A Novel Electrochemical Sensor Modified with a Computer-Simulative Magnetic Ion-Imprinted Membrane for Identification of Uranyl Ion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Design and Calculation of Magnetic Ion Imprinted Membrane by Molecular Simulation
2.4. Fabrication of Ion Imprinted Membrane
2.4.1. Synthesis of Fe3O4@SiO2 Nanoparticles
2.4.2. Preparation of Carbon Paste Electrodes Modified with Fe3O4@SiO2
2.4.3. Preparation of Imprinted Polymer
2.5. Electrochemical Measurements
2.6. Evaluation of the Electroactive Surface Area of the Electrodes
2.7. Actual Sample Analysis
2.8. Statistical Treatment of the Data
3. Results and Discussion
3.1. Simulation Design of Ion Imprinted Polymer
3.2. Electrochemical Characterization of IIP/Fe3O4@SiO2/MCPE Sensors
3.2.1. Differential Pulse Voltammetry (DPV) Characterization
3.2.2. Study on Accessible Surface Area of Different Sensors
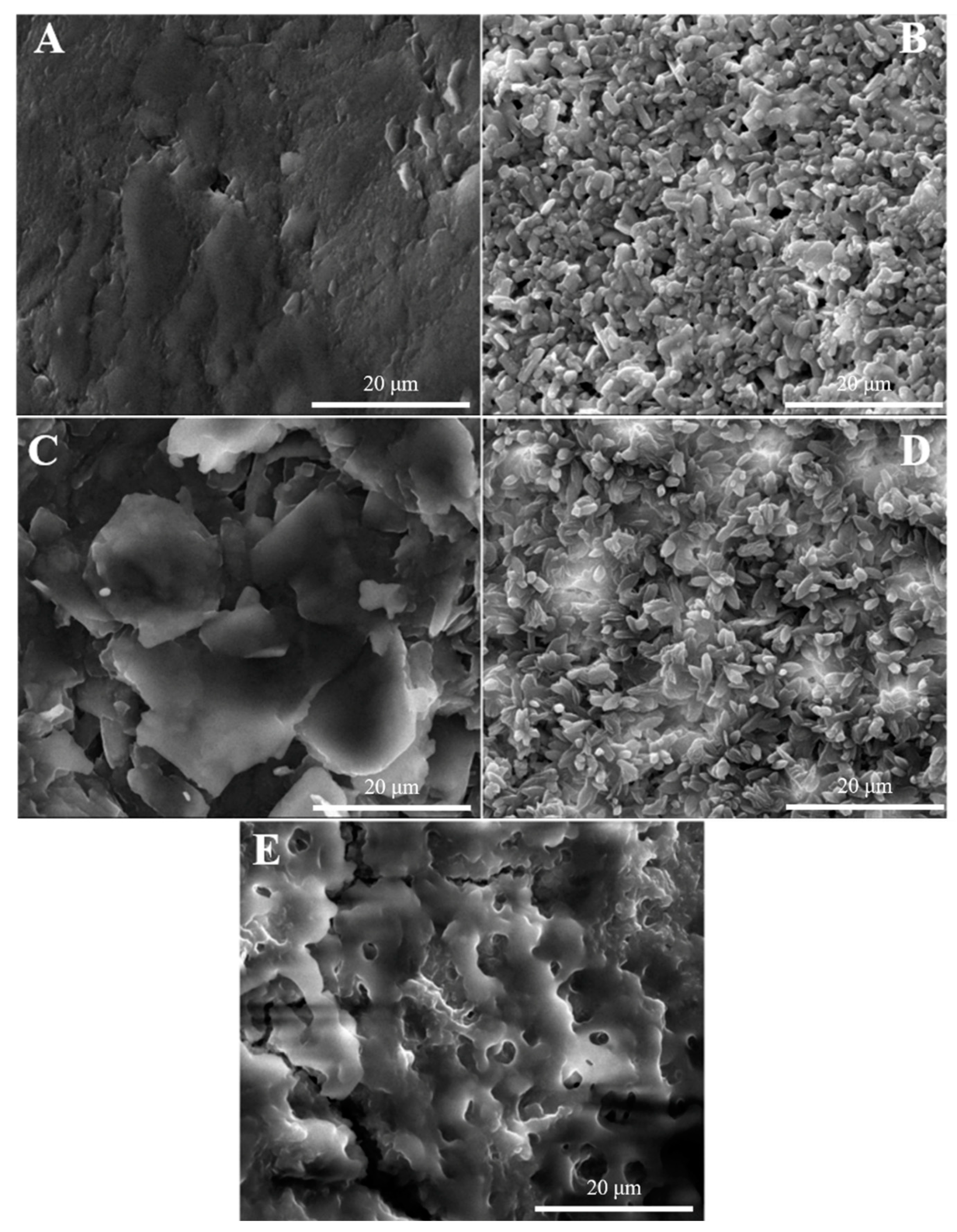
3.2.3. Surface Morphological Characterization of Fe3O4 and Fe3O4@Sio2
3.2.4. Characterization of Sensor Surface Morphology
3.2.5. Electrochemical Impedance Diagram
3.3. Optimization of Experimental Conditions
3.3.1. Dosage Optimization
3.3.2. Optimization of Eluent and Elution Time
3.3.3. Effect of pH
3.3.4. Optimization of Enrichment Time
3.4. Study on the Performance of Uranyl Sensor
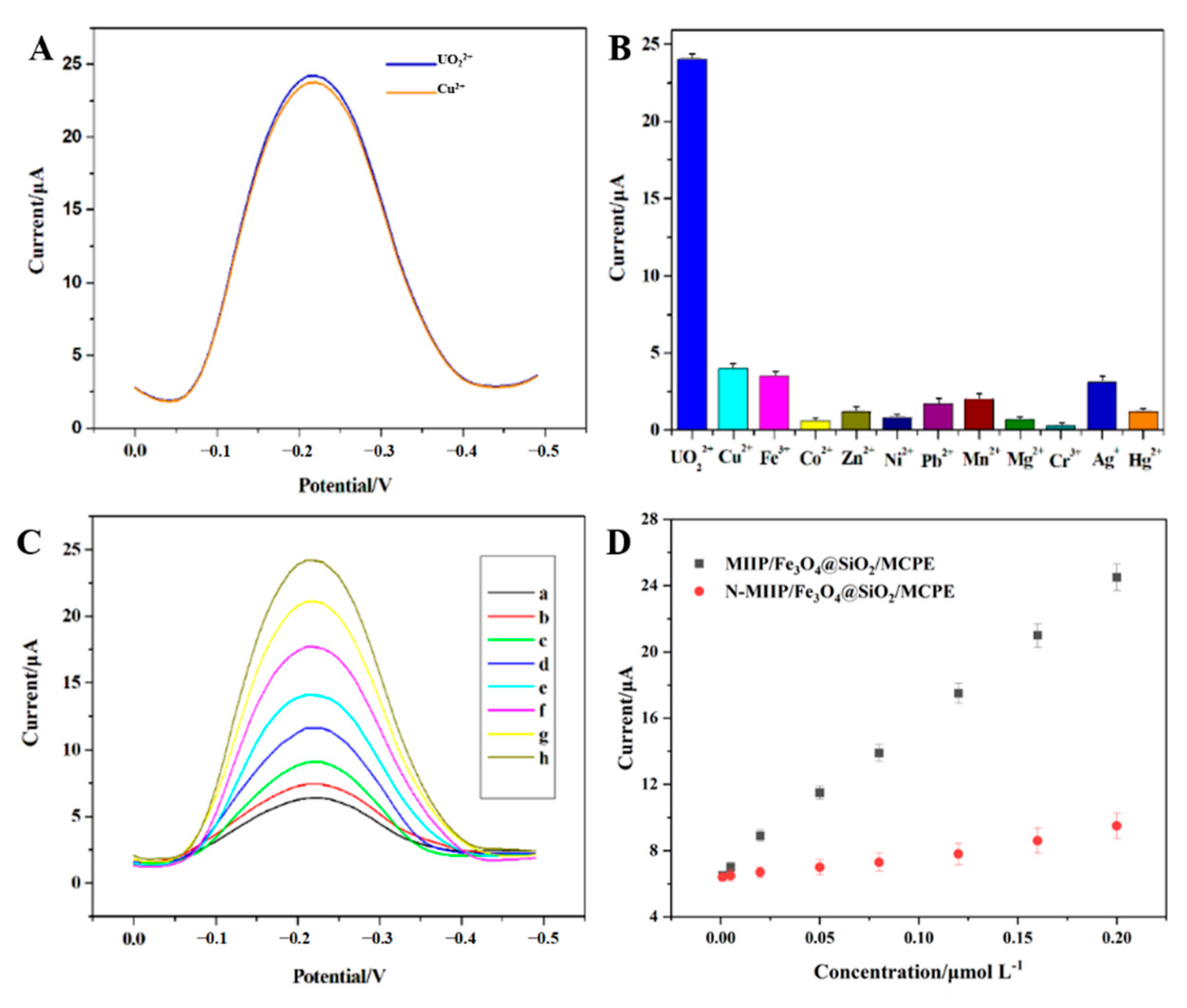
3.4.1. Evaluation of Selectivity of MIIP/Fe3O4@SiO2/MCPE Sensor
3.4.2. Sensor Stability and Repeatability
3.4.3. Calibration Curve and Detection Limit of Sensor
3.5. Actual Sample Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venus, M.; Puntaric, D.; Gvozdic, V.; Vidosavljevic, D.; Bijelic, L.; Puntaric, A.; Puntaric, E.; Vidosavljevic, M.; Matijana, J.; Jasenka, S. Determinations of uranium concentrations in soil, water, vegetables and biological samples from inhabitants of war affected areas in eastern Croatia (ICP-MS method). J. Environ. Radioact. 2019, 203, 147–153. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.X.; Li, G.R.; Liu, Y.J.; Zhou, Z.K.; Wang, X.G.; Zheng, Z.H.; Zhou, Y.P.; Zhao, K.; Xiang, L.; et al. Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China. Minerals 2020, 10, 747. [Google Scholar] [CrossRef]
- Bradley, V.C.S.; Tyler, L.; Metzger, S.C.; Ticknor, B.W.; Dunlap, D.R.; Zirakparvar, N.A.; Roach, B.D.; Hexel, C.R.; Manard, B.T. Direct isotopic analysis of solid uranium particulates on cotton swipes by microextraction-ICP-MS. Anal. Chim. Acta 2022, 1209, 339836. [Google Scholar] [CrossRef]
- El Gammal, E.M.; Ahmed, S.H. Separation and determination of uranium in phosphoric acid medium using high-performance ion chromatography. Radioanal. Nucl. Chem. 2019, 321, 413–419. [Google Scholar] [CrossRef]
- Lincoln, D.R.; Charlton, J.J.; Hatab, N.A.; Skyberg, B.; Lavrik, N.V.; Kravchenko, I.I.; Bradshaw, J.A.; Sepaniak, M.J. Surface Modification of Silicon Pillar Arrays To Enhance Fluorescence Detection of Uranium and DNA. ACS Omega 2017, 2, 7313–7319. [Google Scholar] [CrossRef]
- Wu, X.M.; Huang, Q.X.; Mao, Y.; Wang, X.X.; Wang, Y.Y.; Hu, Q.H.; Wang, H.Q.; Wang, X.K. Sensors for determination of uranium: A review. TrAC Trend Anal. Chem. 2019, 118, 89–111. [Google Scholar] [CrossRef]
- Nazari Serenjeh, F.; Hashemi, P.; Naeimi, H.; Zakerzadeh, E.; Ghiasvand, A.R. Spherical agarose-coated magnetic nanoparticles functionalized with a new salen for magnetic solid-phase extraction of uranyl ion. Microchim. Acta 2016, 183, 2449–2455. [Google Scholar] [CrossRef]
- Lima, N.P.; Saiki, M. Determination of uranium in tree bark samples by epithermal neutron activation analysis. Braz. J. Radiatio Sci. 2019, 7, 15392. [Google Scholar]
- Xing, S.; Zhang, W.C.; Qiao, J.X.; Hou, X.L. Determination of ultra-low level plutonium isotopes (Pu-239,(240)Pu) in environmental samples with high uranium. Talanta 2018, 187, 357–364. [Google Scholar] [CrossRef]
- Banos, A.; Harker, N.J.; Scott, T.B. A review of uranium corrosion by hydrogen and the formation of uranium hydride. Corros. Sci. 2018, 136, 129–147. [Google Scholar] [CrossRef]
- Khattab, M.R.; Tuovinen, H.; Lehto, J.; El Assay, I.E.; El Feky, M.G.; Abd El-Rahman, M.A. Determination of uranium in Egyptian graniteic ore by gamma, alpha, and mass spectrometry. Instrum. Sci. Technol. 2017, 45, 338–348. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC Trend Anal. Chem. 2020, 123, 115772. [Google Scholar] [CrossRef]
- Yun, W.; Jiang, J.L.; Cai, D.Z.; Wang, X.F.; Sang, G.; Liao, J.S.; Lu, T.C.; Yan, K.P. Ultrasensitive electrochemical detection of UO22+ based on DNAzyme and isothermal enzyme-free amplification. RSC Adv. 2016, 6, 3960–3966. [Google Scholar] [CrossRef]
- Zhou, Z.P.; Zhou, Y.M.; Liang, X.Z.; Xie, F.; Liu, S.J.; Ma, J.G. Sensitive detection of uranium in water samples using differential pulse adsorptive stripping voltammetry on glassy carbon electrode. J. Radioanal. Nucl. Chem. 2019, 322, 2049–2056. [Google Scholar] [CrossRef]
- Gęca, I.; Ochab, M.; Korolczuk, M. Application of a solid lead microelectrode as a new voltammetric sensor for adsorptive stripping voltammetry of U (VI). Talanta 2020, 207, 120309. [Google Scholar] [CrossRef]
- Dewangan, P.K.; Khan, F.; Shrivas, K.; Sahu, V. Determination of uranium in environmental sample by nanosensor graphene quantum dots. J. Radioanal. Nucl. Chem. 2019, 320, 757–763. [Google Scholar] [CrossRef]
- Guney, S.; Guney, O. A novel electrochemical sensor for selective determination of uranyl ion based on imprinted polymer sol-gel modified carbon paste electrode. Sens. Actuators B Chem. 2016, 231, 45–53. [Google Scholar] [CrossRef]
- Su, C.L.; Li, Z.Y.; Zhang, D.; Wang, Z.M.; Zhou, X.; Liao, L.F.; Xiao, X.L. A highly sensitive sensor based on a computer-designed magnetic molecularly imprinted membrane for the determination of acetaminophen. Biosens. Bioelectron. 2020, 148, 111819. [Google Scholar] [CrossRef]
- Cao, C.; Liu, J.Q.; Tang, S.Y.; Dai, Z.R.; Xiao, F.B.; Rang, W.Q.; Liu, L.; Chen, T.; Yuan, Y.L.; Li, L. Amplified electrochemical determination of UO22+ based on the cleavage of the DNAzyme and DNA-modified gold nanoparticle network structure. Mikrochim Acta 2020, 187, 1–9. [Google Scholar] [CrossRef]
- Pinaeva, U.; Dietz, T.C.; Al Sheikhly, M.; Balanzat, E.; Castellino, M.; Wade, T.L.; Clochard, M.C. Bis[2-(methacryloyloxy)ethyl] phosphate radiografted into track-etched PVDF for uranium (VI) determination by means of cathodic stripping voltammetry. React. Funct. Polym. 2019, 142, 77–86. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA-modified PANI/SWNTs nanocomposite for differential pulse voltammetry based determination of Cu(II) ions. Sens. Actuators B Chem. 2018, 260, 331–338. [Google Scholar] [CrossRef]
- Wang, Z.M.; Zhang, D.; Xiao, X.L.; Su, C.L.; Li, Z.Y.; Xue, J.H.; Hu, N.; Peng, P.C.; Liao, L.F.; Wang, H.Q. A highly sensitive and selective sensor for trace uranyl (VI) ion based on a graphene-coated carbon paste electrode modified with ion imprinted polymer. Microchem. J. 2020, 155, 104767. [Google Scholar] [CrossRef]
- Silva, W.R.; Sotéa, W.O.; Petruci, J.F.D.; Batista, A.D.; Comar, M.J. The use of in silico models for the rationalization of molecularly imprinted polymer synthesis. Eur. Polym. J. 2022, 166, 111024. [Google Scholar] [CrossRef]
- Liu, J.B.; Wang, G.Y.; Tang, S.S.; Gao, Q.; Liang, D.D.; Jin, R.F. Theoretical and experimental research on self-assembly system of molecularly imprinted polymers formed via chloramphenicol and methacrylic acid. J. Sep. Sci. 2019, 42, 769–777. [Google Scholar] [CrossRef]
- Yang, X.J.; Xu, X.J.; Hou, X.L.; Zhang, P.; Mi, J.L.; Xiao, B.B.; Huang, J.; Stampfl, C. Transition metal-doped tetra-MoN2 monolayers as an electrochemical catalyst for CO2 reduction: A density functional theory study. Catal. Commun. 2021, 149, 106212–106217. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, T.; Gao, P.; Zhao, L.; Zhou, L.; Hua, D.B. Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium. J. Mater. Chem. A 2019, 7, 11214–11222. [Google Scholar] [CrossRef]
- Zhao, W.S.; Liu, J.B.; Tang, S.S.; Jin, R.F. Theoretical research of molecular imprinted polymers formed from formaldehyde and methacrylic acid. J. Mol. Model. 2020, 26, 88–96. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Balouch, A.; Mahesar, S.A.; Kumar, A.; Baloch, A.R.; Abdullah; Bhanger, M.I. Fabrication of cadmium tagged novel ion imprinted polymer for detoxification of the toxic Cd2+ ion from aqueous environment. Microchem. J. 2020, 158, 105247. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Novel ion imprinted polymer electrochemical sensor for the selective detection of lead(II). Food Chem. 2020, 303, 125374. [Google Scholar] [CrossRef]
- Esmali, F.; Mansourpanah, Y.; Farhadi, K.; Amani, S.; Rasoulifard, A.; Ulbricht, M. Fabrication of a novel and highly selective ion-imprinted PES-based porous adsorber membrane for the removal of mercury(II) from water. Sep. Purif. Technol. 2020, 250, 117183. [Google Scholar] [CrossRef]
- Kakavandi, M.G.; Behbahani, M.; Omidi, F.; Hesam, G. Application of Ultrasonic Assisted-Dispersive Solid Phase Extraction Based on Ion-Imprinted Polymer Nanoparticles for Preconcentration and Trace Determination of Lead Ions in Food and Water Samples. Food Anal. Methods 2017, 10, 2454–2466. [Google Scholar] [CrossRef]
- Chen, J.X.; Lei, S.; Zeng, K.; Wang, M.Z.; Asif, A.; Ge, X.W. Catalase-imprinted Fe3O4/Fe@fibrous SiO2/polydopamine nanoparticles: An integrated nanoplatform of magnetic targeting, magnetic resonance imaging, and dual-mode cancer therapy. Nano Res. 2017, 10, 2351–2363. [Google Scholar] [CrossRef]
- Wang, H.J.; Qian, D.; Xiao, X.L.; Gao, S.Q.; Cheng, J.L.; He, B.; Liao, L.F.; Deng, J. A highly sensitive and selective sensor based on a graphene-coated carbon paste electrode modified with a computationally designed boron-embedded duplex molecularly imprinted hybrid membrane for the sensing of lamotrigine. Biosens. Bioelectron. 2017, 94, 663–670. [Google Scholar] [CrossRef]
- Peng, P.C.; Liao, L.F.; Yu, Z.H.; Jiang, M.; Deng, J.; Xiao, X.L. A novel sensor based on multi-walled carbon nanotubes and boron-doped double-layer molecularly imprinted membrane for the analysis of SCZ in pharmaceutical and biological samples. Int. J. Environ. An. Chem. 2019, 99, 1495–1514. [Google Scholar] [CrossRef]
- Wang, H.J.; Qian, D.; Xiao, X.L.; He, B.; Gao, S.Q.; Shi, H.; Liao, L.F.; Deng, J. Enantioselective determination of S-ornidazole by using carbon paste electrode modified with boron-embedded conductive copolymer-polysiloxane-based molecularly imprinted hybrid film. Electrochim. Acta 2017, 246, 338–347. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.N.; Riwu, A.A.P.; Darmokoesoemo, H.; Supriyanto, G.; Holdsworth, C.I.; Amenaghawon, A.N.; Kusuma, H.S. A Cr(VI)-imprinted-poly(4-VP-co-EGDMA) sorbent prepared using precipitation polymerization and its application for selective adsorptive removal and solid phase extraction of Cr(VI) ions from electroplating industrial wastewater. React. Funct. Polym. 2020, 147, 104451. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Behbahani, M.; Najafi, M.; Bagheri, A.; Omidi, F.; Salimi, S. Selective and Sensitive Determination of Uranyl Ions in Complex Matrices by Ion Imprinted Polymers-Based Electrochemical Sensor. Electroanalysis 2015, 27, 2458–2467. [Google Scholar] [CrossRef]
- Ye, L.; Cormack, P.A.G.; Mosbach, K. Molecularly imprinted monodisperse microspheres for competitive radioassay. Anal. Commun. 1999, 36, 35–38. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies-Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Liu, W.F.; Yang, Y.Z.; Liu, X.G. Functional monomer screening and preparation of dibenzothiophene-imprinted polymers on the surface of carbon microsphere. Mon. Chem. 2015, 146, 449–458. [Google Scholar] [CrossRef]
- Lu, C.X.; Tang, Z.G.; Gao, X.X.; Ma, X.M.; Liu, C.B. Computer-aided design of magnetic dummy molecularly imprinted polymers for solid-phase extraction of ten phthalates from food prior to their determination by GC-MS/MS. Microchim. Acta 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Fizir, M.; Wei, L.; Muchuan, N.; Itatahine, A.; Mehdi, Y.A.; He, H.; Dramou, P. QbD approach by computer aided design and response surface methodology for molecularly imprinted polymer based on magnetic halloysite nanotubes for extraction of norfloxacin from real samples. Talanta 2018, 184, 266–276. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.B.; Tang, S.S.; Jin, R.F. Preparation of melamine molecularly imprinted polymer by computer-aided design. J. Sep. Sci. 2015, 38, 2647–2654. [Google Scholar] [CrossRef]
- Knope, K.E.; Cahill, C.L. Uranyl triazolate formation via an in situ Huisgen 1,3-dipolar cycloaddition reaction. Crystengcomm 2011, 13, 153–157. [Google Scholar] [CrossRef]
- Thuery, P. Two uranyl-organic frameworks with pyridinecarboxylate ligands. A novel heterometallic uranyl-copper(II) complex with a cation-cation interaction. Inorg. Chem. 2009, 12, 800–803. [Google Scholar] [CrossRef]
- Chen, X.T.; He, L.F.; Wang, Y.; Liu, B.; Tang, Y.P. Trace analysis of uranyl ion (UO22+) in aqueous solution by fluorescence turn-on detection via aggregation induced emission enhancement effect. Anal. Chim. Acta 2014, 847, 55–60. [Google Scholar] [CrossRef]
- Maji, S.; Viswanathan, K.S. Sensitization of uranium fluorescence using 2,6-pyridinedicarboxylic acid: Application for the determination of uranium in the presence of lanthanides. J. Lumin. 2009, 129, 1242–1248. [Google Scholar] [CrossRef]
- Mostafa, S.I.; El-Maksoud, S.A. Synthesis and characterization of some transition metal complexes of 2-amino-3-hydroxypyridine and its application in corrosion inhibition. Mon. Chem. 1998, 129, 455–466. [Google Scholar] [CrossRef]
- Metilda, P.P.K.; Kala, R.; Gladis, J.M.; Rao, T.P.; Naidu, G.R.K. Ion imprinted polymer based sensor for monitoring toxic uranium in environmental samples. Anal. Chim. Acta 2007, 582, 147–153. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rawashdeh, N.A.F.; Al-Mheidat, I.R.; Nassory, N.S. Preparation and evaluation of new uranyl imprinted polymer electrode sensor for uranyl ion based on uranyl-carboxybezotriazole complex in pvc matrix membrane. Sens. Actuators B Chem. 2016, 227, 336–345. [Google Scholar] [CrossRef]
- Shi, J.C.; Xu, H.F.; Zhao, H.; Lu, L.; Wu, X.X. Preparation of Nd2Fe14B/C magnetic powder and its application in proton exchange membrane fuel cells. J. Power Sources 2014, 252, 189–199. [Google Scholar] [CrossRef]
- Dimovasilis, P.A.; Prodromidis, M.I. An electrochemical sensor for trace uranium determination based on 6-O-palmitoyl-L-ascorbic acid-modified graphite electrodes. Sens. Actuators B Chem. 2011, 156, 689–694. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Mazaheri, S.; Naeimi, H. Uranyl sensor based on a N,N′-bis(salicylidene)-2-hydroxy-phenylmethanediamine and multiwall carbon nanotube electrode. J. Radioanal. Nucl. Chem. 2012, 293, 201–210. [Google Scholar] [CrossRef]
- Shamsipur, M.; Mizani, F.; Mousavi, M.F.; Alizadeh, N.; Alizadeh, K.; Eshghi, H.; Karami, H. A novel flow injection potentiometric graphite coated ion-selective electrode for the low level determination of uranyl ion. Anal. Chim. Acta 2007, 589, 22–32. [Google Scholar] [CrossRef] [PubMed]




| No | Complexes | Binding Energies (∆E/kJ mol−1) |
|---|---|---|
| 1 | 1H-PPCA- UO22+ | −85.07 |
| 2 | 2-HP- UO22+ | −35.28 |
| 3 | 3-HP- UO22+ | −31.19 |
| 4 | H2SA- UO22+ | −42.58 |
| 5 | DHP- UO22+ | −49.13 |
| 6 | 3-PCA- UO22+ | −67.01 |
| 7 | H2Pdc- UO22+ | −100.32 |
| Electrode | Method | Linear Range (mol L−1) | LOD (mol L−1) | References |
|---|---|---|---|---|
| Graphite electrodes | DPV | 1 × 10−8–2.5 × 10−7 | 3.5 × 10−8 | [52] |
| SHPMD/CNT/CPE | DPV | 6 × 10−7–6 × 10−8 | 2 × 10−9 | [53] |
| UO22+−PME/CGE | FIP | 1 × 10−7–1 × 10−1 | 5.4 × 10−8 | [54] |
| UO22+–DCQ–VP | ISE | 2.0×10-8- 1.0×10-2 | 2.0×10-8 | [49] |
| MIIP/Fe3O4@SiO2/MCPE | DPV | 1 × 10−9–2 × 10−7 | 3.23 × 10−10 | This work |
| Sample | Added (μM) | Detected by This Method | ||
|---|---|---|---|---|
| Found (μM) | Recovery (%) | RSD (%) | ||
| Soil 1 | 0.10 | 0.103 | 103 | 1.69 |
| Soil 2 | 0.10 | 0.970 | 97 | 2.01 |
| Soil 3 | 0.10 | 0.101 | 101 | 2.41 |
| Water 4 | 0.15 | 0.156 | 104 | 1.25 |
| Water 5 | 0.15 | 0.147 | 98 | 3.06 |
| Tap water | 0.10 | 0.950 | 95 | 2.15 |
| 0.15 | 0.146 | 97 | 1.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.-Q.; Wang, Z.-M.; Li, Y.-J.; Yang, J.; Liao, L.-F.; Xiao, X.-L.; Liu, Y. A Novel Electrochemical Sensor Modified with a Computer-Simulative Magnetic Ion-Imprinted Membrane for Identification of Uranyl Ion. Sensors 2022, 22, 4410. https://doi.org/10.3390/s22124410
He L-Q, Wang Z-M, Li Y-J, Yang J, Liao L-F, Xiao X-L, Liu Y. A Novel Electrochemical Sensor Modified with a Computer-Simulative Magnetic Ion-Imprinted Membrane for Identification of Uranyl Ion. Sensors. 2022; 22(12):4410. https://doi.org/10.3390/s22124410
Chicago/Turabian StyleHe, Li-Qiong, Zhi-Mei Wang, Yu-Jie Li, Jing Yang, Li-Fu Liao, Xi-Lin Xiao, and Yong Liu. 2022. "A Novel Electrochemical Sensor Modified with a Computer-Simulative Magnetic Ion-Imprinted Membrane for Identification of Uranyl Ion" Sensors 22, no. 12: 4410. https://doi.org/10.3390/s22124410





