Label-Free DNA Detection Using Etched Tilted Bragg Fiber Grating-Based Biosensor
Abstract
:1. Introduction
2. Sensing Principle, Fabrication and Performance of TFBG Length
2.1. Sensing Principle
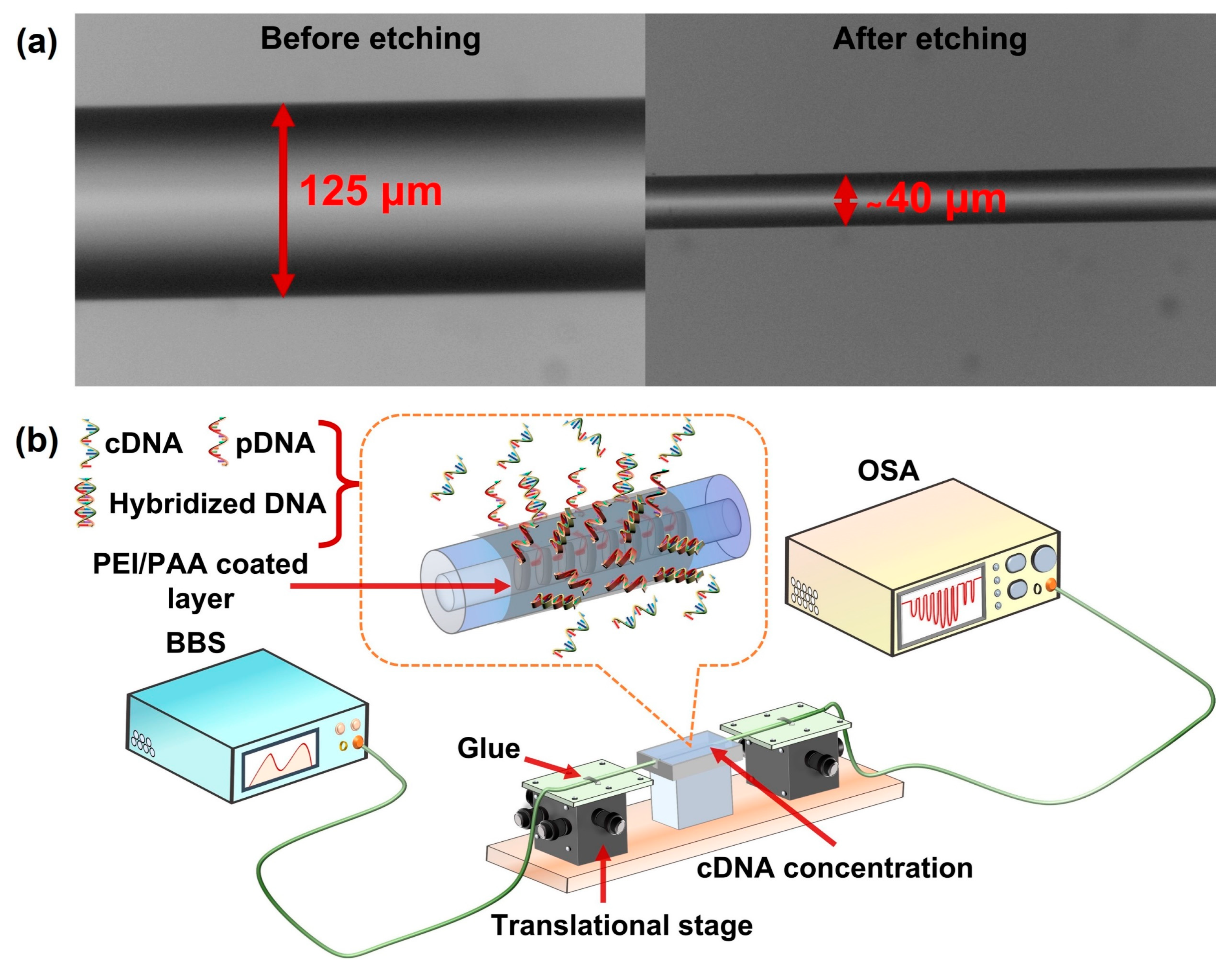
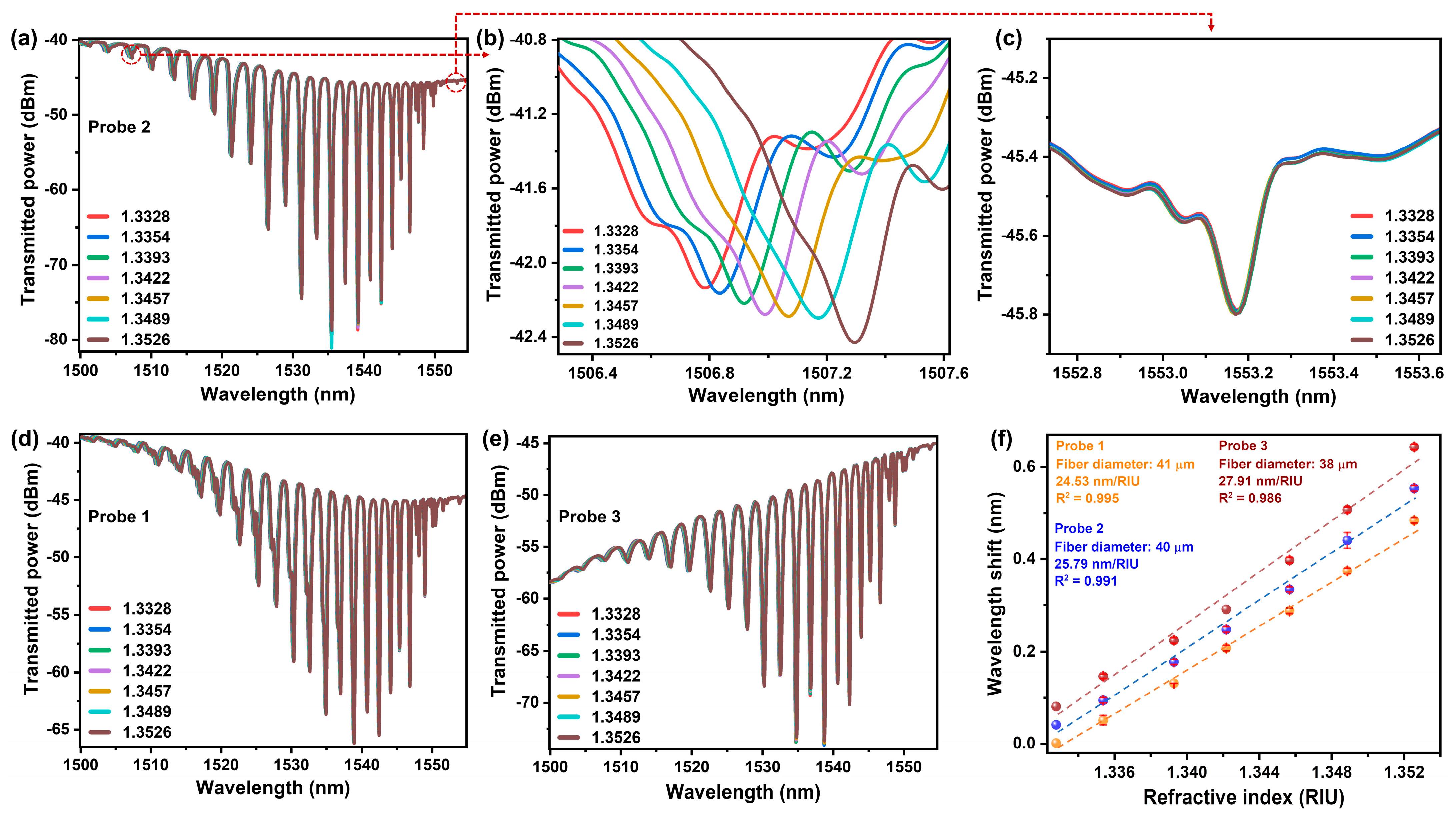
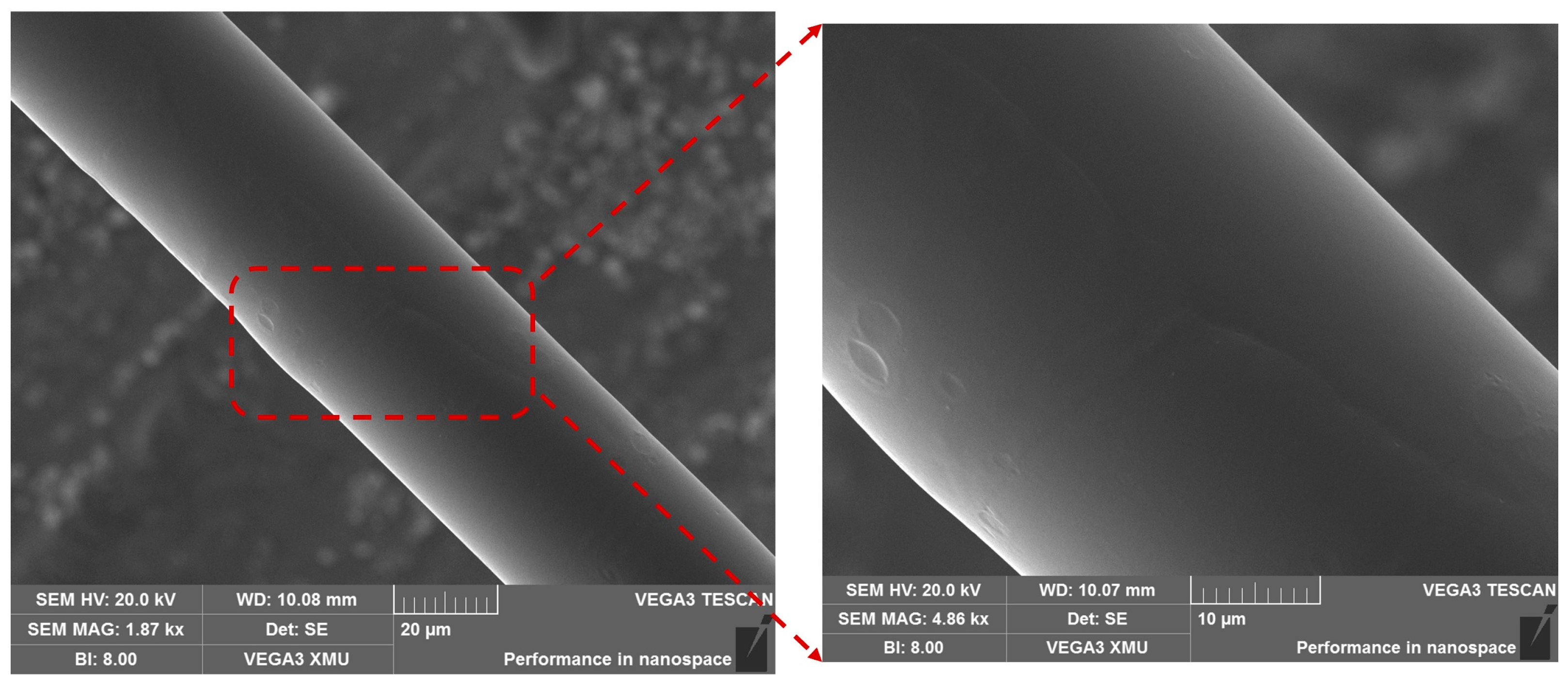
2.2. TFBG Fabrication, Experimental Setup and Response towards Change in RI
3. Materials and DNA Sensor Fabrication
3.1. Materials
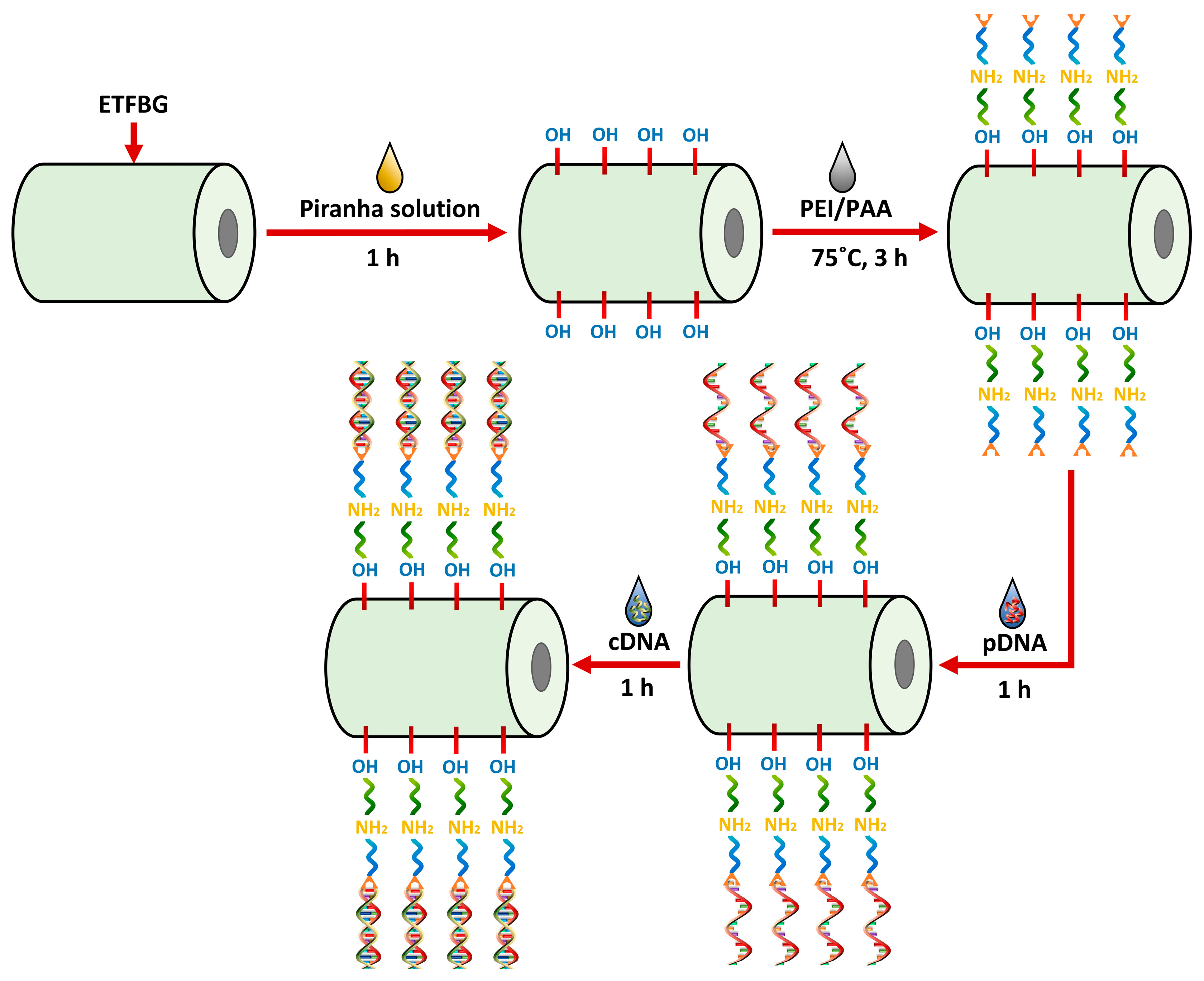
3.2. Sensor Fabrication for DNA
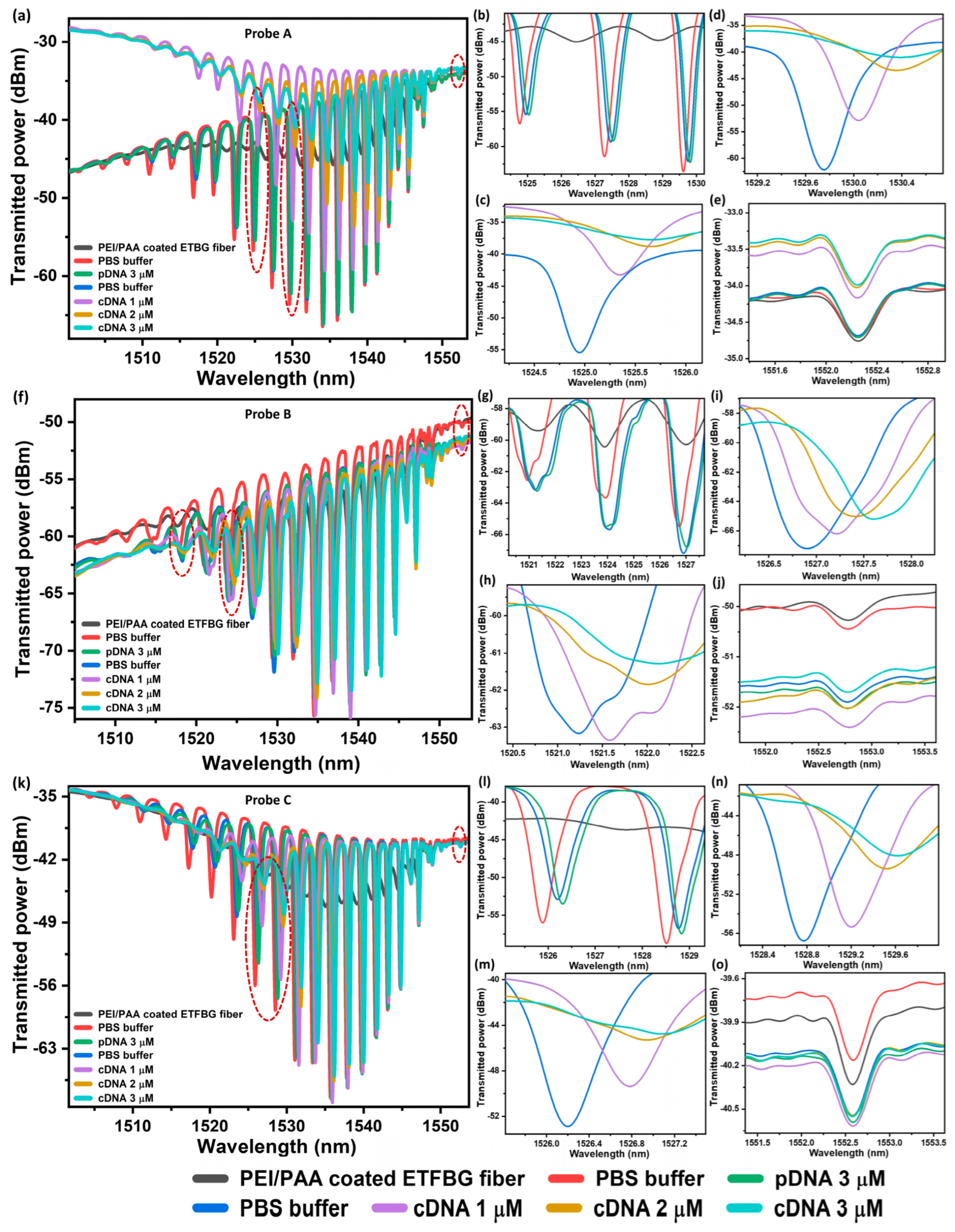
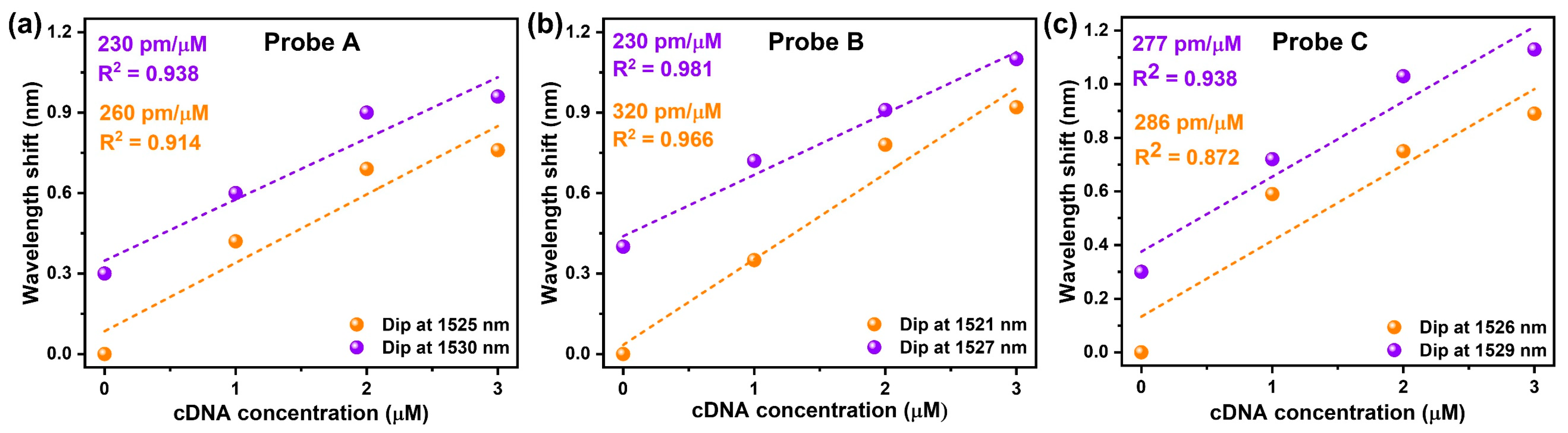
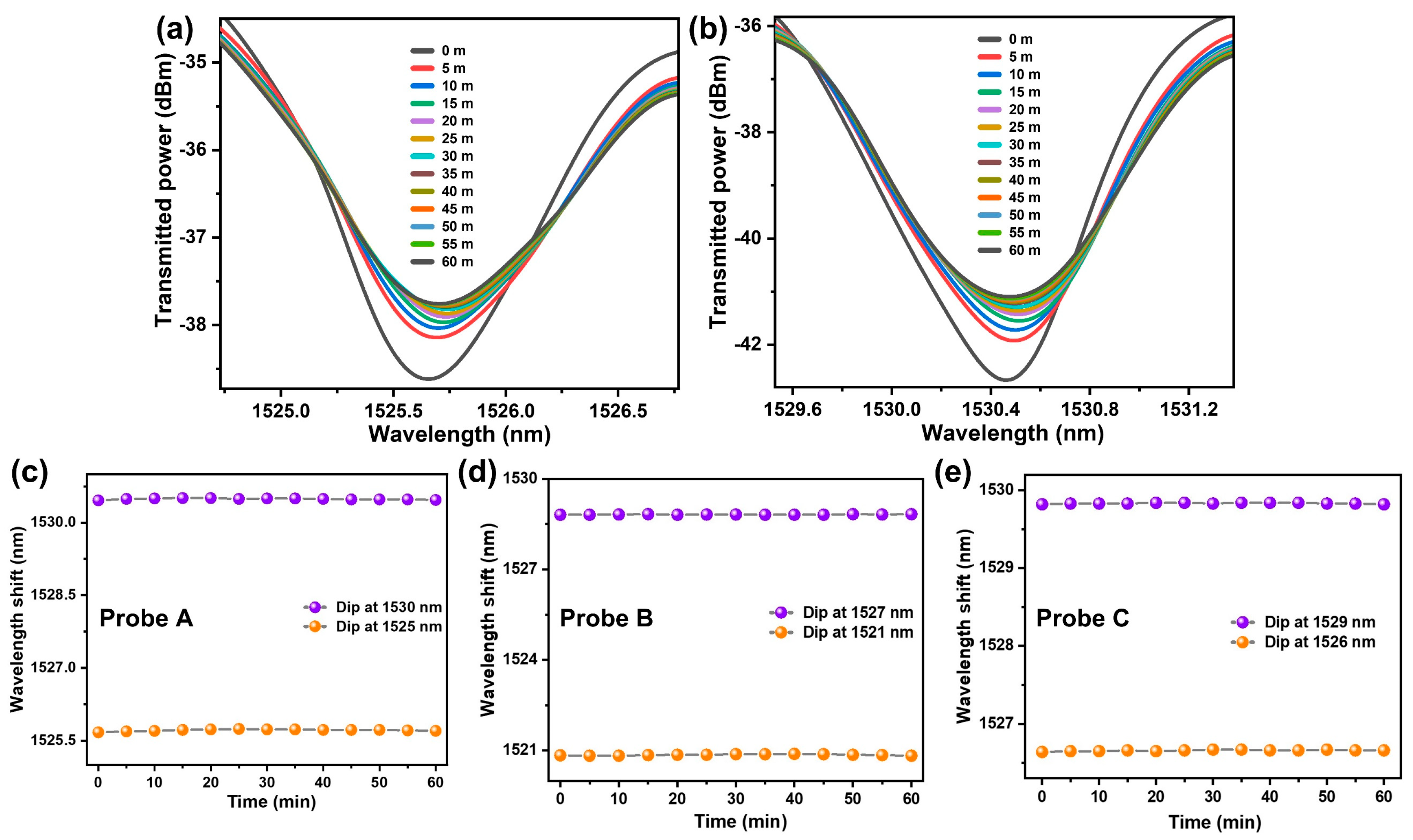
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caygill, R.L.; Blair, G.E.; Millner, P.A. A review on viral biosensors to detect human pathogens. Anal. Chim. Acta 2010, 681, 8–15. [Google Scholar] [CrossRef]
- Cooper, J.; Cass, A. Biosensors; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Rasooly, A.; Herold, K.E.; Herold, K.E. Biosensors and Biodetection; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Parandin, F.; Heidari, F.; Rahimi, Z.; Olyaee, S. Two-Dimensional photonic crystal Biosensors: A review. Opt. Laser Technol. 2021, 144, 107397. [Google Scholar] [CrossRef]
- Li, M.; He, F.; Liao, Q.; Liu, J.; Xu, L.; Jiang, L.; Song, Y.; Wang, S.; Zhu, D. Ultrasensitive DNA detection using photonic crystals. Angew. Chem. 2008, 120, 7368–7372. [Google Scholar] [CrossRef]
- Leung, A.; Shankar, P.M.; Mutharasan, R. A review of fiber-optic biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Porfireva, A.V.; Goida, A.I.; Rogov, A.M.; Evtugyn, G.A. Impedimetric DNA sensor based on poly (proflavine) for determination of anthracycline drugs. Electroanalysis 2020, 32, 827–834. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Benmerkhi, A.; Bouchemat, M.; Bouchemat, T. Design of two-dimensional photonic crystal biosensor using DNA detection. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 960–964. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Paquim, A.-M.C.; Brett, A.M.O. Electrochemical DNA sensors for detection of DNA damage. Sensors 2005, 5, 377–393. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers. Chem. Mater. 2004, 16, 4467–4476. [Google Scholar] [CrossRef]
- Liu, G.; Wan, Y.; Gau, V.; Zhang, J.; Wang, L.; Song, S.; Fan, C. An enzyme-based E-DNA sensor for sequence-specific detection of femtomolar DNA targets. J. Am. Chem. Soc. 2008, 130, 6820–6825. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Jiang, Y.; Zhang, S. Fluorescence detection for DNA using hybridization chain reaction with enzyme-amplification. Chem. Commun. 2010, 46, 3089–3091. [Google Scholar] [CrossRef] [PubMed]
- Al Noman, A.; Dash, J.N.; Cheng, X.; Leong, C.Y.; Tam, H.-Y.; Yu, C. Hydrogel based Fabry-Pérot cavity for a pH sensor. Opt. Express 2020, 28, 39640–39648. [Google Scholar] [CrossRef] [PubMed]
- Al Noman, A.; Dash, J.N.; Cheng, X.; Tam, H.-Y.; Yu, C. Mach-Zehnder interferometer based fiber-optic nitrate sensor. Opt. Express 2022, 30, 38966–38974. [Google Scholar] [CrossRef]
- Johari, M.A.M.; Al Noman, A.; Khudus, M.A.; Jali, M.H.; Yusof, H.H.M.; Harun, S.W.; Yasin, M. Microbottle resonator for formaldehyde liquid sensing. Optik 2018, 173, 180–184. [Google Scholar] [CrossRef]
- Pollet, J.; Delport, F.; Janssen, K.P.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA–protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Zhou, X.; Gong, P.; Zhang, Y.; Zhao, Y.; Nguyen, L.V.; Ebendorff-Heidepriem, H.; Warren-Smith, S.C. Plug-in label-free optical fiber DNA hybridization sensor based on C-type fiber Vernier effect. Sens. Actuators B Chem. 2022, 354, 131212. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Zhou, X.; Zhang, Y.; Zhao, Y.; Nguyen, L.V.; Ebendorff-Heidepriem, H.; Warren-Smith, S.C. In-situ DNA detection with an interferometric-type optical sensor based on tapered exposed core microstructured optical fiber. Sens. Actuators B Chem. 2022, 351, 130942. [Google Scholar] [CrossRef]
- Kashif, M.; Bakar, A.A.A.; Arsad, N.; Shaari, S. Development of phase detection schemes based on surface plasmon resonance using interferometry. Sensors 2014, 14, 15914–15938. [Google Scholar] [CrossRef] [Green Version]
- Ran, Z.; He, X.; Rao, Y.; Sun, D.; Qin, X.; Zeng, D.; Chu, W.; Li, X.; Wei, Y. Fiber-optic microstructure sensors: A review. Photonic Sens. 2021, 11, 227–261. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Baldini, F.; Tombelli, S.; Trono, C.; Giannetti, A. Biosensing with optical fiber gratings. Nanophotonics 2017, 6, 663–679. [Google Scholar] [CrossRef]
- Jang, H.S.; Park, K.N.; Kim, J.P.; Sim, S.J.; Kwon, O.J.; Han, Y.-G.; Lee, K.S. Sensitive DNA biosensor based on a long-period grating formed on the side-polished fiber surface. Opt. Express 2009, 17, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, C.; Hughes, M.D.; Nagel, D.A.; Hine, A.V.; Zhang, L. EDC-mediated oligonucleotide immobilization on a long period grating optical biosensor. J. Biosens. Bioelectron. 2015, 6, 1000173. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Guo, T.; Ran, Y.; Huang, Y.; Guan, B.-O. In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 2014, 61, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Guo, T.; Guan, B.-O. Label-free detection of DNA hybridization using a reflective microfiber bragg grating biosensor with self-assembly technique. J. Light. Technol. 2017, 35, 3354–3359. [Google Scholar] [CrossRef]
- Miao, Y.-p.; Liu, B. Refractive index sensor based on measuring the transmission power of tilted fiber Bragg grating. Opt. Fiber Technol. 2009, 15, 233–236. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, X.; Gong, H.; Shen, C. Refractive-index sensor based on tilted fiber Bragg grating interacting with multimode fiber. Microw. Opt. Technol. Lett. 2010, 52, 1375–1377. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, S.; Chi, H.; Jin, X.; Zhang, X. Refractive index sensor based on tilted fiber Bragg grating and stimulated Brillouin scattering. Opt. Express 2012, 20, 10853–10858. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, M.; Jiang, S.; Du, L.; Cheng, Y.; Li, P.; Wang, C. Design and fabrication of Gr/Ag-coated tilted grating sensor for ultra-sensitive detection of DNA hybridization. Sens. Actuators B Chem. 2022, 359, 131587. [Google Scholar] [CrossRef]
- Baker, A.; Saltik, M.; Lehrmann, H.; Killisch, I.; Mautner, V.; Lamm, G.; Christofori, G.; Cotten, M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997, 4, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Raghuwanshi, S.K.; Prakash, O.; Saini, P.K. Design and development of tilted fiber Bragg grating (TFBG) chemical sensor with regression analysis of grating parameters for sensitivity optimization. Opt. Quantum Electron. 2021, 53, 664. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Hu, Z.; Zhang, Y.; Wu, C.; Yang, M.; Wang, P. A novel electrochemical biosensor based on dynamic polymerase-extending hybridization for E. coli O157: H7 DNA detection. Talanta 2009, 78, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hussain, A.; Bai, S.; Khalil, I.A.; Harashima, H.; Ahsan, F. Positively charged polyethylenimines enhance nasal absorption of the negatively charged drug, low molecular weight heparin. J. Control. Release 2006, 115, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Zhang, A.P.; He, S.; Gu, B. Cladding-mode-recoupling-based tilted fiber Bragg grating sensor with a core-diameter-mismatched fiber section. IEEE Photonics J. 2010, 2, 152–157. [Google Scholar]

| Sensing Mechanism | Optical Fiber Structure | Coating Materials | Detection Range | Sensitivity | LOD | Ref. |
|---|---|---|---|---|---|---|
| FPI | C-type fiber + SMF | Piranha solution/APTEs | 1 μM | 4310 pm/μM | 67.5 nM | [19] |
| MZI | Tapered exposed core fiber + SMF | Piranha solution/APTEs | 0–30 nM | 61.8 pm/nM | 0.31 nM | [20] |
| LPG | Side-polished fiber | PLL | 1 μM | 1182 pm/μM | - | [24] |
| LPG | SMF | APTEs/EDC | 2 μM | 245 pm/μM | 4 nM | [25] |
| FBG | Microfiber | PLL | 0.5–1 μM | 456 pm/μM | 0.5 μM | [26] |
| SPR + TFBG | SMF | Gr/Ag | 0.0001–2 nM | - | 3 pM | [31] |
| TFBG | Etched SMF | PEI/PAA | 0–3 μM | 320 pm/μM | 0.65 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noman, A.A.; Dash, J.N.; Maruf, M.A.A.; Xin, C.; Tam, H.-Y.; Yu, C. Label-Free DNA Detection Using Etched Tilted Bragg Fiber Grating-Based Biosensor. Sensors 2023, 23, 7019. https://doi.org/10.3390/s23167019
Noman AA, Dash JN, Maruf MAA, Xin C, Tam H-Y, Yu C. Label-Free DNA Detection Using Etched Tilted Bragg Fiber Grating-Based Biosensor. Sensors. 2023; 23(16):7019. https://doi.org/10.3390/s23167019
Chicago/Turabian StyleNoman, Abdullah Al, Jitendra Narayan Dash, Md Abdullah Al Maruf, Cheng Xin, Hwa-Yam Tam, and Changyuan Yu. 2023. "Label-Free DNA Detection Using Etched Tilted Bragg Fiber Grating-Based Biosensor" Sensors 23, no. 16: 7019. https://doi.org/10.3390/s23167019






