Electrochemical L-Tyrosine Sensor Based on a Glassy Carbon Electrode Modified with Exfoliated Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Chemicals
2.3. Electrochemical Exfoliation of Graphite Rods for Graphene Synthesis (EGr)
2.4. Preparation of Graphene-Modified Electrode (EGr/GC)
3. Results and Discussion
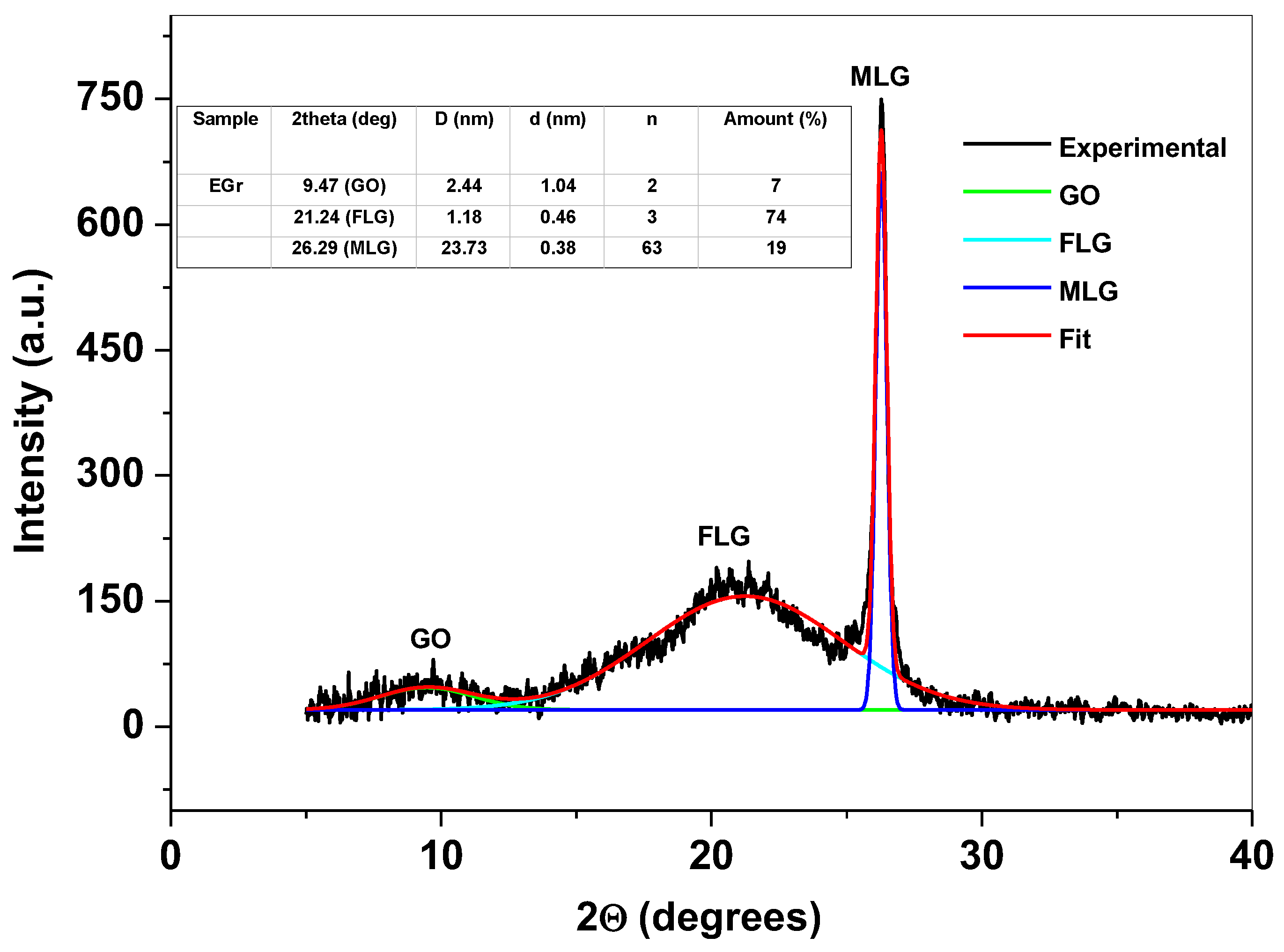
3.1. Morphological and Structural Characterization of EGr Sample
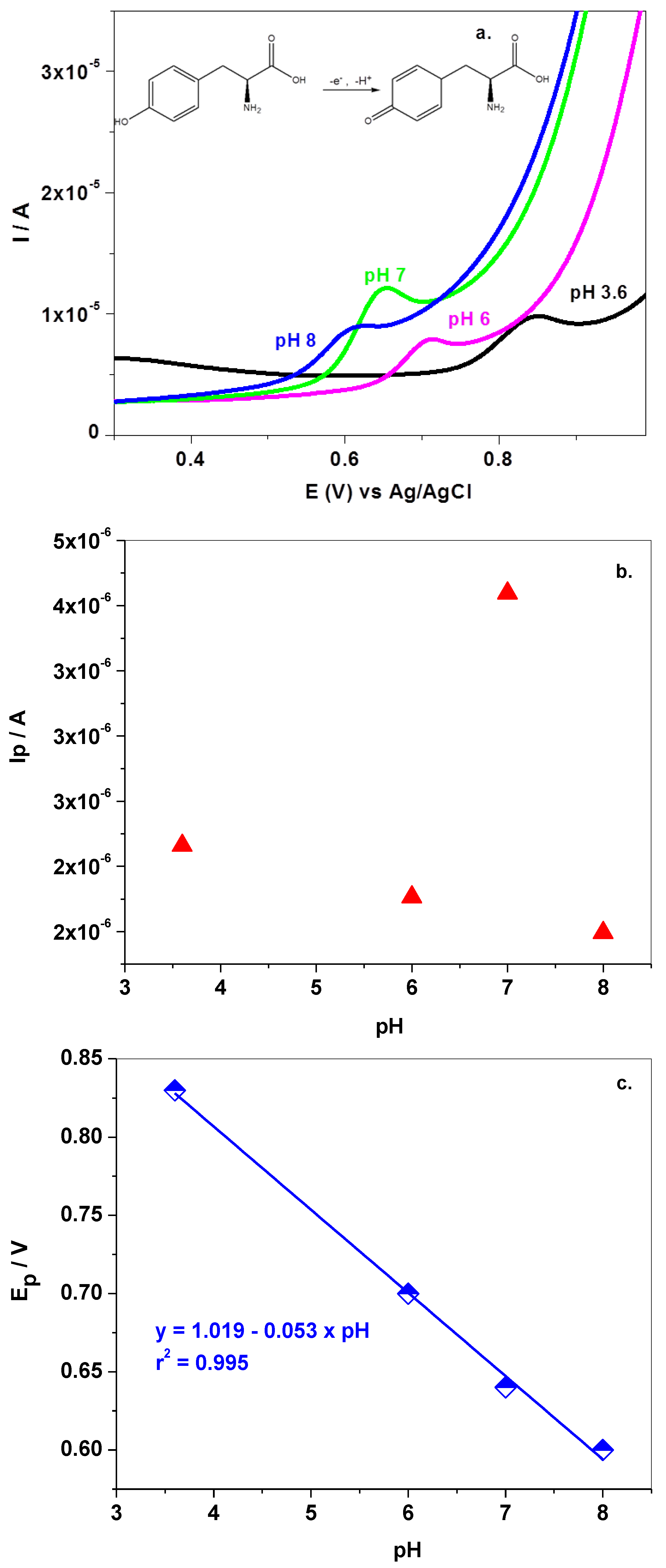
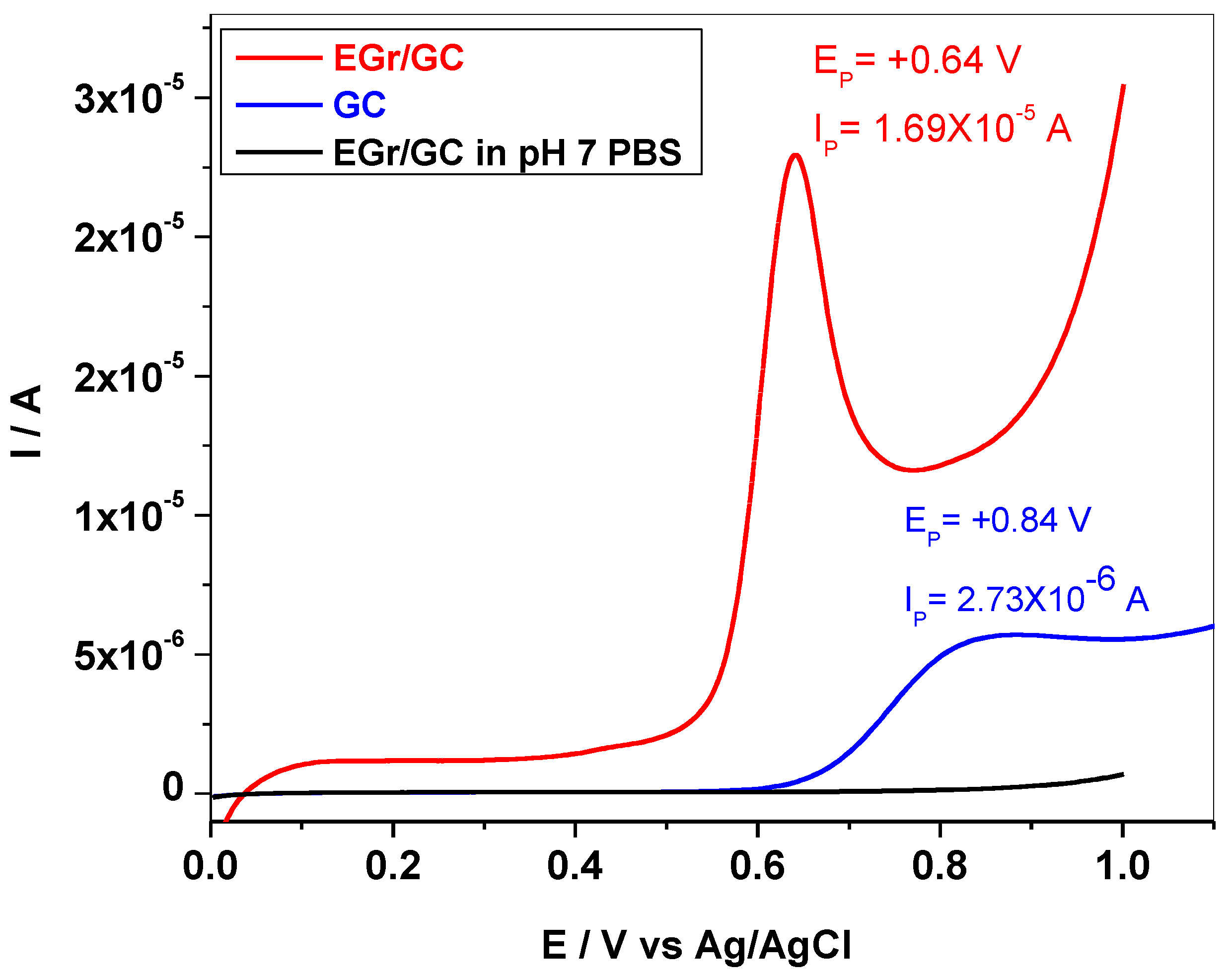
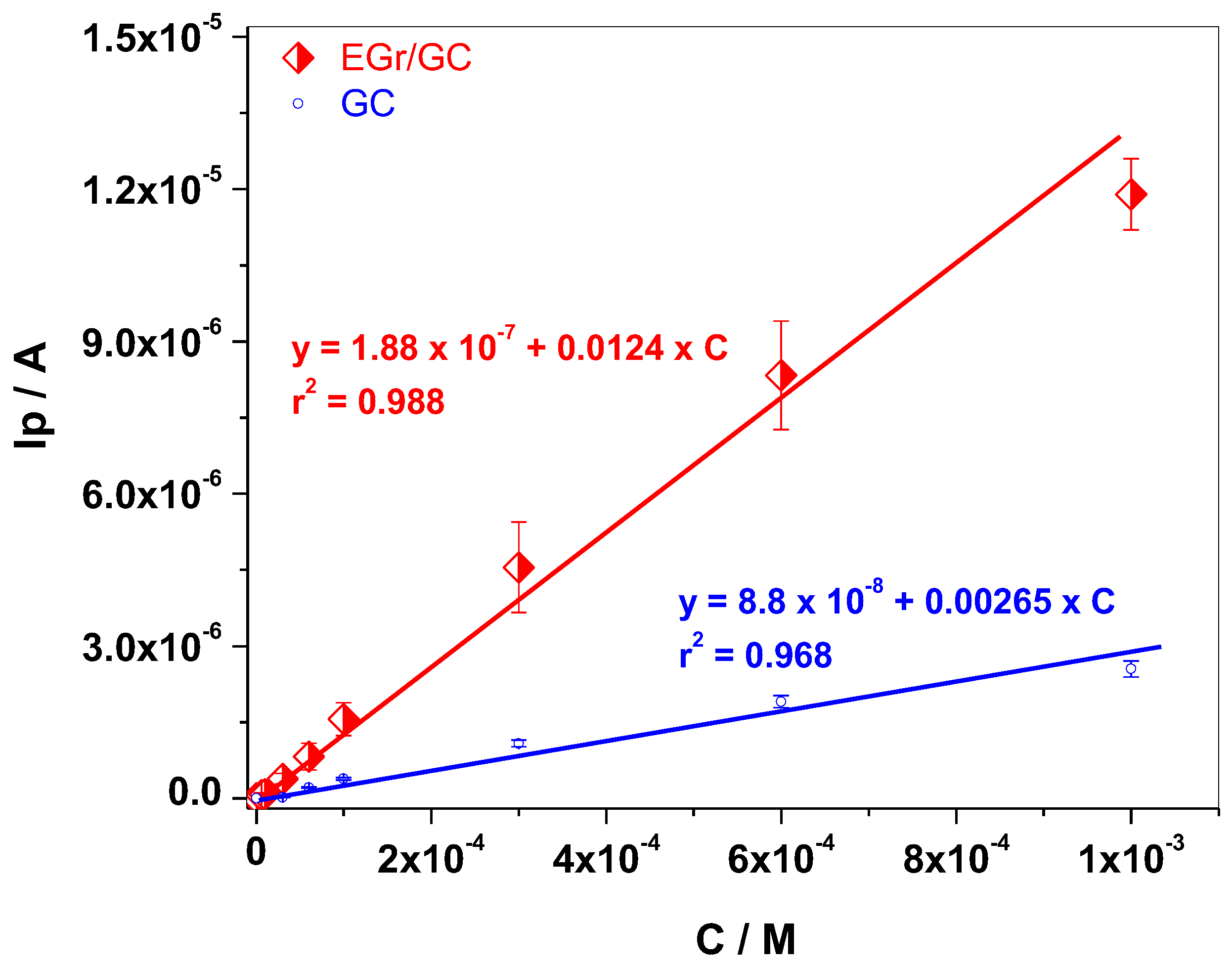
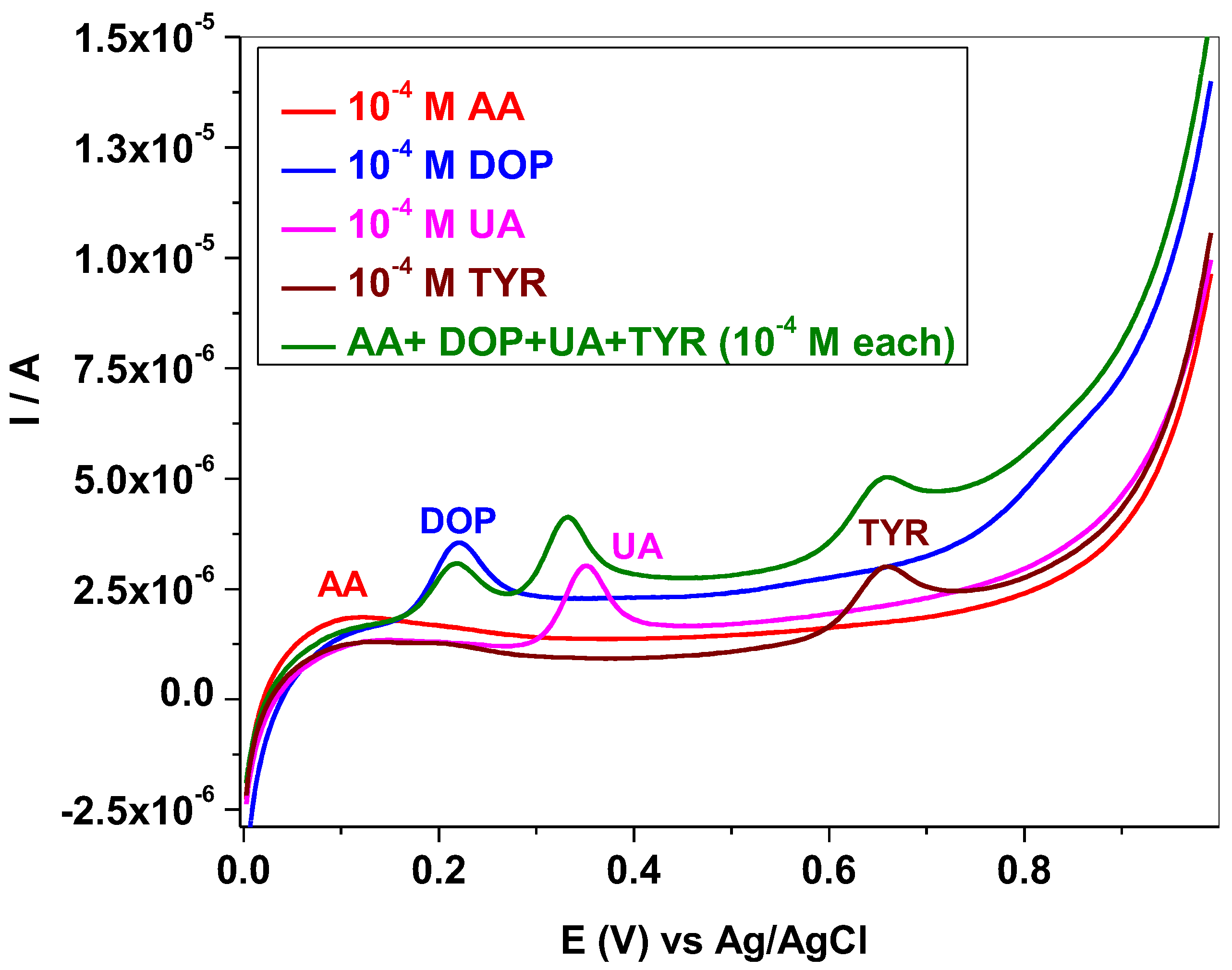
3.2. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Attipoe, S.; Zeno, S.A.; Lee, C.; Crawford, C.; Khorsan, R.; Walter, A.R.; Deuster, P.A. Tyrosine for mitigating stress and enhancing performance in healthy adult humans, a rapid evidence assessment of the literature. Mil. Med. 2015, 180, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrea, G.D.; Ostuzzi, R.; Bolner, A.; Francesconi, F.; Musco, F.; Davide, O. Study of tyrosine metabolism in eating disorders. Possible correlation with migraine. Neurol. Sci. 2008, 29, 88–92. [Google Scholar] [CrossRef]
- Barone, H.; Bliksrud, Y.T.; Elgen, I.B.; Szigetvari, P.D.; Ghorbani, S.; Haavik, J. Tyrosinemia Type 1 and symptoms of ADHD: Biochemical mechanisms and implications for treatment and prognosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Molina, A.; Jimenez-Jimenez, J.; Vargas, C.; Navarro, A.; Ortı-pareja, M.; Gasalla, T. Decreased cerebrospinal fluid levels of neutral and basic amino acids in patients with Parkinson’s disease. J. Neurol. Sci. 1997, 150, 123–127. [Google Scholar] [CrossRef]
- Ayhan, M.E. CVD graphene-based flexible and transparent SERS substrate towards L-tyrosine detection. Microelectron. Eng. 2021, 241, 111546. [Google Scholar] [CrossRef]
- Neurauter, G.; Scholl-bürgi, S.; Haara, A.; Geisler, S.; Mayersbach, P.; Schennach, H.; Fuchs, D. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fl uorescence detection. Clin. Biochem. 2013, 46, 1848–1851. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, W.; Huang, H.; Su, X. Determination of L-tyrosine based on luminescence quenching of Mn-Doped ZnSe quantum dots in enzyme catalysis system. J. Fluoresc. 2011, 21, 125–131. [Google Scholar] [CrossRef]
- Gu, W.; Wang, M.; Mao, X.; Wang, Y.; Li, L.; Xia, W. A facile sensitive l-tyrosine electrochemical sensor based on a coupled CuO/Cu2O nanoparticles and multi-walled carbon nanotubes nanocomposite film. Anal. Methods 2014, 7, 1313–1320. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, C.; Zhang, S. Electrochemical behavior and sensitive determination of L -tyrosine with a gold nanoparticles modified glassy carbon electrode. Anal. Sci. 2009, 25, 1221–1225. [Google Scholar] [CrossRef] [Green Version]
- Dinu, A.; Apetrei, C. Quantification of tyrosine in pharmaceuticals with the new biosensor based on laccase-modified polypyrrole polymeric thin film. Polymers 2022, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Phelane, L.; Gouveia-Caridade, C.; Barsan, M.M.; Priscilla, G.L.; Brett, C.M.A.; Iwuoha, E.I. Electrochemical determination of tyrosine using a novel tyrosinase Multi-Walled Carbon Nanotube (MWCNT) polysulfone modified glassy carbon electrode (GCE). Anal. Lett. 2019, 53, 308–321. [Google Scholar] [CrossRef]
- Moulaee, K.; Neri, G. Electrochemical amino acid sensing: A review on challenges and achievements. Biosensors 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and thermal properties of graphene and recent advances in graphene based electronics applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, S.; Tamura, K.; Kato, M.; Asaoka, H.; Yagi, I. Electrochemically driven specific alkaline metal cation adsorption on a graphene interface. J. Phys. Chem. C 2021, 125, 22154–22162. [Google Scholar] [CrossRef]
- Qiu, H.; Jiang, T.; Wang, X.; Zhu, L.; Wang, Q.; Zhao, Y. Electrochemical investigation of adsorption of graphene oxide at an interface between two immiscible electrolyte solutions. RSC Adv. 2020, 10, 25817–25827. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, S.A.Y.; Bonannia, A.; Pumera, M. Graphene and its electrochemistry: An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. Recent Progress in the Electrochemical Exfoliation of Colloidal Graphene: A Review; IntechOpen Book Series; IntechOpen: London, UK, 2021; pp. 3–11. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef] [Green Version]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon 2021, 168, 737–747. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Sonochemical preparation of functionalized graphenes. J. Am. Chem. Soc. 2011, 133, 9148–9151. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Tillotson, E.; Conlan, A.P.; Ding, F.; Haigh, S.J.; et al. Mechanisms of liquid-phase exfoliation for the production of graphene. ACS Nano 2020, 9, 10976–10985. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, M.; Jin, L.; Liu, L.; Mo, Y.; Li, X.; Mo, Z.; Liu, Z.; You, S.; Zhu, H. Research progress of the liquid-phase exfoliation and stable dispersion mechanism and method of graphene. Front. Mater. 2019, 6, 325. [Google Scholar] [CrossRef]
- Moosa, A.A.; Abed, M.S. Graphene preparation and graphite exfoliation. Turk. J. Chem. 2021, 45, 493–519. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, A.; Wang, L. A Sensitive Electrochemical Sensor Based on Graphene/Pt Nanoparticles for Simultaneous Determination of Tyrosine and Tryptophan in the Presence of 5-hydroxytryptophan. Int. J. Electrochem. Sci. 2020, 15, 12599–12609. [Google Scholar] [CrossRef]
- Kavitha, C.; Bramhaiah, K.; John, N.S. Low-cost electrochemical detection of l-tyrosine using an rGO-Cu modified pencil graphite electrode and its surface orientation on a Ag electrode using an: Ex situ spectroelectrochemical method. RSC Adv. 2020, 10, 22871–22880. [Google Scholar] [CrossRef]
- Liu, M.; Lao, J.; Wang, H.; Xu, Z.; Li, J.; Wen, L.; Yin, Z.; Luo, C.; Peng, H. Electrochemical Determination of Tyrosine Using Graphene and Gold Nanoparticle Composite Modified Glassy Carbon Electrode. Russ. J. Electrochem. 2021, 57, 41–50. [Google Scholar] [CrossRef]
- Razeghi, M.; Pircheraghi, G. TPU/graphene nanocomposites: Effect of graphene functionality on the morphology of separated hard domains in thermoplastic polyurethane. Polymer 2018, 148, 169–180. [Google Scholar] [CrossRef]
- Udayabhaskar, R.; Mangalaraja, R.V.; Pandiyarajan, T.; Karthikeyan, B.; Mansilla, H.D.; Contreras, D. Spectroscopic investigation on graphene-copper nanocomposites with strong UV emission and high catalytic activity. Carbon 2017, 124, 256–262. [Google Scholar] [CrossRef]
- Coroş, M.; Pogăcean, F.; Roşu, M.-C.; Socaci, C.; Borodi, G.; Mageruşan, L.; Biriş, A.R.; Pruneanu, S. Simple and cost-effective synthesis of graphene by electrochemical exfoliation of graphite rods. RSC Adv. 2016, 6, 2651–2661. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 1–23. [Google Scholar] [CrossRef]
- Bisquert, J. Influence of the boundaries in the impedance of porous film electrodes. Phys. Chem. Chem. Phys. 2000, 2, 4185–4192. [Google Scholar] [CrossRef]
- Beitollahi, H.; Garkani Nejad, F. Graphene Oxide/ZnO Nano Composite for Sensitive and Selective Electrochemical Sensing of Levodopa and Tyrosine Using Modified Graphite Screen Printed Electrode. Electroanalysis 2016, 28, 2237–2244. [Google Scholar] [CrossRef]
- Deng, K.Q.; Zhou, J.H.; Li, X.F. Direct electrochemical reduction of graphene oxide and its application to determination of l-tryptophan and l-tyrosine. Colloids Surf. B Biointerfaces 2013, 101, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Dinu, A.; Apetrei, C. Development of polypyrrole modified screen-printed carbon electrode based sensors for determination of L-tyrosine in pharmaceutical products. Int. J. Mol. Sci. 2021, 22, 7528. [Google Scholar] [CrossRef] [PubMed]
- Murtada, K.; Salghi, R.; Ríos, A.; Zougagh, M. A sensitive electrochemical sensor based on aluminium doped copper selenide nanoparticles-modified screen printed carbon electrode for determination of L-tyrosine in pharmaceutical samples. J. Electroanal. Chem. 2020, 874, 114466. [Google Scholar] [CrossRef]





| Sensing Material/Electrode | Linear Range (M) | DL (M) | Sensitivity A/M | Recovery % | Ref. |
|---|---|---|---|---|---|
| Nano-Au-/CA/GCE | 10−7 M−3 × 10−4 | 4 × 10−8 | - | 99.9–102.4 | [10] |
| GR/Au NPs/GCE | 0.1–100 × 10−6 | 47 × 10−9 | 0.918 | - | [29] |
| GR/ZnO/SPE | 10−6–8 × 10−4 | 3.4 × 10−7 | 0.016 | 97.5–103 | [35] |
| ERGO/GCE | 0.5–80 × 10−6 | 0.2 × −10−6 | 0.026 | - | [36] |
| PPy/FeCN-SPCE | 0.5–27 × 10−6 | 8.20 × 10−8 | 1.46 | 99.92–103.97 | [37] |
| PPy/NP-SPCE | 4.30 × 10−7 | 0.278 | |||
| PPy/SDS-SPCE | 3.51 × 10−7 | 0.341 | |||
| Al-CuSe-NPs/SPCE | 0.15–10 × 10−6 | 0.04 × 10−6 | 0.053 | 97.6–101 | [38] |
| EGr/GC | 6 × 10−6–10−3 | 1.81 × 10−6 | 0.0124 | 98.5–103.1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varodi, C.; Pogăcean, F.; Coroş, M.; Ciorîță, A.; Pruneanu, S. Electrochemical L-Tyrosine Sensor Based on a Glassy Carbon Electrode Modified with Exfoliated Graphene. Sensors 2022, 22, 3606. https://doi.org/10.3390/s22103606
Varodi C, Pogăcean F, Coroş M, Ciorîță A, Pruneanu S. Electrochemical L-Tyrosine Sensor Based on a Glassy Carbon Electrode Modified with Exfoliated Graphene. Sensors. 2022; 22(10):3606. https://doi.org/10.3390/s22103606
Chicago/Turabian StyleVarodi, Codruţa, Florina Pogăcean, Maria Coroş, Alexandra Ciorîță, and Stela Pruneanu. 2022. "Electrochemical L-Tyrosine Sensor Based on a Glassy Carbon Electrode Modified with Exfoliated Graphene" Sensors 22, no. 10: 3606. https://doi.org/10.3390/s22103606







