. Apache Cordova in Action; Manning: New York, NY, USA, 2015. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service, U.S. Government Print Office: Washington, DC, USA, 2014.
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 25 May 2023).
- Kendall Rank Correlation and Mann-Kendall Trend Test. Available online: https://cran.r-project.org/web/packages/Kendall/ (accessed on 25 May 2023).
- Li, B.; Yu, Q. A nonparametric test of independence between 2 variables. Stat. Anal. Data Min. 2017, 10, 422–435. [Google Scholar] [CrossRef]
- Krige, D.G. Two-dimensional weighted moving average trend surfaces for ore valuations. J. S. Afr. Inst. Min. Metall. 1966, 66, 13–38. [Google Scholar]
- Duda, B.M.; Weindorf, D.C.; Chakraborty, S.; Li, B.; Man, T.; Paulette, L.; Deb, S. Soil characterization across catenas via advanced proximal sensors. Geoderma 2017, 298, 78–91. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef]
- Agu, E.; Pedersen, P.; Strong, D.; Tulu, B.; He, Q.; Wang, L.; Li, Y. The smartphone as a medical device: Assessing enablers, benefits and challenges. In Proceedings of the 2013 IEEE International Workshop of Internet-of-Things Networking and Control (IoT-NC), New Orleans, LA, USA, 24 June 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 48–52. [Google Scholar]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Daeid, N.N. Using the iPhone as a device for a rapid quantitative analysis of trinitrotoluene in soil. Talanta 2013, 115, 143–149. [Google Scholar] [CrossRef]
- Kwon, L.; Long, K.D.; Wan, Y.; Yu, H.; Cunningham, B.T. Medical diagnostics with mobile devices: Comparison of intrinsic and extrinsic sensing. Biotechnol. Adv. 2016, 34, 291–304. [Google Scholar] [CrossRef]
- Jones, D.L.; Cross, P.; Withers, P.J.; DeLuca, T.H.; Robinson, D.A.; Quilliam, R.S.; Harris, I.M.; Chadwick, D.R.; Edwards-Jones, G. Nutrient stripping: The global disparity between food security and soil nutrient stocks. J. Appl. Ecol. 2013, 50, 851–862. [Google Scholar] [CrossRef]
- Dasgupta, S.; Debnath, S.; Das, A.; Biswas, A.; Weindorf, D.C.; Li, B.; Shukla, A.K.; Das, S.; Saha, S.; Chakraborty, S. Developing regional soil micronutrient management strategies through ensemble learning based digital soil mapping. Geoderma 2023, 433, 116457. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Ma, K.; Zhou, H.; Wang, J. The relationships between land use and soil conditions in the hilly area of the loess plateau in northern Shaanxi, China. Catena 2000, 39, 69–78. [Google Scholar] [CrossRef]
- Venteris, E.; Basta, N.; Bigham, J.; Rea, R. Modeling spatial patterns in soil arsenic to estimate natural baseline concentrations. J. Environ. Qual. 2014, 43, 936–946. [Google Scholar] [CrossRef]
- Scavo, A.; Fontanazza, S.; Restuccia, A.; Pesce, G.R.; Abbate, C.; Mauromicale, G. The role of cover crops in improving soil fertility and plant nutritional status in temperate climates. A review. Agron. Sustain. Dev. 2022, 42, 93. [Google Scholar] [CrossRef]
- Liu, B.; Gumpertz, M.L.; Hu, S.; Ristaino, J.B. Long-term effects of organic and synthetic soil fertility amendments on soil microbial communities and the development of southern blight. Soil Biol. Biochem. 2007, 39, 2302–2316. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Chaudhari, S.K. Soil and water management in India: Challenges and opportunities. In Soil Science: Fundamentals to Recent Advances; Rakshit, A., Singh, S.K., Abhilash, P.C., Biswas, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 751–764. [Google Scholar]
- Venter, C. Regenerative farming: When agriculture and technology connect. Stockfarm 2021, 11, 15–17. [Google Scholar]
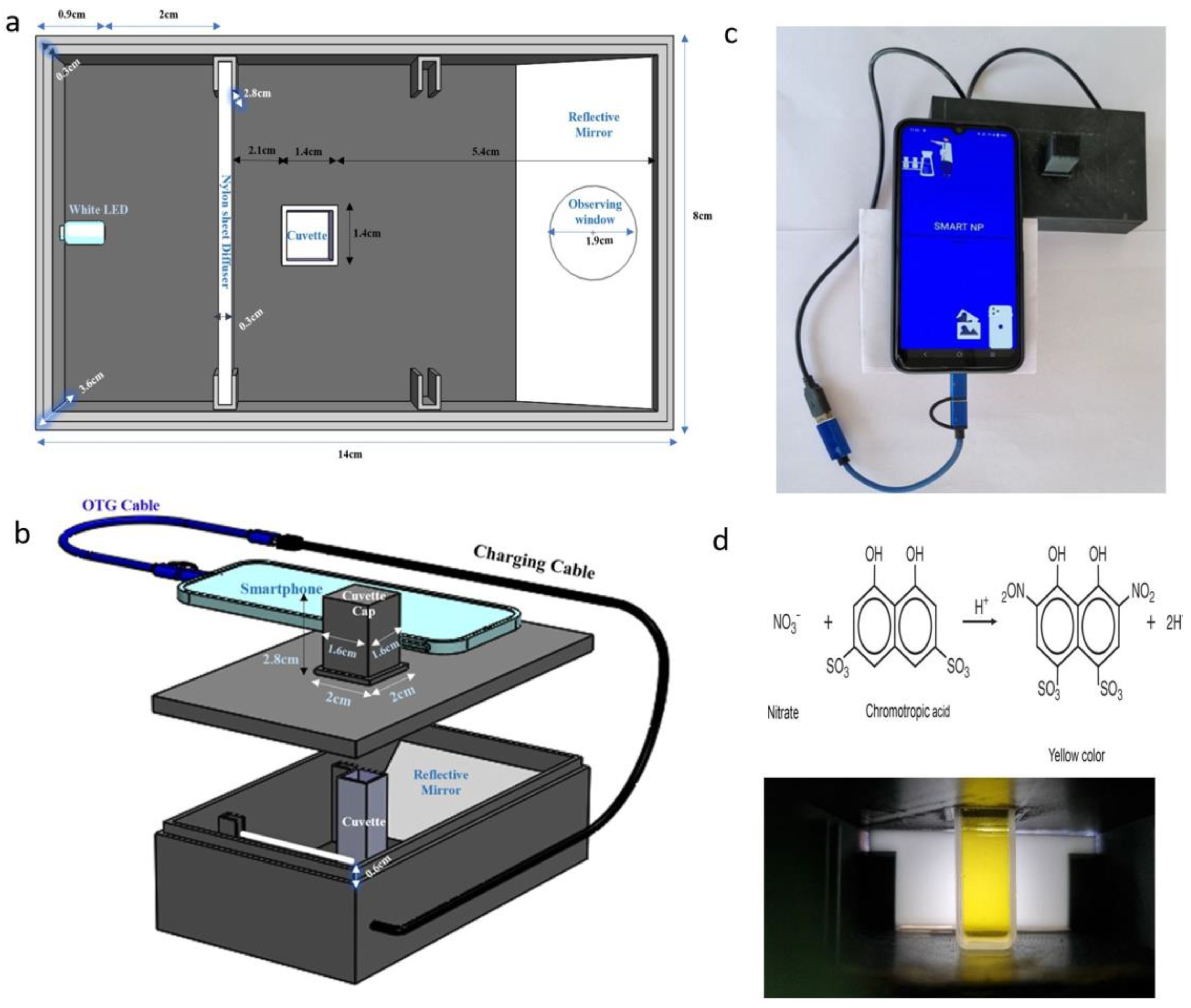


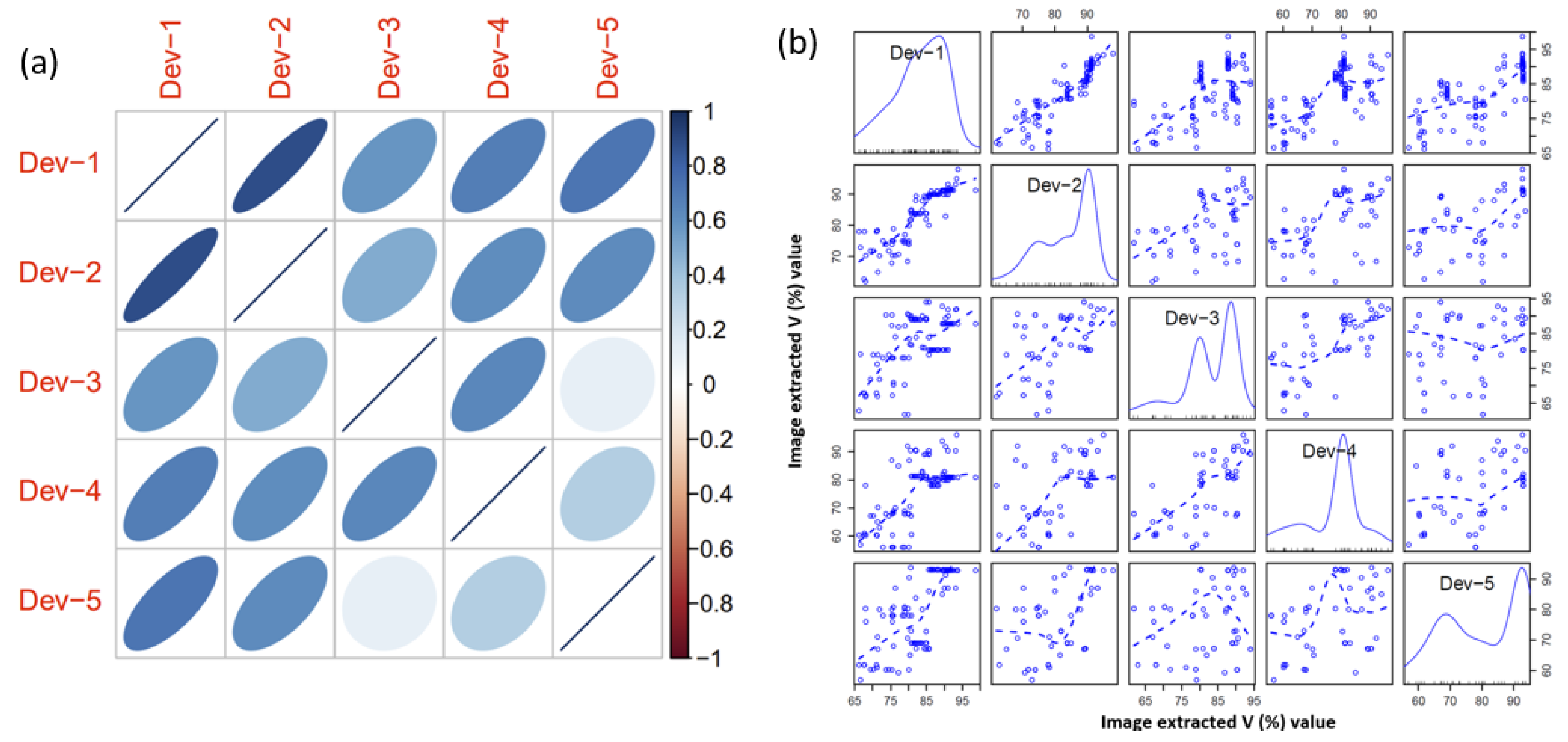
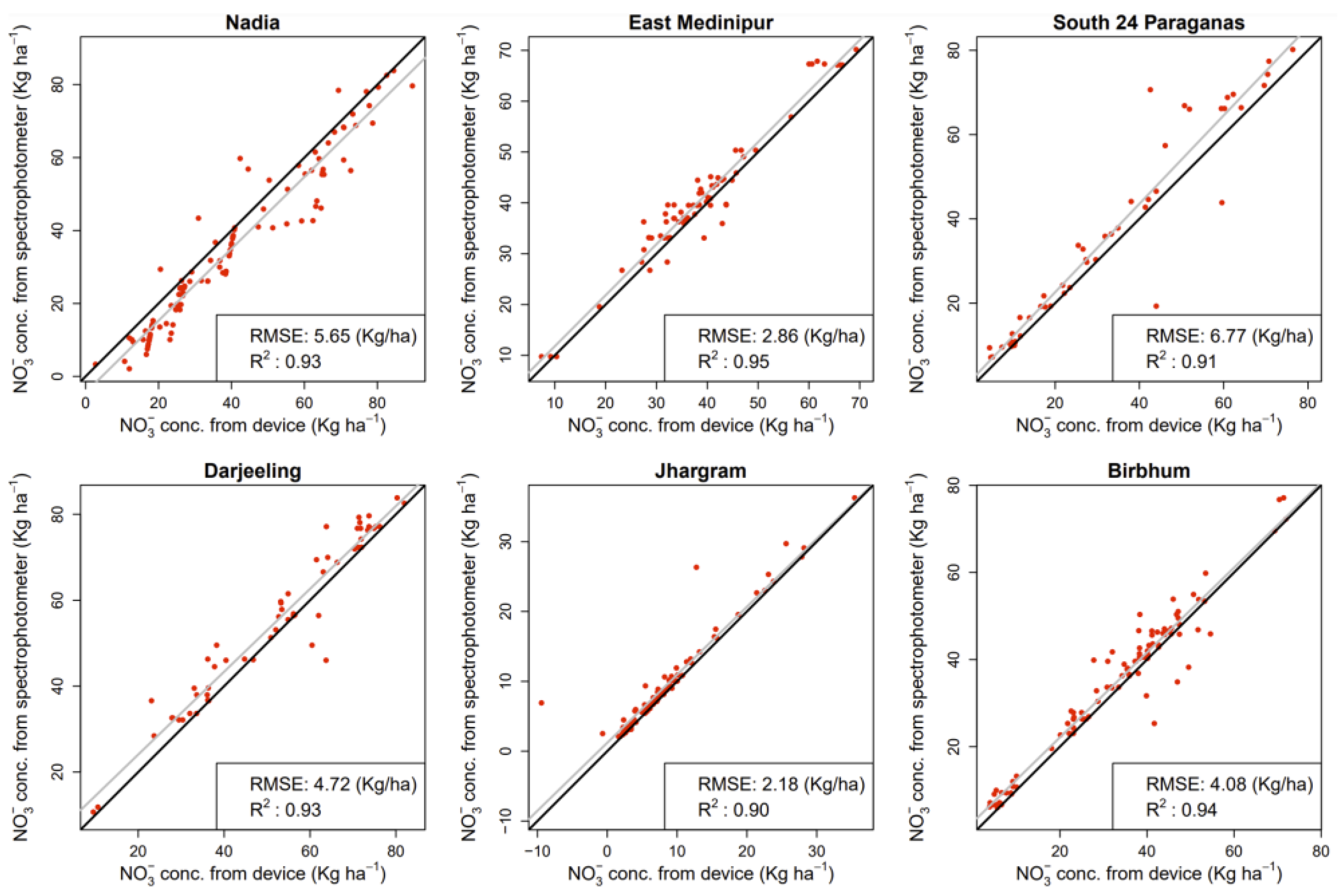
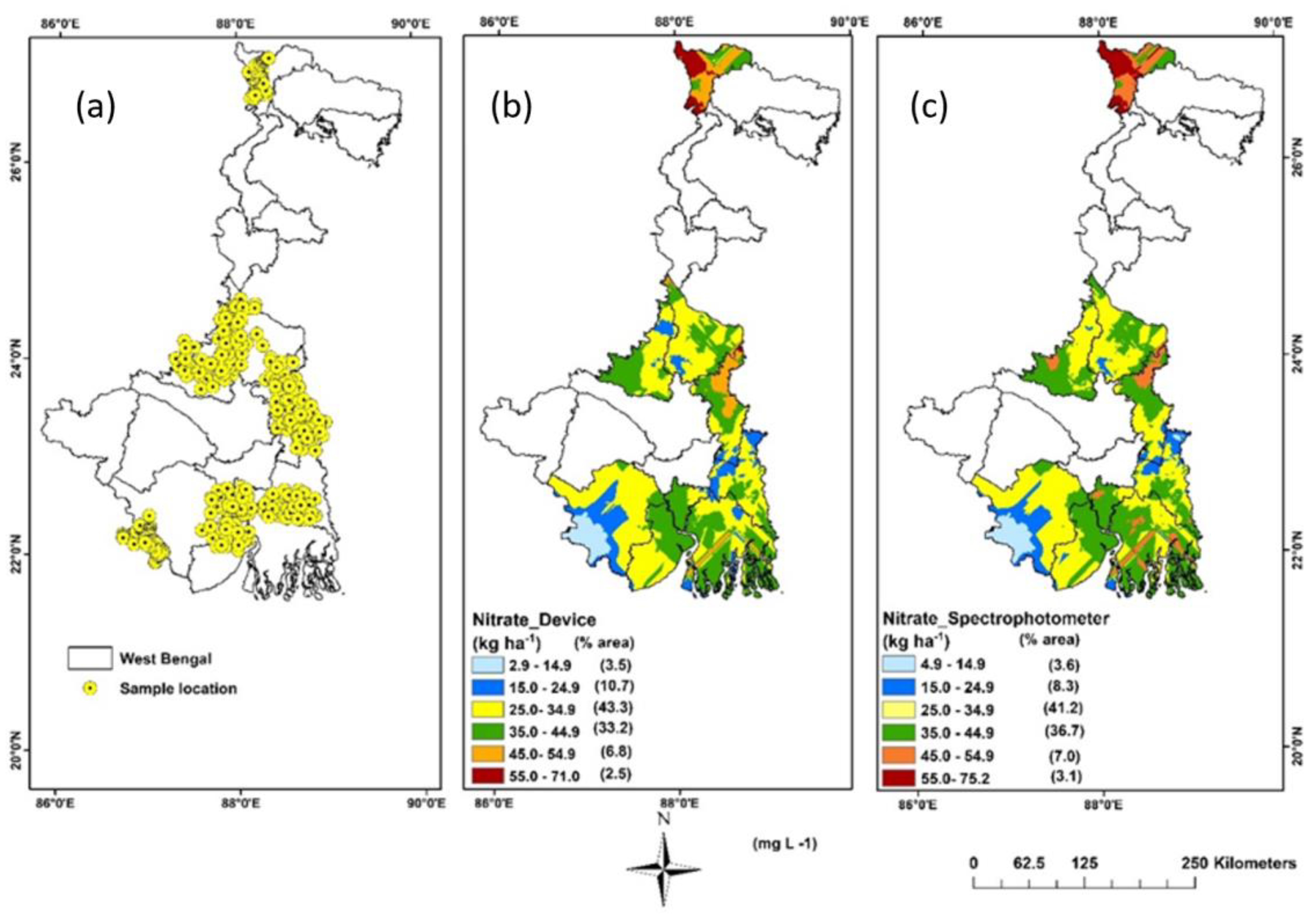
| Smartphone | Camera (Megapixels) | Media | R2 | Equation |
|---|---|---|---|---|
| HONOR-20i | 24 | Soil | 0.98 | NO3− = 24.82 − 0.26x 1 |
| Redmi 12 Pro | 50 | 0.98 | NO3− = 231.75 − 2.34x | |
| Vivo Y1S | 13 | 0.90 | NO3− = 171.12 − 1.70x |
| Authors | Media | Analyte | R2 | Equation |
|---|---|---|---|---|
| Present study | Soil | NO3− | 0.98 | NO3− = 24.82 − 0.26x 1 |
| [16] | NO3− | 0.98 | NO3− = 16.94 − 0.17x | |
| [16] | PO43− | 0.96 | PO43− = 2.49 − 0.026x | |
| [14] | PO43− | 0.99 | PO43− = 0.3930 × exp(−x/1.854) + 0.3978 | |
| [16] | Water | NO3− | 0.97 | NO3− = 11.87 − 0.12x |
| [16] | PO43− | 0.98 | PO43− = 45.74 − 0.49x | |
| [14] | PO43− | 0.99 | PO43− = 0.3930 × exp(−x/1.854) + 0.3978 | |
| [13] | Cl | 0.99 | Cl = 86.008z 5,2 − 359.04z 4 + 556.14z 3 − 402.96z 2 + 135.15z − 15.804 |
| Kriging Parameters | Device-Predicted Values | Laboratory-Measured Values |
|---|---|---|
| Variogram model | Gaussian | Gaussian |
| Nugget | 204.84 | 222.64 |
| Partial Sill | 47.65 | 39.68 |
| Nugget/Sill Ratio | 0.81 | 0.85 |
| Regression equation | Soil NO3− = 0.51x 1 + 15.12 | Soil NO3− = 0.49x + 16.35 |
| RMSE | 1.07 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavanya, V.; Nayak, A.; Deb Roy, P.; Dasgupta, S.; Dey, S.; Li, B.; Weindorf, D.C.; Chakraborty, S. A Smartphone-Enabled Imaging Device for Chromotropic Acid-Based Measurement of Nitrate in Soil Samples. Sensors 2023, 23, 7345. https://doi.org/10.3390/s23177345
Lavanya V, Nayak A, Deb Roy P, Dasgupta S, Dey S, Li B, Weindorf DC, Chakraborty S. A Smartphone-Enabled Imaging Device for Chromotropic Acid-Based Measurement of Nitrate in Soil Samples. Sensors. 2023; 23(17):7345. https://doi.org/10.3390/s23177345
Chicago/Turabian StyleLavanya, Veerabhadrappa, Anshuman Nayak, Partha Deb Roy, Shubhadip Dasgupta, Subhadip Dey, Bin Li, David C. Weindorf, and Somsubhra Chakraborty. 2023. "A Smartphone-Enabled Imaging Device for Chromotropic Acid-Based Measurement of Nitrate in Soil Samples" Sensors 23, no. 17: 7345. https://doi.org/10.3390/s23177345






