Passive Millimeter-Wave Imaging for Burns Diagnostics under Dressing Materials
Abstract
:1. Introduction

Related Work
2. Materials and Methods
2.1. Porcine Skin Samples
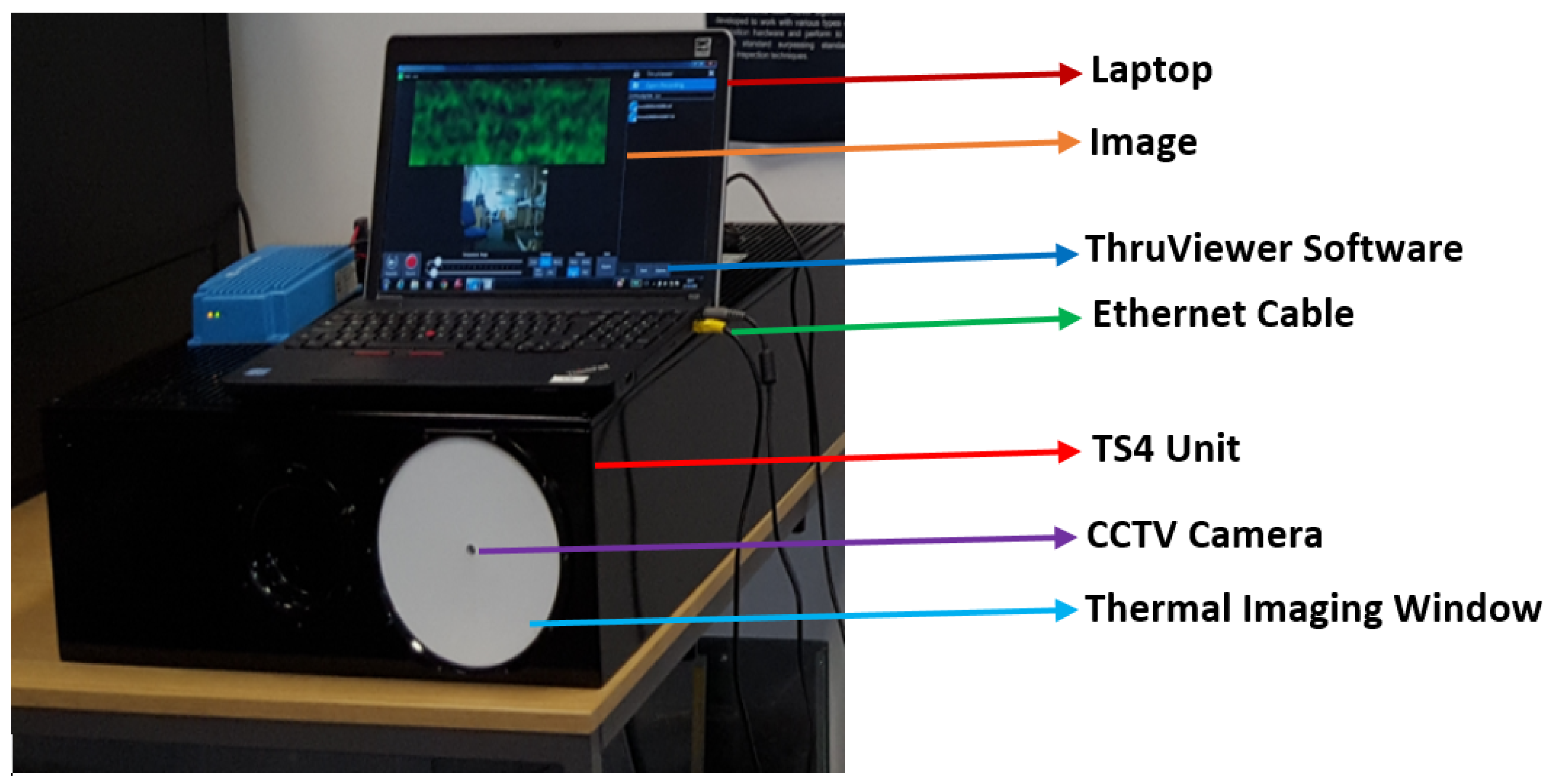
2.2. Experimental Setup and Description
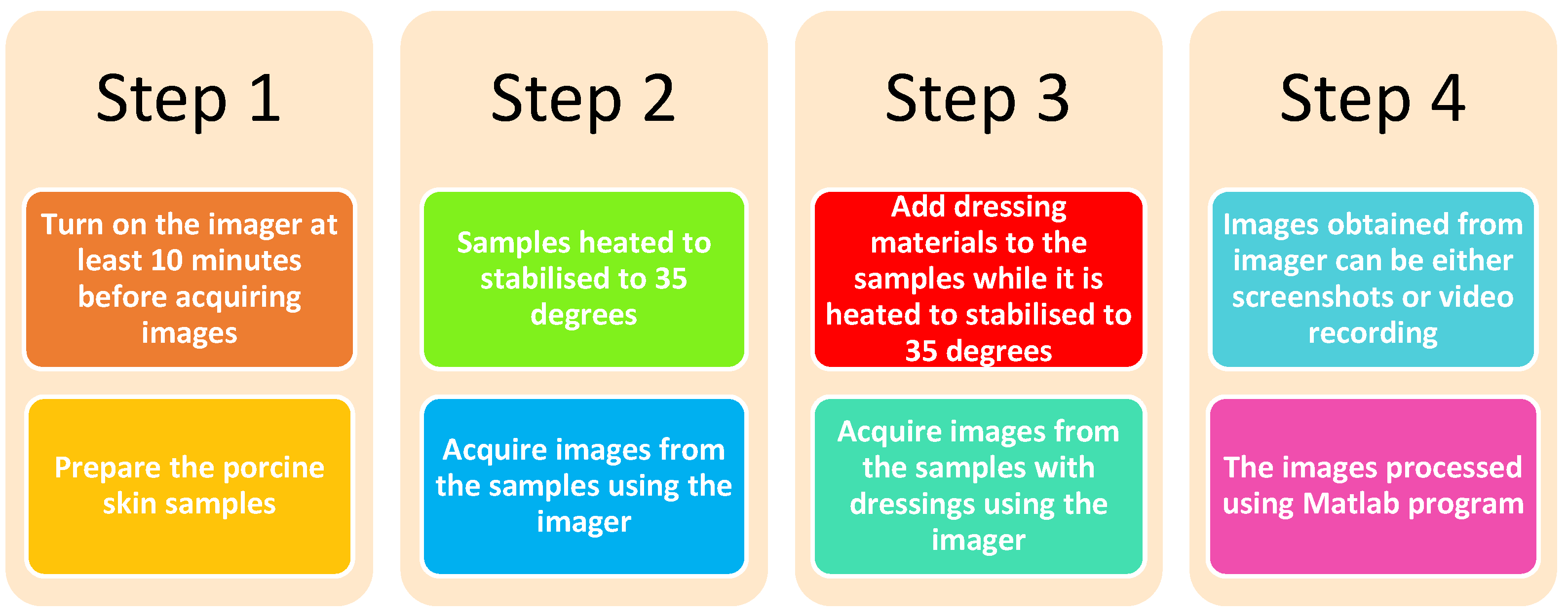
2.3. Methodology of Conducting the Experimental Work
2.3.1. Methodology 1: Porcine Skin without Burns
2.3.2. Methodology 2: Porcine Skin with Burns
2.4. Methodology of Processing Images
3. Results
3.1. Initial Measurements
3.2. Unburned Skin
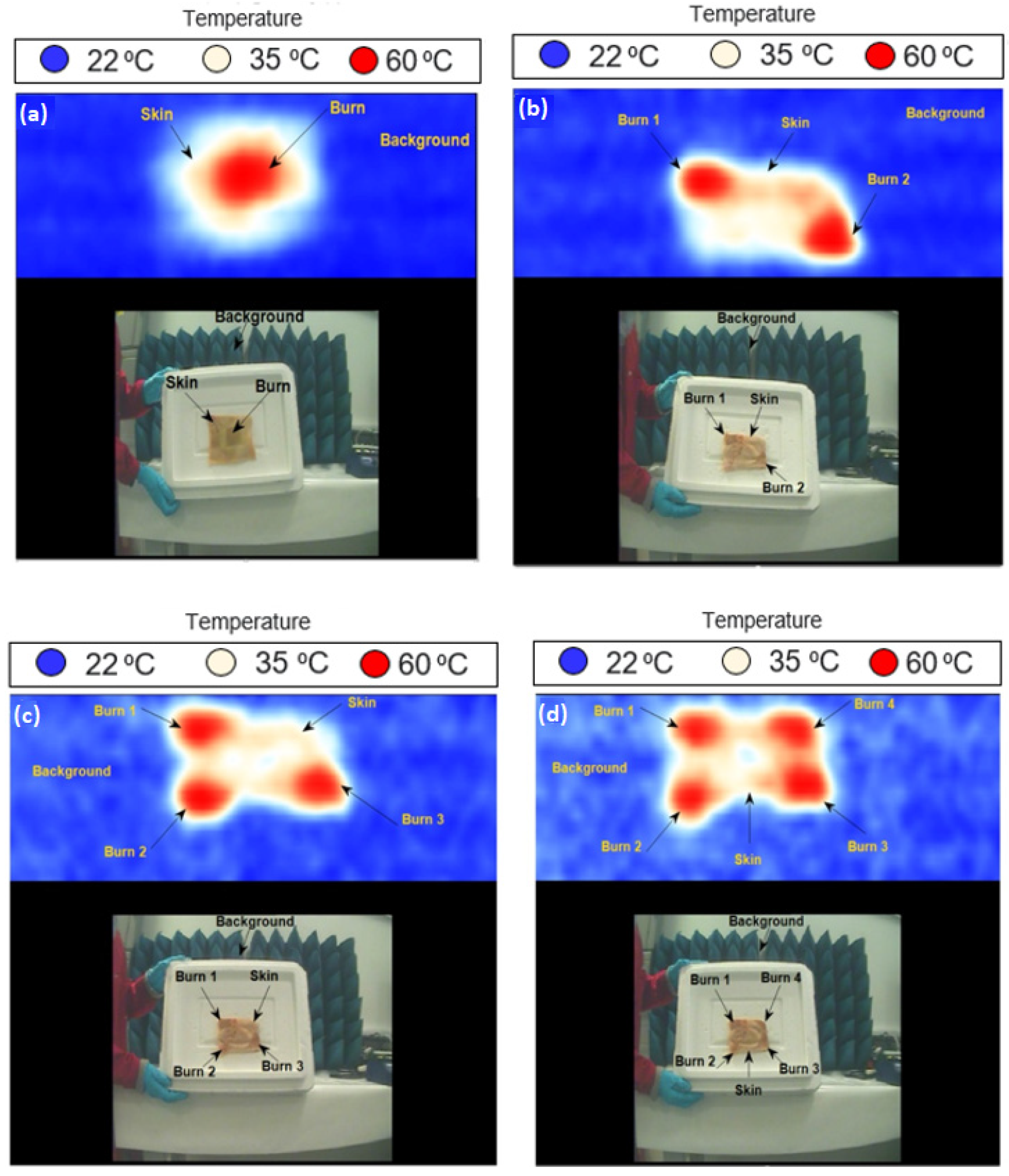
3.3. Skin with Single and Multiple Burns
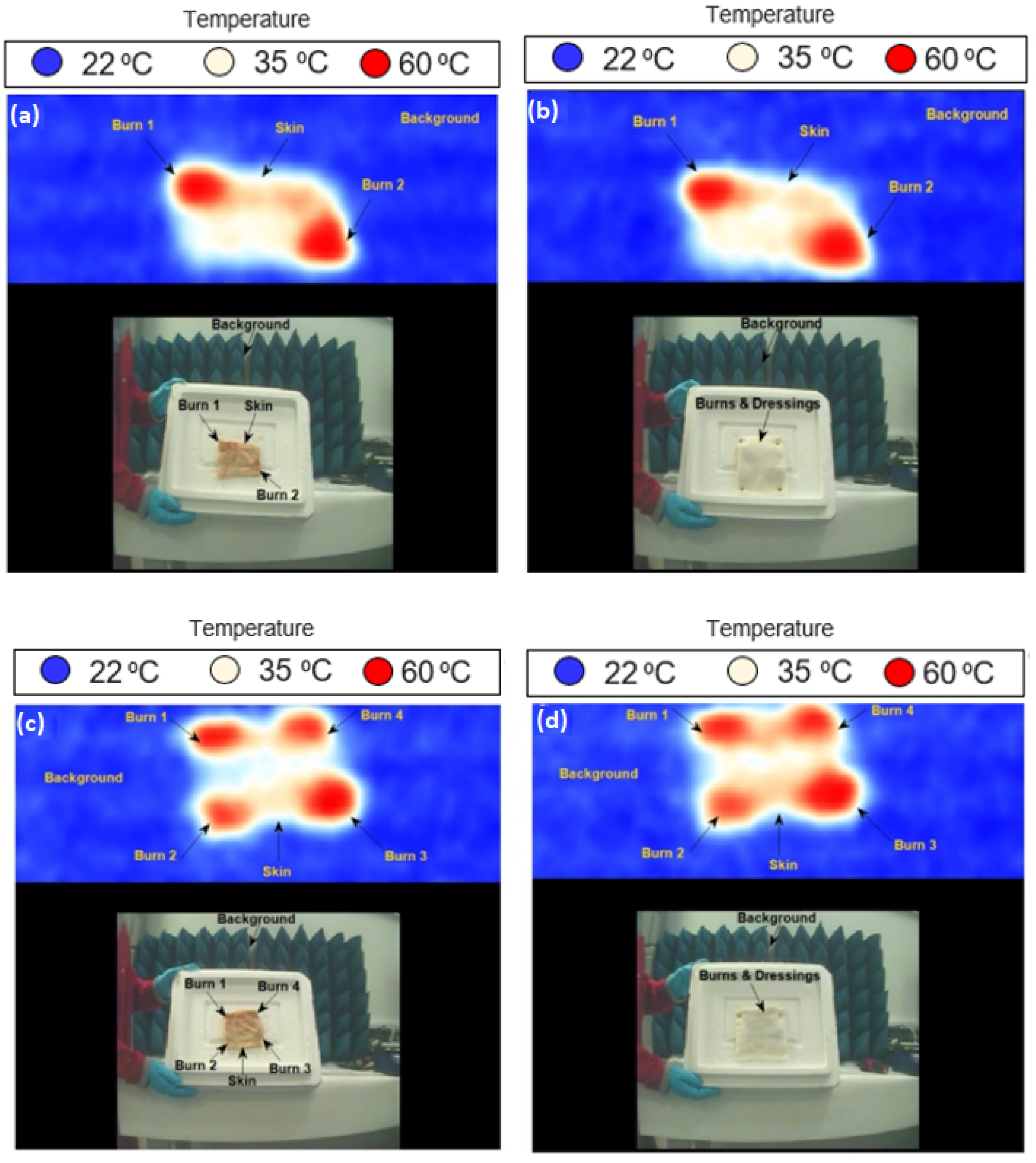
3.4. Skin with Burns and Dressing Materials
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zohra, Z.; Lanigan, S.W. Skin: Structure and Function. In Dermatology in Clinical Practice, 1st ed.; Springer London Limited Publisher: London, UK, 2010; pp. 1–15. [Google Scholar]
- Xu, F.; Lu, T.J. Introduction to Skin Biothermomechanics and Thermal Pain, 1st ed.; Springer Berlin Heidelberg Publisher: Berlin, UK, 2011. [Google Scholar]
- Berzina, Z. Skin Stories: Charting and Mapping the Skin. Research Using Analogies of Human Skin Tissue in Relation to My Textile Practice. Ph.D. Thesis, University of the Arts London, London, UK, 2004. [Google Scholar]
- Owda, A.Y.; Owda, M.; Rezgui, N.-D. Synthetic Aperture Radar Imaging for Burn Wounds Diagnostics. Sensors 2020, 20, 847. [Google Scholar] [CrossRef] [Green Version]
- Standring, S.; Borley, N.R.; Collins, P.; Crossman, A.R.; Gatzoulis, M.A. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed.; Elsevier Limited Publisher: Beijing, China, 2008. [Google Scholar]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Harmer, S.W.; Shylo, S.; Shah, M.; Bowring, N.J.; Owda, A.Y. On the feasibility of assessing burn wound healing without removal of dressings using radiometric millimeter-wave sensing. Prog. Electromagn. Res. M 2016, 45, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Seia, Z.; Musso, L.; Palazzini, S.; Bertero, M. Skin Biopsy Procedures: How and Where to Perform a Proper Biopsy, Skin Biopsy-Perspectives. In Skin Biopsy; Intech Publisher: Shanghai, China, 2011; pp. 1–18. [Google Scholar]
- Essen, H.; Essen, J.M.; Nuessler, D.; Hommes, A.; Krebs, C.; Fatihi, N.; Buzug, T. Monitoring of wound healing by millimeter wave imaging. In Proceedings of the 35th International Conference on Infrared, Millimeter, and Terahertz Waves, Rome, Italy, 5–10 September 2010. [Google Scholar]
- Mattsson, M.-O.; Simkó, M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices Evid. Res. 2019, 12, 347–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owda, A.Y.; Salmon, N.; Harmer, S.W.; Shylo, S.; Bowring, N.J.; Rezgu, N.D.; Shah, M. Millimeter-wave emissivity as a metric for the non-contact diagnosis of human skin conditions. Bioelectromagnetics 2017, 38, 559–569. [Google Scholar] [CrossRef]
- Lubecke, O.B.; Nikawa, Y.; Snyder, W.; Lin, J.; Mizuno, K. Novel microwave and millimeter-wave biomedical applications. In Proceedings of the 4th International Conference, In Telecommunications in Modern Satellite, Cable and Broadcasting Services, Nis, Yugoslavia, 13–15 October 1999. [Google Scholar]
- Owda, A.Y.; Salmon, N.; Owda, M. Indoor passive sensing for detecting hidden objects under clothing. In SPIE 11868, Emerging Imaging and Sensing Technologies for Security and Defence VI; SPIE: Madrid, Spain, 2021. [Google Scholar]
- Ebling, F.J.G.; Montagna, W. Human Skin. Encyclopædia Britannica, Inc. 11 April 2016. Available online: https://www.britannica.com/science/human-skin (accessed on 10 February 2022).
- Joung, M.; Suzuki, Y.; Tanaka, T.; Kagaya, S.; Watanabe, K.; Matono, H.; Wagatsuma, Y.; Mizuno, K. Development of passive millimeter-wave imaging systems and its applications to medical-and bio-objects imaging. In Proceedings of the Terahertz for Military and Security Applications II, Orlando, FL, USA, 8 September 2004. [Google Scholar]
- Ahmed, S.S. Microwave Imaging in Security Two Decades of Innovation. IEEE J. Microw. 2021, 1, 191–201. [Google Scholar] [CrossRef]
- Owda, A.Y.; Salmon, N.; Rezgui, N.-D. Electromagnetic Signatures of Human Skin in the Millimeter Wave Band 80–100 GHz. Prog. Electromagn. Res. B 2018, 80, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Luukanen, A.; Appleby, R.; Kemp, M.; Salmon, N. Millimeter-Wave and Terahertz Imaging in Security Applications. In Terahertz Spectroscopy and Imaging; Springer Series in Optical Science Publisher: Berlin, UK, 2012; Volume 171, pp. 491–520. [Google Scholar]
- Anderton, R. Design of Manufacturing Concepts for a Real Time Passive Millimeter Wave Imager; Technical Report; University of Reading: Reading, UK, 1999. [Google Scholar]
- Luxin, Y.; Tianxu, Z.; Sheng, Z.; Jian, H.; Jianmao, Z. Study on multichannel passive millimeter-wave radiometer imaging and superresolution. Int. J. Infrared Millim. Waves 2006, 27, 1403–1414. [Google Scholar]
- Ulaby, F.T.; Moore, R.K.; Fung, A.K. Microwave Remote Sensing: Active and Passive Volume I: Microwave Remote Sensing Fundamentals and Radiometry; Artech House Publisher: London, UK, 1981; Volume I. [Google Scholar]
- Salmon, N.A.; Borrill, J.R.; Gleed, D.G. Absolute temperature stability of passive imaging radiometers. In Proceedings of the SPIE Passive Millimeter-Wave Imaging Technology, Orlando, FL, USA, 27 June 1997. [Google Scholar]
- Pozar, D.M. Microwave Engineering, 4th ed.; John Wiley & Sons Publisher: Hoboken, NJ, USA, 2011. [Google Scholar]
- Owda, A.Y.; Salmon, N. Variation in the electromagnetic signatures of the human skin with physical activity and hydration level of the skin. In Proceedings of the SPIE 11164, Millimetre Wave and Terahertz Sensors and Technology XII, Strasbourg, France, 9–12 September 2019. [Google Scholar]
- Appleby, R. Passive millimeter-wave imaging and how it differs from terahertz imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2004, 362, 379–392. [Google Scholar] [CrossRef]
- Harmer, S.W.; Bowring, N.; Andrews, D.; Rezgui, N.D.; Southgate, M.; Smith, S. A Review of Nonimaging Stand-Off Concealed Threat Detection with Millimeter-Wave Radar. IEEE Microw. Mag. 2012, 13, 160–167. [Google Scholar] [CrossRef]
- Wiltse, J. History of millimeter and submillimeter waves. Microw. Theory Tech. IEEE Trans. 1984, 32, 1118–1127. [Google Scholar] [CrossRef]
- Owda, A.Y.; Salmon, N.; Shylo, S.; Owda, M. Assessment of Bandaged Burn Wounds Using Porcine Skin and Millimetric Radiometry. Sensors 2019, 19, 2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zoughi, R. Millimeter Wave Reflectometry and Imaging for Noninvasive Diagnosis of Skin Burn Injuries. IEEE Trans. Instrum. Meas. 2017, 66, 77–84. [Google Scholar] [CrossRef]
- Owda, A.Y.; Salmon, N.; Andrews, D.; Rezgui, N.-D. Active millimeter-wave radar for sensing and imaging through dressing materials. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017. [Google Scholar]
- Mattsson, M.-O.; Zeni, O.; Simkó, M. Is there a Biological Basis for Therapeutic Applications of Millimetre Waves and THz Waves? J. Infrared Millim. Terahertz Waves 2018, 39, 863–878. [Google Scholar] [CrossRef]
- Leszczynski, D. Physiological effects of millimeter-waves on skin and skin cells: An overview of the to-date published studies. Rev. Environ. Health 2020, 35, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Castro-Camus, E.; Koch, M.; Mittleman, D.M. Recent advances in terahertz imaging: 1999 to 2021. Appl. Phys. B 2022, 128, 12. [Google Scholar] [CrossRef]
- Woodward, R.M.; Wallace, V.P.; Cole, B.E.; Pye, R.J.; Arnone, D.D.; Linfield, E.H.; Pepper, M. Terahertz pulse imaging in reflection geometry of skin tissue using time-domain analysis techniques. In Proceedings of the SPIE Clinical Diagnostic Systems: Technologies and Instrumentation, San Jose, CA, USA, 19–25 January 2002. [Google Scholar]
- Arbab, M.H.; Winebrenner, D.P.; Dickey, T.C.; Chen, A.; Klein, M.B.; Mourad, P.D. Terahertz spectroscopy for the assessment of burn injuries In Vivo. J. Biomed. Opt. 2013, 18, 077004. [Google Scholar] [CrossRef] [Green Version]
- Tewari, P.; Bajwa, N.; Singh, R.S.; Culjat, M.O.; Grundgest, W.S.; Taylor, Z.D.; Kealey, C.P.; Bennett, D.B.; Barnett, K.S.; Stojadinovic, A. In Vivo terahertz imaging of rat skin burns. J. Biomed. Opt. 2012, 17, 040503. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, S.M.; de Boer, J.F.; Park, H.; Keikhanzadeh, K.; Huang, H.-E.L.; Zhang, J.; Jung, W.G.; Chen, Z.; Nelson, J.S. Determination of burn depth by polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2004, 9, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Park, B.H.; Saxer, C.; Srinivas, S.M.; Nelson, J.S.; de Boer, J.F. In Vivo burn depth determination by high-speed fiber-based polarization sensitive optical coherence tomography. J. Biomed. Opt. 2001, 6, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Pierce, M.C.; Maguluri, G.; Park, B.H.; Yoon, S.J.; Lydon, M.; Sheridan, R.; de Boer, J.F. In Vivo imaging of human burn injuries with polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2012, 17, 066012. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Maher, J.R.; Kim, J.; Selim, M.A.; Levinson, H.; Wax, A. Evaluation of burn severity In Vivo in a mouse model using spectroscopic optical coherence tomography. Biomed. Opt. Express 2015, 6, 3339–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, D.P.; Choo-Smith, L.-P.; Flueraru, C.; Mao, Y.; Chang, S.; Disano, J.; Sherif, S.; Sowa, M.G. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys. Rev. 2011, 3, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Strutt, H.J.W. LVIII. On the scattering of light by small particles. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 41, 447–454. [Google Scholar] [CrossRef]
- Blackledge, J.M. Scattering Theory. In Digital Image Processing Mathematical and Computational Methods; Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2006; pp. 160–197. [Google Scholar]
- Dziewonski, M. Planimetry of thermograms in diagnosis of burn wounds. Sci. Res. Inst. Math. Comput. Sci. 2009, 8, 33–38. [Google Scholar]
- Liddington, M.I.; Shakespeare, P.G. Timing of the thermographic assessment of burns. Burns 1996, 22, 26–28. [Google Scholar] [CrossRef]
- Miccio, J.; Parikh, S.; Marinaro, X.; Prasad, A.; McClain, S.; Singer, A.J.; Clark, R.A.F. Forward-looking infrared imaging predicts ultimate burn depth in a porcine vertical injury progression model. Burns 2016, 42, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Ruminski, J.; Kaczmarek, M.; Renkielska, A.; Nowakowski, A. Thermal parametric imaging in the evaluation of skin burn depth. IEEE Trans. Biomed. Eng. 2007, 54, 303–312. [Google Scholar] [CrossRef]
- Digital Barriers. SafeSearch (ThruVision and ThruViewer), User Manual; Digital Barriers Company: London, UK, 2014. [Google Scholar]
- Andrews, C.J.; Kempf, M.; Kimble, R.; Cuttle, L. Development of a Consistent and Reproducible Porcine Scald Burn Model. PLoS ONE 2016, 11, e0162888. [Google Scholar] [CrossRef] [Green Version]
- Andrews, C.J.; Cuttle, L.; Simpson, M.J. Quantifying the role of burn temperature, burn duration and skin thickness in an In Vivo animal skin model of heat conduction. Int. J. Heat Mass Transf. 2016, 101, 542–549. [Google Scholar] [CrossRef]




| Imager Specification | Value |
|---|---|
| Frequency band | 232–268 GHz |
| Center frequency | 250 GHz |
| Band width | 36 GHz |
| Depth of field | 200 mm |
| Special resolution | 5.5 mm |
| Operating wave length | 1.2 mm |
| Lens diameter | 175 mm |
| Field of view | 150 mm (w) × 300 mm (h) |
| Frame rate | 4 Hz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owda, A.Y. Passive Millimeter-Wave Imaging for Burns Diagnostics under Dressing Materials. Sensors 2022, 22, 2428. https://doi.org/10.3390/s22072428
Owda AY. Passive Millimeter-Wave Imaging for Burns Diagnostics under Dressing Materials. Sensors. 2022; 22(7):2428. https://doi.org/10.3390/s22072428
Chicago/Turabian StyleOwda, Amani Yousef. 2022. "Passive Millimeter-Wave Imaging for Burns Diagnostics under Dressing Materials" Sensors 22, no. 7: 2428. https://doi.org/10.3390/s22072428






