Electrocortical Dynamics of Usual Walking and the Planning to Step over Obstacles in Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Clinical Assessments
2.2. Experimental Design and Gait Assessment
2.3. EEG Recordings and Processing
2.3.1. Independent Component Analysis (ICA)
2.3.2. EEG Clustering
2.3.3. EEG Absolute Power from Independent Components
2.4. Statistical Analysis
3. Results
3.1. Gait Parameters
3.2. Electroencephalography
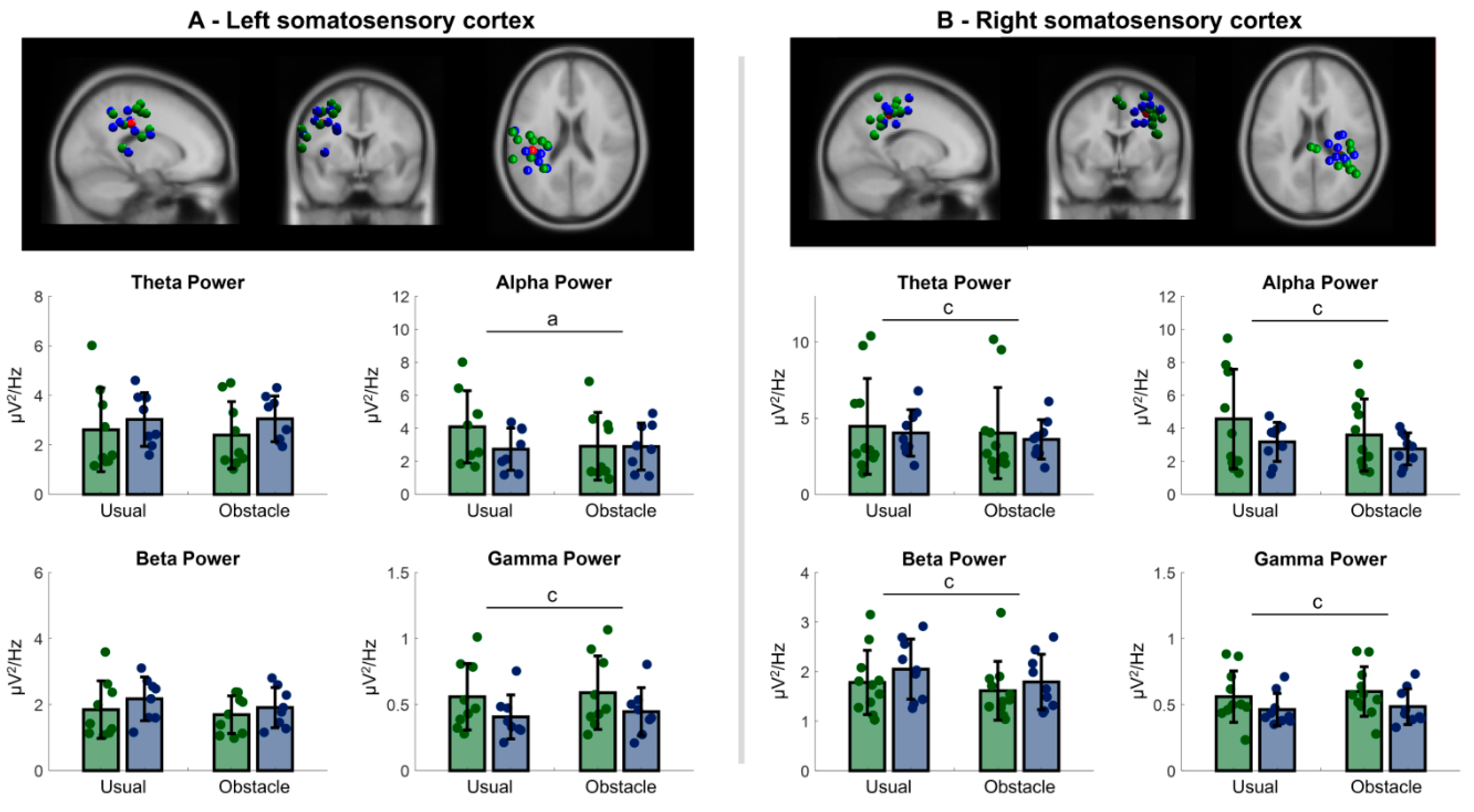
3.2.1. Left Sensorimotor Cortex
3.2.2. Right Sensorimotor Cortex
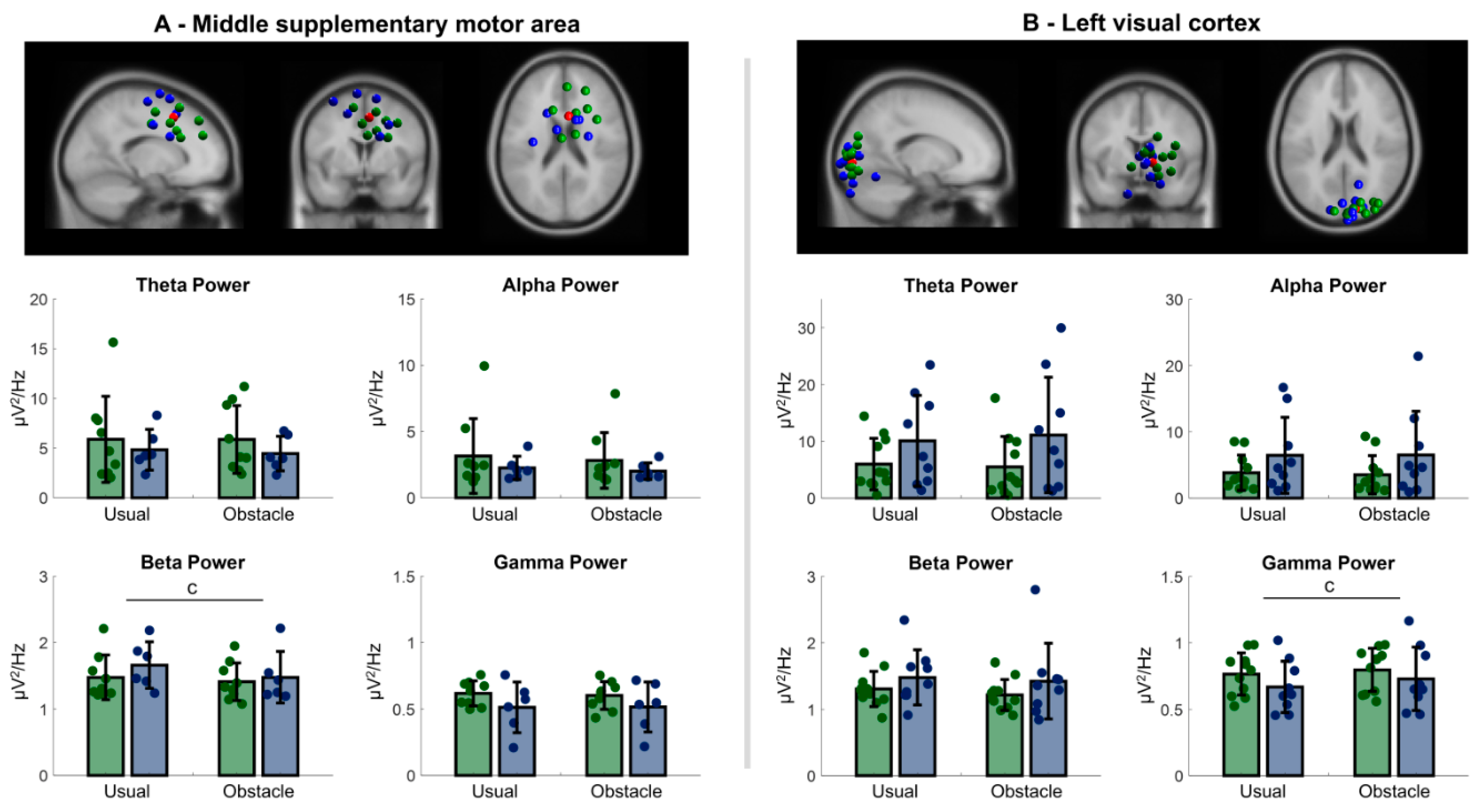
3.2.3. Central Premotor and Supplementary Motor Area
3.2.4. Primary Visual Cortex
4. Discussion
4.1. PD-Related Changes in the Cortical Control of Locomotion
4.2. Gait and Electrocortical Modulations Required for Obstacle Avoidance
4.3. Clinical Implication and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Anidi, C.; O’Day, J.J.; Anderson, R.W.; Afzal, M.F.; Syrkin-Nikolau, J.; Velisar, A.; Bronte-Stewart, H.M. Neuromodulation Targets Pathological Not Physiological Beta Bursts during Gait in Parkinson’s Disease. Neurobiol. Dis. 2018, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-X.; Wang, J.; Yi, G.-S.; Che, Y.-Q. Investigation of EEG Abnormalities in the Early Stage of Parkinson’s Disease. Cogn. Neurodyn. 2013, 7, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bockova, M.; Rektor, I. Impairment of Brain Functions in Parkinson’s Disease Reflected by Alterations in Neural Connectivity in EEG Studies: A Viewpoint. Clin. Neurophysiol. 2019, 130, 239–247. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Human Electrocortical Dynamics While Stepping over Obstacles. Sci. Rep. 2019, 9, 4693. [Google Scholar] [CrossRef]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical Activity Is Coupled to Gait Cycle Phase during Treadmill Walking. Neuroimage 2011, 54, 1289–1296. [Google Scholar] [CrossRef]
- Sipp, A.R.; Gwin, J.T.; Makeig, S.; Ferris, D.P. Loss of Balance during Balance Beam Walking Elicits a Multifocal Theta Band Electrocortical Response. J. Neurophysiol. 2013, 110, 2050–2060. [Google Scholar] [CrossRef]
- Hairston, W.D.; König, P.; Ferris, D.P.; Oliveira, A.S.; Schlink, B.R. Restricted Vision Increases Sensorimotor Cortex Involvement in Human Walking. J. Neurophysiol. 2017, 118, 1943–1951. [Google Scholar] [CrossRef]
- Roeder, L.; Boonstra, T.W.; Kerr, G.K. Corticomuscular Control of Walking in Older People and People with Parkinson’s Disease. Sci. Rep. 2020, 10, 2980. [Google Scholar] [CrossRef]
- Peterson, D.S.; Horak, F.B. Neural Control of Walking in People with Parkinsonism. Physiology 2016, 31, 95–107. [Google Scholar] [CrossRef]
- Stuart, S.; Vitorio, R.; Morris, R.; Martini, D.N.; Fino, P.C.; Mancini, M. Cortical Activity during Walking and Balance Tasks in Older Adults and in People with Parkinson’s Disease: A Structured Review. Maturitas 2018, 113, 53–72. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Vitorio, R.; Lirani-Silva, E.; Baptista, A.M.; Barbieri, F.A.; dos Santos, P.C.R.; Teixeira-Arroyo, C.; Gobbi, L.T.B. Disease Severity Affects Obstacle Crossing in People with Parkinson’s Disease. Gait Posture 2014, 40, 266–269. [Google Scholar] [CrossRef]
- Vitório, R.; Pieruccini-Faria, F.; Stella, F.; Gobbi, S.; Gobbi, L.T.B. Effects of Obstacle Height on Obstacle Crossing in Mild Parkinson’s Disease. Gait Posture 2010, 31, 143–146. [Google Scholar] [CrossRef]
- Orcioli-Silva, D.; Barbieri, F.A.; dos Santos, P.C.R.; Beretta, V.S.; Simieli, L.; Vitorio, R.; Lirani-Silva, E.; Gobbi, L.T.B. Double Obstacles Increase Gait Asymmetry during Obstacle Crossing in People with Parkinson’s Disease and Healthy Older Adults: A Pilot Study. Sci. Rep. 2020, 10, 2272. [Google Scholar] [CrossRef]
- Ashburn, A.; Stack, E.; Ballinger, C.; Fazakarley, L.; Fitton, C. The Circumstances of Falls among People with Parkinson’s Disease and the Use of Falls Diaries to Facilitate Reporting. Disabil. Rehabil. 2008, 30, 1205–1212. [Google Scholar] [CrossRef]
- Moraca, G.A.G.; Beretta, V.S.; Dos Santos, P.C.R.; Nóbrega-Sousa, P.; Orcioli-Silva, D.; Vitório, R.; Gobbi, L.T.B. Center of Pressure Responses to Unpredictable External Perturbations Indicate Low Accuracy in Predicting Fall Risk in People with Parkinson’s Disease. Eur. J. Neurosci. 2021, 53, 2901–2911. [Google Scholar] [CrossRef]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Reelick, M.F.; Bloem, B.R.; Giladi, N.; Deutsch, J.E.; Hausdorff, J.M.; Claassen, J.A.H.; Mirelman, A. The Role of the Frontal Lobe in Complex Walking among Patients with Parkinson’s Disease and Healthy Older Adults: An FNIRS Study. Neurorehabil. Neural Repair 2016, 30, 963–971. [Google Scholar] [CrossRef]
- Orcioli-Silva, D.; Vitório, R.; Beretta, V.S.; da Conceição, N.R.; Nóbrega-Sousa, P.; Oliveira, A.S.; Gobbi, L.T.B. Is Cortical Activation During Walking Different between Parkinson’s Disease Motor Subtypes? J. Gerontol. Ser. A 2021, 76, 561–567. [Google Scholar] [CrossRef]
- Orcioli-Silva, D.; Vitório, R.; Nóbrega-Sousa, P.; Beretta, V.S.; da Conceição, N.R.; Oliveira, A.S.; Pereira, M.P.; Gobbi, L.T.B. Cortical Activity Underlying Gait Improvements Achieved with Dopaminergic Medication during Usual Walking and Obstacle Avoidance in Parkinson Disease. Neurorehabil. Neural Repair 2021, 35, 406–418. [Google Scholar] [CrossRef]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of Participation in Robotic-Assisted Treadmill Walking Modulates Midline Sensorimotor EEG Rhythms in Able-Bodied Subjects. Neuroimage 2012, 63, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, S.M.; Van Dieën, J.H.; Daffertshofer, A. Beta Activity in the Premotor Cortex Is Increased during Stabilized as Compared to Normal Walking. Front. Hum. Neurosci. 2015, 9, 593. [Google Scholar] [CrossRef] [PubMed]
- Mustile, M.; Kourtis, D.; Ladouce, S.; Learmonth, G.; Edwards, M.G.; Donaldson, D.I.; Ietswaart, M. Mobile EEG Reveals Functionally Dissociable Dynamic Processes Supporting Real-world Ambulatory Obstacle Avoidance: Evidence for Early Proactive Control. Eur. J. Neurosci. 2021, 54, 8106–8119. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.; Wagner, J.; Makeig, S.; Mancini, M. Brain Activity Response to Visual Cues for Gait Impairment in Parkinson’s Disease: An EEG Study. Neurorehabil. Neural Repair 2021, 35, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Palmer, J.; Onton, J.; Oostenveld, R.; Makeig, S. Independent EEG Sources Are Dipolar. PLoS ONE 2012, 7, e30135. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Schlink, B.R.; David Hairston, W.; König, P.; Ferris, D.P. A Channel Rejection Method for Attenuating Motion-Related Artifacts in EEG Recordings during Walking. Front. Neurosci. 2017, 11, 225. [Google Scholar] [CrossRef]
- Donoghue, T.; Dominguez, J.; Voytek, B. Electrophysiological Frequency Band Ratio Measures Conflate Periodic and Aperiodic Neural Activity. eNeuro 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Vitorio, R.; Lirani-Silva, E.; Barbieri, F.A.; Raile, V.; Stella, F.; Gobbi, L.T.B. Influence of Visual Feedback Sampling on Obstacle Crossing Behavior in People with Parkinson’s Disease. Gait Posture 2013, 38, 330–334. [Google Scholar] [CrossRef]
- Almeida, O.P. Mini Exame Do Estado Mental e o Diagnóstico de Demência No Brasil. Arquivos Neuro-Psiquiatria 1998, 56, 605–612. [Google Scholar] [CrossRef]
- Chaumon, M.; Bishop, D.V.M.; Busch, N.A. A Practical Guide to the Selection of Independent Components of the Electroencephalogram for Artifact Correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F.; Jones, J.A.; Almeida, Q.J. Motor Planning in Parkinson’s Disease Patients Experiencing Freezing of Gait: The Influence of Cognitive Load When Approaching Obstacles. Brain Cogn. 2014, 87, 76–85. [Google Scholar] [CrossRef]
- Martens, K.A.E.; Almeida, Q.J. Dissociating between Sensory and Perceptual Deficits in PD: More than Simply a Motor Deficit. Mov. Disord. 2012, 27, 387–392. [Google Scholar] [CrossRef]
- Crémers, J.; D’Ostilio, K.; Stamatakis, J.; Delvaux, V.; Garraux, G. Brain Activation Pattern Related to Gait Disturbances in Parkinson’s Disease. Mov. Disord. 2012, 27, 1498–1505. [Google Scholar] [CrossRef]
- Hanakawa, T.; Katsumi, Y.; Fukuyama, H.; Honda, M.; Hayashi, T.; Kimura, J.; Shibasaki, H. Mechanisms Underlying Gait Disturbance in Parkinson’s Disease. Brain 1999, 122, 1271–1282. [Google Scholar] [CrossRef]
- Viaene, A.N.; Petrof, I.; Sherman, S.M. Properties of the Thalamic Projection from the Posterior Medial Nucleus to Primary and Secondary Somatosensory Cortices in the Mouse. Proc. Natl. Acad. Sci. USA 2011, 108, 18156–18161. [Google Scholar] [CrossRef]
- Mease, R.A.; Metz, M.; Groh, A. Cortical Sensory Responses Are Enhanced by the Higher-Order Thalamus. Cell Rep. 2016, 14, 208–215. [Google Scholar] [CrossRef]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain Activity during Walking: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- Neuper, C.; Wortz, M.; Pfurtscheller, G. ERD/ERS Patterns Reflecting Sensorimotor Activation and Deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-Related EEG/MEG Synchronization and Desynchronization: Basic Principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Galna, B.; Murphy, A.T.; Morris, M.E. Obstacle Crossing in People with Parkinson’s Disease: Foot Clearance and Spatiotemporal Deficits. Hum. Mov. Sci. 2010, 29, 843–852. [Google Scholar] [CrossRef]
- Wrobel, A. Beta Activity: A Carrier for Visual Attention. Acta Neurobiol. Exp. 2000, 60, 247–260. [Google Scholar]
- Kaminski, J.; Brzezicka, A.; Gola, M.; Wrobel, A. Beta Band Oscillations Engagement in Human Alertness Process. Int. J. Psychophysiol. 2012, 85, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Guntekin, B.; Emek-Savas, D.D.; Kurt, P.; Yener, G.G.; Basar, E. Beta Oscillatory Responses in Healthy Subjects and Subjects with Mild Cognitive Impairment. NeuroImage Clin. 2013, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Gobbi, L.T.B.; Lirani-Silva, E.; Moraes, R.; Almeida, Q.J. Synchrony of Gaze and Stepping Patterns in People with Parkinson’s Disease. Behav. Brain Res. 2016, 307, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Ianez, E.; Ubeda, A.; Hortal, E.; Del-Ama, A.J.; Gil-Agudo, A.; Azorin, J.M. Decoding the Attentional Demands of Gait through EEG Gamma Band Features. PLoS ONE 2016, 11, e0154136. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Arguissain, F.G.; Andersen, O.K. Cognitive Processing for Step Precision Increases Beta and Gamma Band Modulation During Overground Walking. Brain Topogr. 2018, 31, 661–671. [Google Scholar] [CrossRef]
- Casarotto, S.; Turco, F.; Comanducci, A.; Perretti, A.; Marotta, G.; Pezzoli, G.; Rosanova, M.; Isaias, I.U. Excitability of the Supplementary Motor Area in Parkinson’s Disease Depends on Subcortical Damage. Brain Stimul. 2019, 12, 152–160. [Google Scholar] [CrossRef]
- Conceição, N.R.; Gobbi, L.T.B.; Nóbrega-Sousa, P.; Orcioli-Silva, D.; Beretta, V.S.; Lirani-Silva, E.; Okano, A.H.; Vitório, R. Aerobic Exercise Combined with Transcranial Direct Current Stimulation over the Prefrontal Cortex in Parkinson Disease: Effects on Cortical Activity, Gait, and Cognition. Neurorehabil. Neural Repair 2021, 35, 717–728. [Google Scholar] [CrossRef]
- Beretta, V.S.; Santos, P.C.R.; Orcioli-Silva, D.; Zampier, V.C.; Vitório, R.; Gobbi, L.T.B. Transcranial Direct Current Stimulation for Balance Rehabilitation in Neurological Disorders: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 81, 101736. [Google Scholar] [CrossRef]
- Lee, H.K.; Ahn, S.J.; Shin, Y.M.; Kang, N.; Cauraugh, J.H. Does Transcranial Direct Current Stimulation Improve Functional Locomotion in People with Parkinson’s Disease? A Systematic Review and Meta-Analysis. J. Neuroeng. Rehabil. 2019, 16, 84. [Google Scholar] [CrossRef]

| Variables | Parkinson | Control | Statistics |
|---|---|---|---|
| Sex (male/female) | 6/9 | 5/9 | X2 = 0.056, p = 0.812 |
| Age (years) | 70.8 (10.5) | 70.9 (4.9) | t = −0.02, p = 0.984 |
| Body mass (kg) | 69.6 (12.4) | 69.4 (12.1) | t = 0.046, p = 0.964 |
| Height (cm) | 162.2 (7.5) | 160.8 (8.6) | t = 0.449, p = 0.657 |
| MMSE (0–30 score) | 27.1 (1.5) | 28.7 (1.1) | Z = −2.786, p = 0.005 * |
| UPDRS I (0–16 score) | 3.3 (1.9) | - | - |
| UPDRS II (0–52 score) | 8.8 (4.8) | - | - |
| UPDRS III (0–108 score) | 25.8 (9.3) | - | - |
| Hoehn and Yahr [1/1.5/2/2.5/3] | 1/4/5/4/1 | - | - |
| Functional Area | Brodmann | No. of Participants | No. of ICs |
|---|---|---|---|
| (Centroid Location) | Area | (PD/Control) | (PD/Control) |
| Left sensorimotor cortex | 2 | 9/8 | 9/8 |
| Right sensorimotor cortex | 2 | 11/9 | 11/9 |
| Visual cortex | 17 | 11/9 | 11/9 |
| Central premotor and SMA | 6 | 9/7 | 9/7 |
| Variables | Parkinson | Control | Group | Condition | Group × Condition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Step | USU | OBT | USU | OBT | F | p | F | p | F | p | Post Hoc |
| Length (cm) | 56.0 (6.6) | 55.8 (7.3) | 63.3 (6.6) | 63.7 (8.2) | 8.331 | 0.008 | 0.058 | 0.811 | 0.501 | 0.485 | |
| Duration (s) | 0.52 (0.03) | 0.56 (0.03) | 0.53 (0.05) | 0.56 (0.05) | 0.137 | 0.714 | 176.968 | 0.001 | 2.622 | 0.117 | |
| Velocity (cm/s) | 108.4 (14.2) | 100.5 (15.5) | 120.9 (17.4) | 114.9 (17.7) | 5.083 | 0.032 | 57.496 | 0.001 | 1.035 | 0.318 | |
| Width (cm) | 9.2 (2.5) | 10.1 (2.7) | 8.6 (1.9) | 8.6 (2.1) | 1.440 | 0.241 | 9.737 | 0.004 | 11.070 | 0.003 | PD: USU < OBT |
| Step variability | |||||||||||
| Length (cm) | 2.42 (1.01) | 6.50 (2.04) | 1.93 (0.71) | 5.30 (1.49) | 4.064 | 0.054 t | 132.582 | 0.001 | 2.623 | 0.117 | |
| Duration (s) | 0.021 (0.011) | 0.080 (0.025) | 0.016 (0.004) | 0.067 (0.017) | 2.972 | 0.096 | 267.767 | 0.001 | 1.353 | 0.255 | |
| Velocity (cm/s) | 6.8 (4.0) | 11.2 (2.9) | 5.3 (1.5) | 11.1 (3.1) | 0.682 | 0.416 | 71.509 | 0.001 | 1.601 | 0.217 | |
| Width (cm) | 2.05 (0.62) | 2.72 (0.86) | 2.04 (0.49) | 2.55 (0.60) | 0.170 | 0.684 | 41.485 | 0.001 | 0.756 | 0.392 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitório, R.; Lirani-Silva, E.; Orcioli-Silva, D.; Beretta, V.S.; Oliveira, A.S.; Gobbi, L.T.B. Electrocortical Dynamics of Usual Walking and the Planning to Step over Obstacles in Parkinson’s Disease. Sensors 2023, 23, 4866. https://doi.org/10.3390/s23104866
Vitório R, Lirani-Silva E, Orcioli-Silva D, Beretta VS, Oliveira AS, Gobbi LTB. Electrocortical Dynamics of Usual Walking and the Planning to Step over Obstacles in Parkinson’s Disease. Sensors. 2023; 23(10):4866. https://doi.org/10.3390/s23104866
Chicago/Turabian StyleVitório, Rodrigo, Ellen Lirani-Silva, Diego Orcioli-Silva, Victor Spiandor Beretta, Anderson Souza Oliveira, and Lilian Teresa Bucken Gobbi. 2023. "Electrocortical Dynamics of Usual Walking and the Planning to Step over Obstacles in Parkinson’s Disease" Sensors 23, no. 10: 4866. https://doi.org/10.3390/s23104866







