Heart Rate Variability as a Potential Indicator of Cancer Pain in a Mouse Model of Peritoneal Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
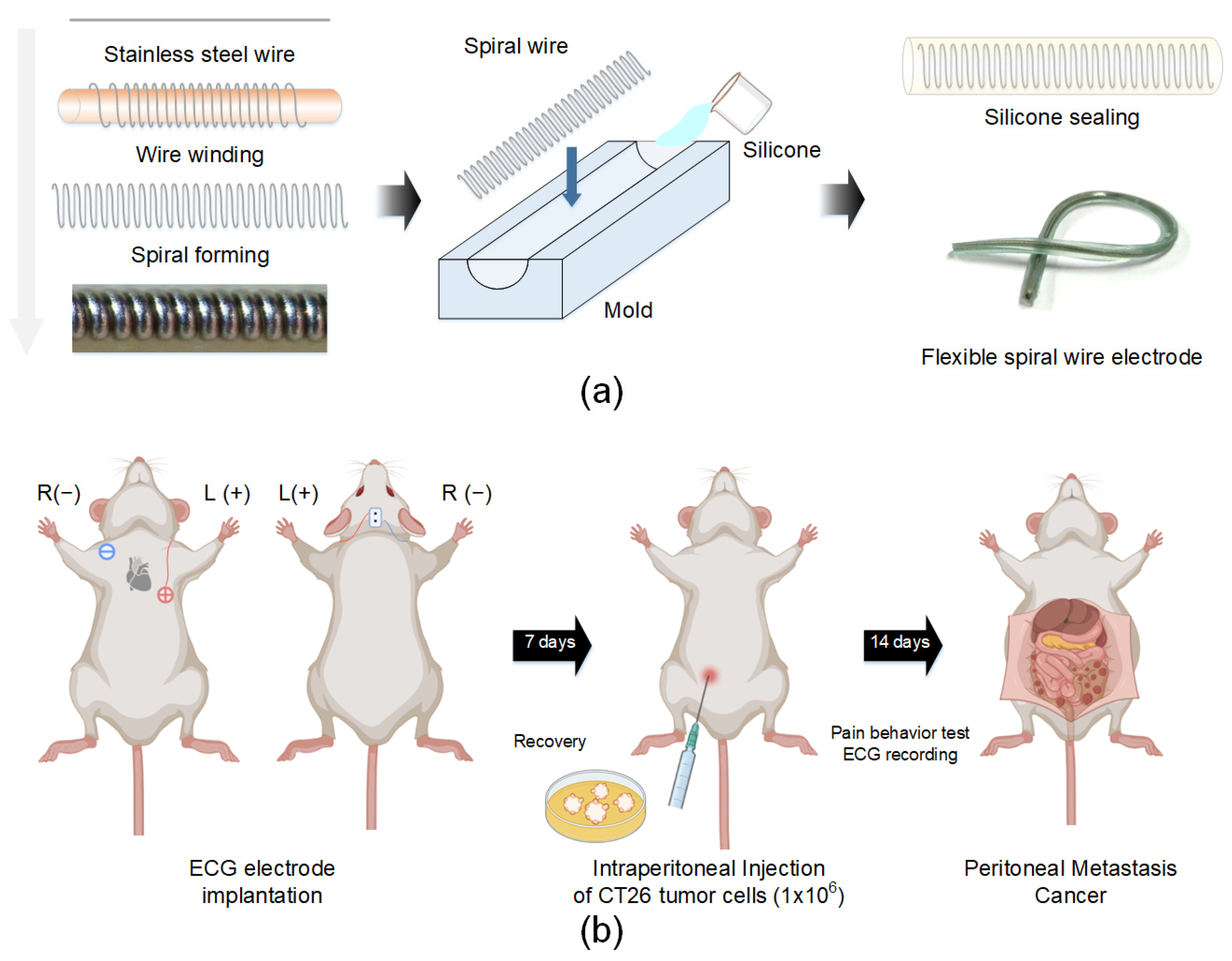
2.2. ECG Electrode Fabrication and Implantation
2.3. Peritoneal Metastasis Cancer Model
2.4. ECG Recording and HRV Parameter Extraction
2.5. Pain Assessment
2.6. Analgesic Evaluation
2.7. Statistical Analysis
3. Results
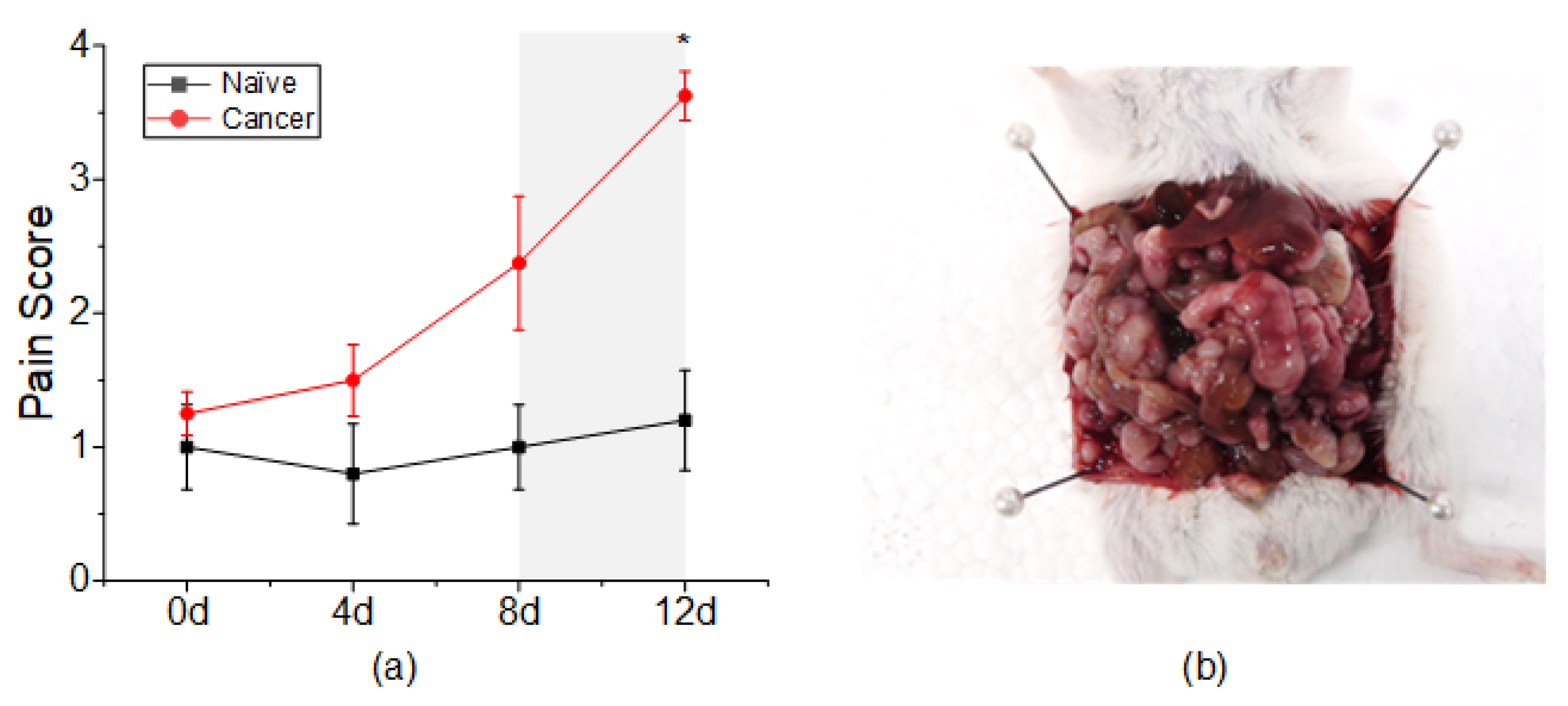
3.1. Pain Assessment
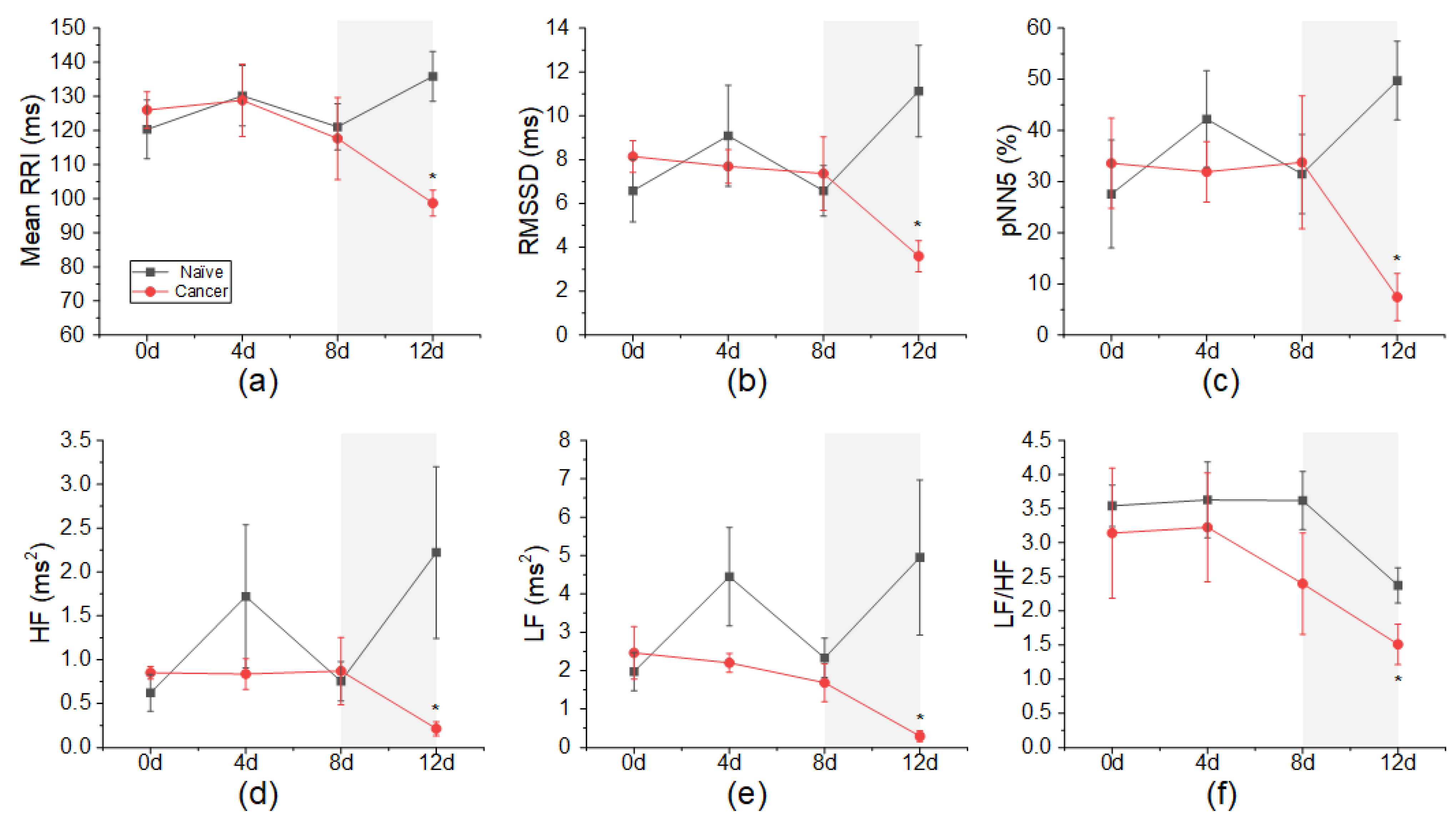
3.2. Changes in HRV According to Cancer Induction
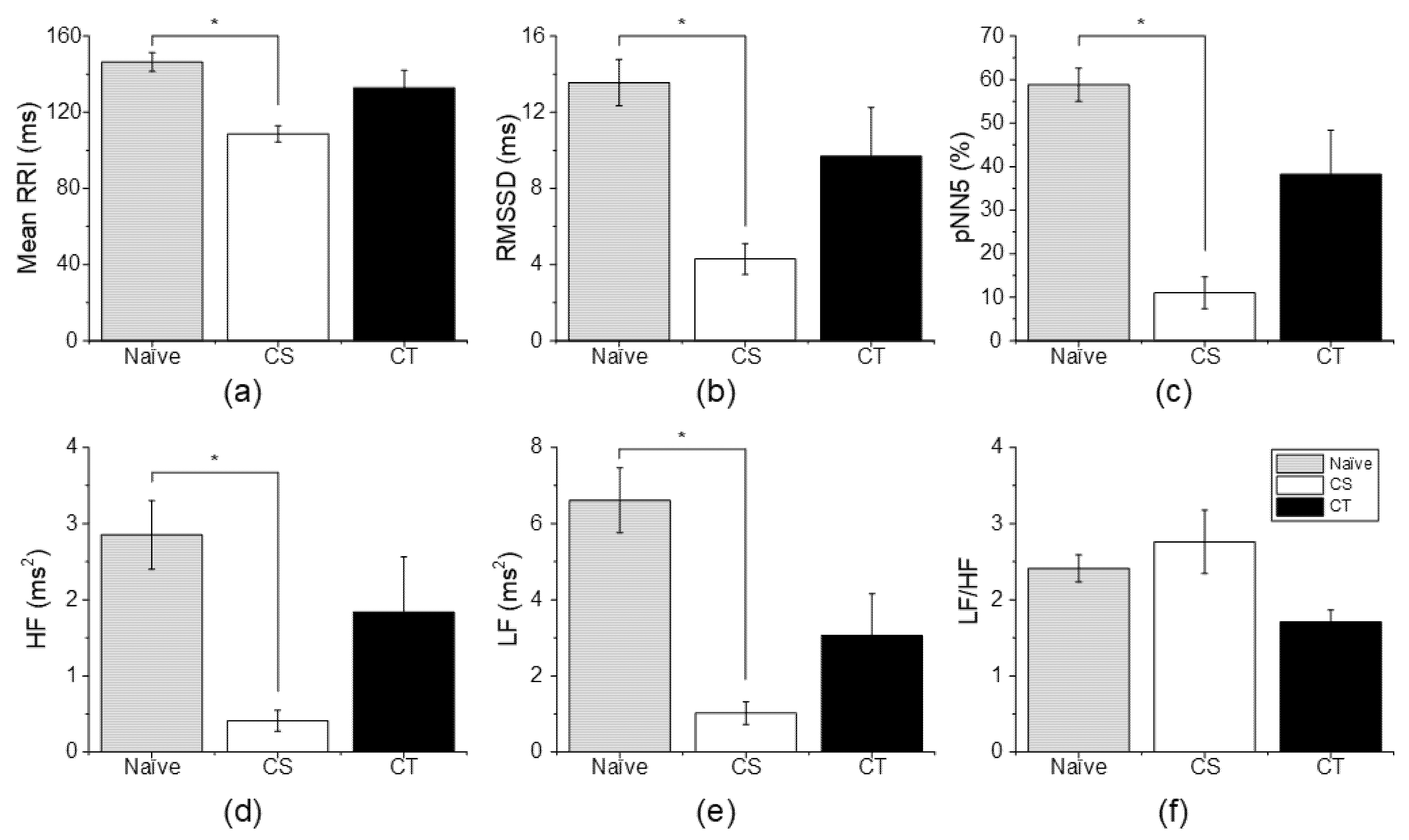
3.3. Changes in HRV According to Analgesic Administration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grayson, M. Pain. Nature 2016, 535, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paice, J.A.; Ferrell, B. The Management of Cancer Pain. CA Cancer J. Clin. 2011, 61, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Turk, D.C. Chronic back pain and rheumatoid arthritis: Predicting pain and disability from cognitive variables. J. Behav. Med. 1988, 11, 251–265. [Google Scholar] [CrossRef]
- Lee, Y.C.; Nassikas, N.J.; Clauw, D.J. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res. Ther. 2011, 13, 211. [Google Scholar] [CrossRef] [Green Version]
- Ledowski, T.; Stein, J.; Albus, S.; MacDonald, B. The influence of age and sex on the relationship between heart rate variability, haemodynamic variables and subjective measures of acute post-operative pain. Eur. J. Anaesthesiol. 2011, 28, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F.; Chatelle, C.; Schnakers, C.; Laureys, S. Heart Rate Variability as an Indicator of Nociceptive Pain in Disorders of Consciousness? J. Pain Symptom. Manag. 2019, 57, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability and pain: Associations of two interrelated homeostatic processes. Biol. Psychol. 2008, 77, 174–182. [Google Scholar] [CrossRef]
- Arras, M.; Rettich, A.; Cinelli, P.; Kasermann, H.P.; Burki, K. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet. Res. 2007, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Charlet, A.; Rodeau, J.L.; Poisbeau, P. Poincare plot descriptors of heart rate variability as markers of persistent pain expression in freely moving rats. Physiol. Behav. 2011, 104, 694–701. [Google Scholar] [CrossRef]
- Jin, Y.; Sato, J.; Yamazaki, M.; Omura, S.; Funakubo, M.; Senoo, S.; Aoyama, M.; Mizumura, K. Changes in cardiovascular parameters and plasma norepinephrine level in rats after chronic constriction injury on the sciatic nerve. Pain 2008, 135, 221–231. [Google Scholar] [CrossRef]
- Jess, G.; Pogatzki-Zahn, E.M.; Zahn, P.K.; Meyer-Friessem, C.H. Monitoring heart rate variability to assess experimentally induced pain using the analgesia nociception index A randomised volunteer study. Eur. J. Anaesthesiol. 2016, 33, 118–125. [Google Scholar] [CrossRef] [PubMed]
- De Jonckheere, J.; Logier, R.; Jounwaz, R.; Vidal, R.; Jeanne, M. From pain to stress evaluation using heart rate variability analysis: Development of an evaluation platform. In Proceedings of the 2010 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3852–3855. [Google Scholar] [CrossRef]
- Funcke, S.; Sauerlaender, S.; Pinnschmidt, H.O.; Saugel, B.; Bremer, K.; Reuter, D.A.; Nitzschke, R. Validation of Innovative Techniques for Monitoring Nociception during General Anesthesia A Clinical Study Using Tetanic and Intracutaneous Electrical Stimulation. Anesthesiology 2017, 127, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Faye, P.M.; De Jonckheere, J.; Logier, R.; Kuissi, E.; Jeanne, M.; Rakza, T.; Storme, L. Newborn Infant Pain Assessment Using Heart Rate Variability Analysis. Clin. J. Pain 2010, 26, 777–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallman, D.M.; Olsson, E.M.; von Scheele, B.; Melin, L.; Lyskov, E. Effects of heart rate variability biofeedback in subjects with stress-related chronic neck pain: A pilot study. Appl. Psychophysiol. Biofeedback 2011, 36, 71–80. [Google Scholar] [CrossRef]
- Xie, L.; Chen, X.; Wen, Z.; Yang, Y.; Shi, J.; Chen, C.; Peng, M.; Liu, Y.; Sun, X. Spiral Steel Wire Based Fiber-Shaped Stretchable and Tailorable Triboelectric Nanogenerator for Wearable Power Source and Active Gesture Sensor. Nanomicro Lett. 2019, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Mali, B.; Zulj, S.; Magjarevic, R.; Miklavcic, D.; Jarm, T. Matlab-based tool for ECG and HRV analysis. Biomed. Signal Process. Control. 2014, 10, 108–116. [Google Scholar] [CrossRef]
- Rowan, W.H.; Campen, M.J.; Wichers, L.B.; Watkinson, W.P. Heart rate variability in rodents: Uses and caveats in toxicological studies. Cardiovasc. Toxicol. 2007, 7, 28–51. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time Qrs Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Wolfe, A.M.; Kennedy, L.H.; Na, J.J.; Nemzek-Hamlin, J.A. Efficacy of Tramadol as a Sole Analgesic for Postoperative Pain in Male and Female Mice. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 411–419. [Google Scholar]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef]
- Portenoy, R.K. Treatment of cancer pain. Lancet 2011, 377, 2236–2247. [Google Scholar] [CrossRef]
- Park, H.; Oh, S.; Noh, Y.; Kim, J.Y.; Kim, J.H. Heart Rate Variability as a Marker of Distress and Recovery: The Effect of Brief Supportive Expressive Group Therapy With Mindfulness in Cancer Patients. Integr. Cancer Ther. 2018, 17, 825–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, S.; Seidman, L.C.; Tsao, J.C.I.; Lung, K.C.; Zeltzer, L.K.; Naliboff, B.D. Heart rate variability as a biomarker for autonomic nervous system response differences between children with chronic pain and healthy control children. J. Pain Res. 2013, 6, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.A.; Boucher, J.P.; Comtois, A.S. Heart Rate Variability Modulation after Manipulation in Pain-Free Patients Vs Patients in Pain Reply. J. Manip. Physiol. Ther. 2010, 33, 321–322. [Google Scholar] [CrossRef]
- Zegre Cannon, C.; Kissling, G.E.; Goulding, D.R.; King-Herbert, A.P.; Blankenship-Paris, T. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab. Anim. 2011, 40, 85–93. [Google Scholar] [CrossRef]
- Meeuse, J.J.; Lowik, M.S.; Lowik, S.A.; Aarden, E.; van Roon, A.M.; Gans, R.O.; van Wijhe, M.; Lefrandt, J.D.; Reyners, A.K. Heart rate variability parameters do not correlate with pain intensity in healthy volunteers. Pain Med. 2013, 14, 1192–1201. [Google Scholar] [CrossRef]
- de Geus, E.J.C.; Gianaros, P.J.; Brindle, R.C.; Jennings, J.R.; Berntson, G.G. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 2019, 56, e13287. [Google Scholar] [CrossRef] [Green Version]
- Platisa, M.M.; Gal, V. Dependence of heart rate variability on heart period in disease and aging. Physiol. Meas. 2006, 27, 989–998. [Google Scholar] [CrossRef]
- Chon, K.H.; Yang, B.; Posada-Quintero, H.F.; Siu, K.L.; Rolle, M.; Brink, P.; Birzgalis, A.; Moore, L.C. A novel quantitative method for diabetic cardiac autonomic neuropathy assessment in type 1 diabetic mice. J. Diabetes Sci. Technol. 2014, 8, 1157–1167. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Unit | Formula | Description |
|---|---|---|---|
| Time-domain HRV parameters | |||
| MeanRRI | ms | Mean variability of interbeat interval | |
| RMSSD | ms | Reflects parasympathetic activity | |
| pNN5 | % | Reflects parasympathetic activity | |
| Frequency-domain HRV parameters | |||
| LF | ms2 | Reflects both parasympathetic and sympathetic activity | |
| HF | ms2 | Reflects parasympathetic activity | |
| LF/HF | Reflects parasympathetic and sympathetic balance | ||
| Categories | Pain Sign Parameter (+/−) |
|---|---|
| Physiological characteristics | 10% or more body weight change Above normal body temperature (35.8~37.4 °C) Diarrhea |
| Posture | Hunched posture |
| Appearance | Rough hair coat Pinched face Distended abdomen/swollen Reluctance to move |
| Activity | Not grooming |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Yoon, H.Y.; Kwon, I.K.; Youn, I.; Han, S. Heart Rate Variability as a Potential Indicator of Cancer Pain in a Mouse Model of Peritoneal Metastasis. Sensors 2022, 22, 2152. https://doi.org/10.3390/s22062152
Kim Y, Yoon HY, Kwon IK, Youn I, Han S. Heart Rate Variability as a Potential Indicator of Cancer Pain in a Mouse Model of Peritoneal Metastasis. Sensors. 2022; 22(6):2152. https://doi.org/10.3390/s22062152
Chicago/Turabian StyleKim, Yurim, Hong Yeol Yoon, Il Keun Kwon, Inchan Youn, and Sungmin Han. 2022. "Heart Rate Variability as a Potential Indicator of Cancer Pain in a Mouse Model of Peritoneal Metastasis" Sensors 22, no. 6: 2152. https://doi.org/10.3390/s22062152






