Optical Gas-Cell Dynamic Adsorption in a Photoacoustic Spectroscopy-Based SOF2 and SO2F2 Gas Sensor
Abstract
:1. Introduction
2. Photoacoustic Spectroscopy Principle
3. Analysis and Simulation
3.1. Gas-Cell Design and Simulation
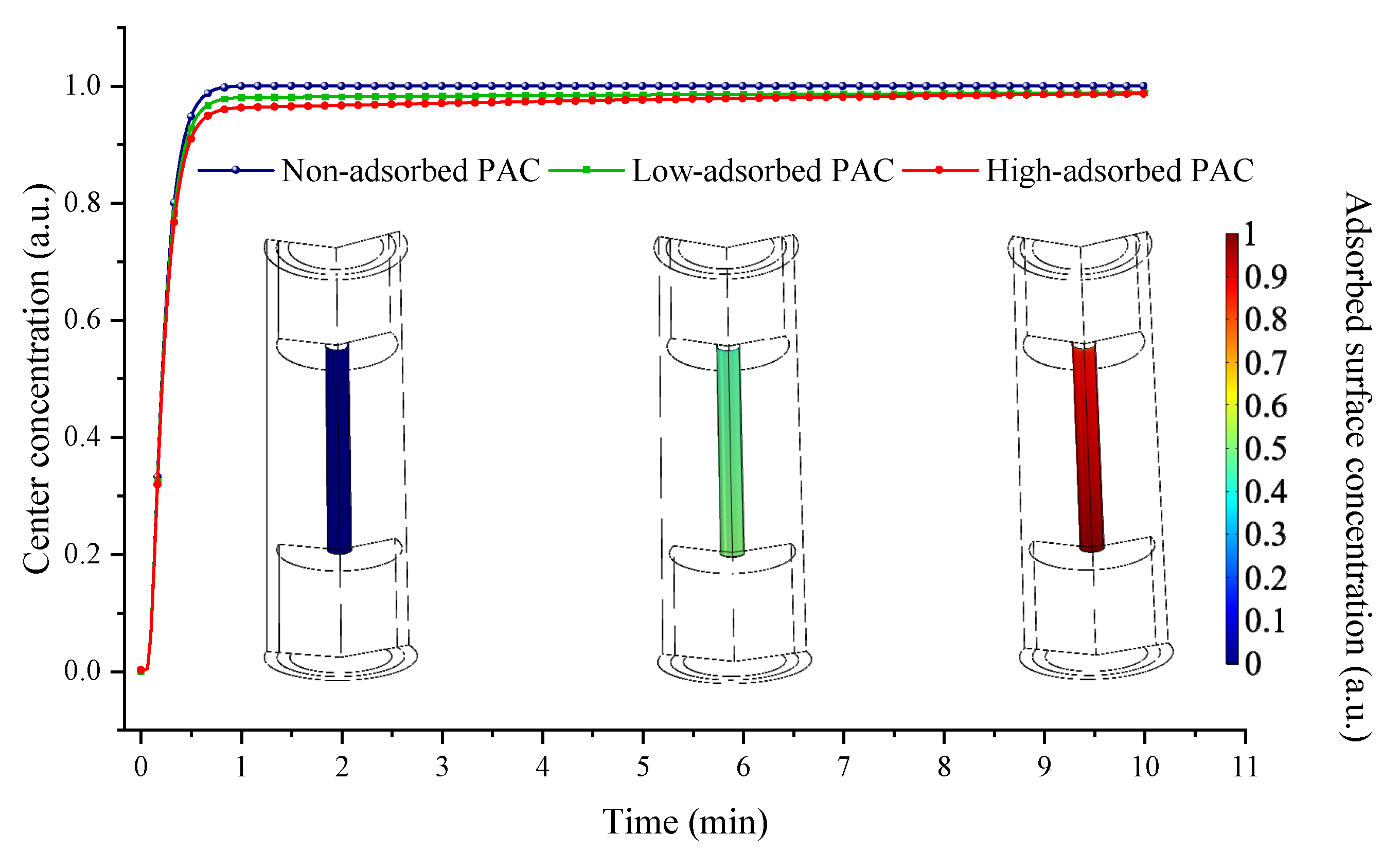
3.2. Gas Molecule and Adsorption Material
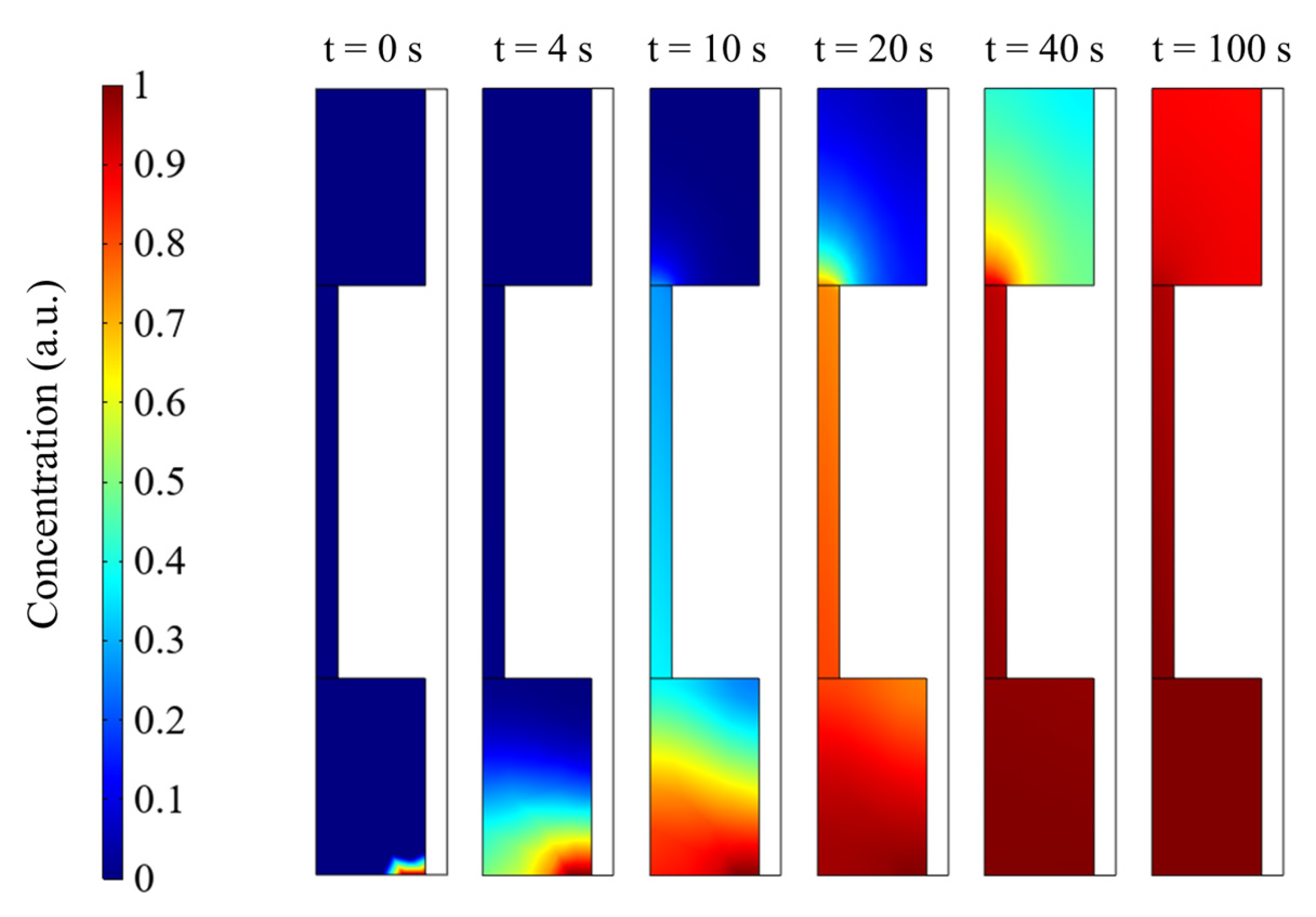
3.3. Gas Adsorption and Diffusing
4. Experiment and Discussion
4.1. Experimental PAS System
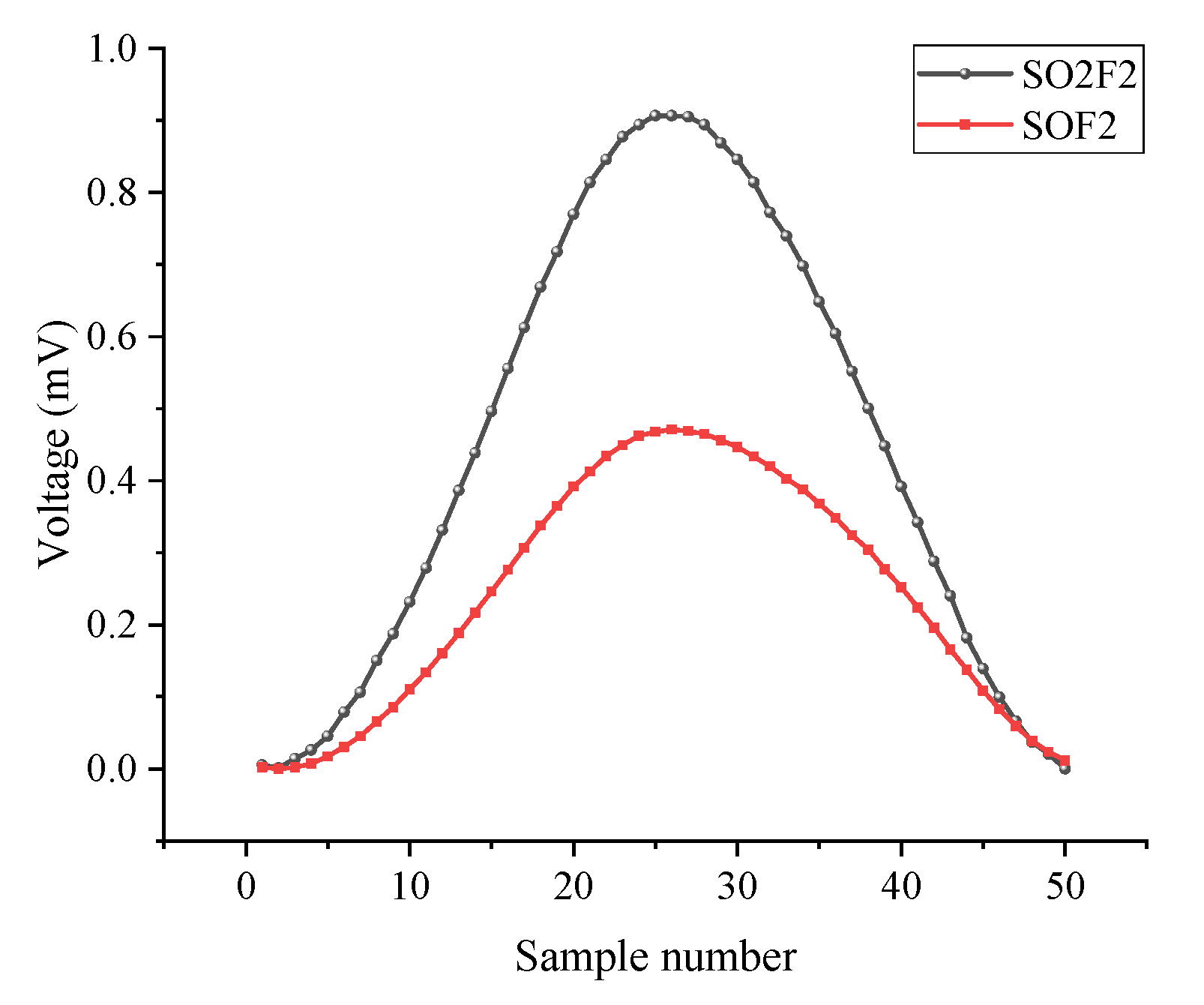
4.2. Adsorption Analysis for SO2F2
4.3. Adsorption Analysis for SOF2
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.G.; Lu, Z.; Liu, H.T.; Gao, Q.; Li, B.; Wu, X.J. Emission spectrum characteristics of SF6 plasma based on femtosecond laser-guided high-voltage discharge. Appl. Phys. B 2022, 128, 1–7. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.P.; Pan, J.Y.; Zhang, X.X.; Yao, Q.; He, J.J.; Hou, X.Z. Correlation Analysis between Formation Process of SF6 Decomposed Components and Partial Discharge Qualities. IEEE Trans. Dielectr. Electr. Insulation. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Heise, H.M.; Kurte, R.; Fischer, P.; Klockow, D.; Janissek, P.R. Gas analysis by infrared spectroscopy as a tool for electrical fault diagnostics in SF6 insulated equipment. Fresenius’ J. Anal. Chemistry. 1997, 358, 793–799. [Google Scholar] [CrossRef]
- Deng, B.T.; Sima, C.; Xiao, Y.F.; Wang, X.F.; Ai, Y.; Li, T.L.; Lu, P.; Liu, D.M. Modified laser scanning technique in wavelength modulation spectroscopy for advanced TDLAS gas sensing. Opt. Lasers Eng. 2022, 151, 106906. [Google Scholar] [CrossRef]
- Jaworski, P. A Review of Antiresonant Hollow-Core Fiber-Assisted Spectroscopy of Gases. Sensors 2021, 21, 5640. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.T.; Sima, C.T.; Tan, H.Y.; Zhang, X.H.; Lian, Z.G.; Chen, G.Q.; Yu, Q.Q.; Xu, J.H.; Liu, D.M. Design of hollow core step-index antiresonant fiber with stepped refractive indices cladding. Front. Optoelectron. 2021, 14, 407–413. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sima, C.T.; Deng, B.T.; Zhang, X.H.; Chen, G.Q.; Yu, Q.Q.; Xu, J.H.; Lian, Z.G.; Liu, D.M. Design and Analysis of Ultra-Wideband Highly-Birefringent Bragg Layered Photonic Bandgap Fiber With Concave-Index Cladding. IEEE Photonics J. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Sima, C.; Lu, P.; Long, Y.; Ai, Y.; Zhang, W.; Pan, Y.; Zhang, J.; Liu, D. Ultra-sensitive ppb-level methane detection based on NIR all-optical photoacoustic spectroscopy by using differential fiber-optic microphones with gold-chromium composite nanomembrane. Photoacoustics 2022, 26, 100353. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lu, P.; Sima, C.; Zhao, J.; Pan, Y.; Li, T.; Zhang, X.; Liu, D. Small-volume highly-sensitive all-optical gas sensor using non-resonant photoacoustic spectroscopy with dual silicon cantilever optical microphones. Photoacoustics 2022, 27, 100382. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, W.; Zhang, Y.; Liu, J.; Kan, R.; Wang, M.; Chen, J.; Cui, Y. H2S detection by tunable diode laser absorption spectroscopy. Proceedings of 2006 IEEE International Conference on Information Acquisition, Weihai, China; 2006; pp. 754–758. [Google Scholar]
- Chen, T.A.; Ma, F.X.; Zhao, Y.; Liao, Z.H.; Qiu, Z.J.; Zhang, G.Q. Cantilever enhanced based photoacoustic detection of SF6 decomposition component SO2 using UV LED. Sens. Rev. 2022, 42, 70–75. [Google Scholar] [CrossRef]
- He, Y.; Ma, Y.F.; Tong, Y.; Yu, X.; Tittel, F.K. A portable gas sensor for sensitive CO detection based on quartz-enhanced photoacoustic spectroscopy. Opt. Laser Technol. 2019, 115, 129–133. [Google Scholar] [CrossRef]
- Ilke, M.; Bauer, R.; Lengden, M. A Calibration-Free Methodology for Resonantly Enhanced Photoacoustic Spectroscopy Using Quantum Cascade Lasers. IEEE Sens. J. 2020, 20, 10530–10538. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Tajima, T.; Seyama, M.; Waki, K. Differential Continuous Wave Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring. IEEE Sens. J. 2020, 20, 4453–4458. [Google Scholar] [CrossRef]
- Lassen, M.; Harder, D.B.; Brusch, A.; Nielsen, O.S.; Heikens, D.; Persijn, S.; Petersen, J.C. Photo-acoustic sensor for detection of oil contamination in compressed air systems. Opt. Express. 2017, 25, 1806–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, C.; Dai, F.; Cheng, J.; Gan, Q.; Zhang, Z.; Cui, G.; Tan, T.; Li, D.; Xie, J. Analysis of Infrared Characteristics of SO2F2 and SOF2 of SF6 Decomposition Components. Proceedings of 2021 International Conference on Electrical Materials and Power Equipment (ICEMPE), Chongqing, China; 2021; pp. 1–4. [Google Scholar]
- Photoacoustic Resonator. Available online: https://cn.comsol.com/model/photoacoustic-resonator-12435 (accessed on 20 March 2022).
- National Library of Medicine. CID 17607. PubChem Compound Summary for CID 17607, Sulfuryl fluoride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sulfuryl-fluoride (accessed on 6 June 2022).
- National Library of Medicine. CID 24548. PubChem Compound Summary for CID 24548, Thionyl fluoride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thionyl-fluoride (accessed on 6 June 2022).
- Qian, H.; Deng, J.; Xie, Z.C.; Pan, Z.C.; Zhang, J.Y.; Zhou, H.B. Adsorption and Gas Sensing Properties of the Pt-3-MoSe2 Monolayer to SOF2 and SO2F2. ACS Omega 2020, 5, 7722–7728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, F.; Lei, Z.; Yang, X.; Tang, J.; Yao, Q.; Miao, Y. Evaluating DC Partial Discharge with SF6 Decomposition Characteristics. IEEE Trans. Power Delivery. 2019, 34, 1383–1392. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Yu, P.; Liu, Z. Optical gas sensing of sub-ppm SO2F2 and SOF2from SF6 decomposition based on photoacoustic spectroscopy. IET Optoelectron. 2022, 1–6. [Google Scholar] [CrossRef]




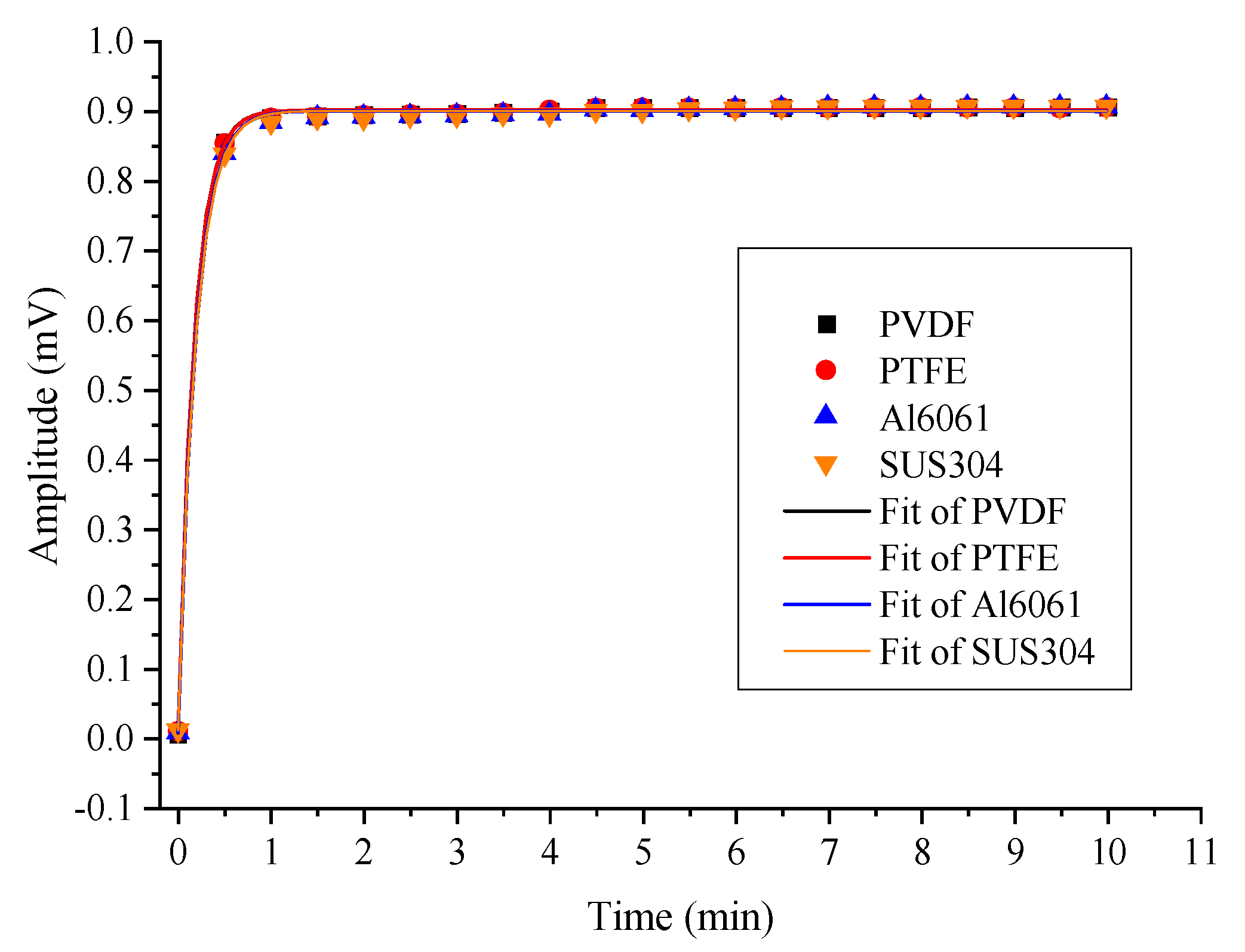

| Materials | Max Value | 90% Max | 90% Response Time |
|---|---|---|---|
| PVDF | 0.9055 | 0.81495 | 24 |
| PTFE | 0.9058 | 0.81522 | 25 |
| Al6061 | 0.9063 | 0.81567 | 27 |
| SUS304 | 0.9065 | 0.81585 | 27 |
| Materials | Max Value | 90% Max | 90% Response Time |
|---|---|---|---|
| PVDF | 0.47076 | 0.42368 | 28 |
| PTFE | 0.47092 | 0.42383 | 28 |
| Al6061 | 0.47112 | 0.42347 | 34 |
| SUS304 | 0.47116 | 0.42404 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, M.; Yu, P.; Liu, Z. Optical Gas-Cell Dynamic Adsorption in a Photoacoustic Spectroscopy-Based SOF2 and SO2F2 Gas Sensor. Sensors 2022, 22, 7949. https://doi.org/10.3390/s22207949
Zhang Y, Wang M, Yu P, Liu Z. Optical Gas-Cell Dynamic Adsorption in a Photoacoustic Spectroscopy-Based SOF2 and SO2F2 Gas Sensor. Sensors. 2022; 22(20):7949. https://doi.org/10.3390/s22207949
Chicago/Turabian StyleZhang, Ying, Mingwei Wang, Pengcheng Yu, and Zhe Liu. 2022. "Optical Gas-Cell Dynamic Adsorption in a Photoacoustic Spectroscopy-Based SOF2 and SO2F2 Gas Sensor" Sensors 22, no. 20: 7949. https://doi.org/10.3390/s22207949







