Synthetic Aperture Imaging Using High-Frequency Convex Array for Ophthalmic Ultrasound Applications
Abstract
:1. Introduction
2. Methods
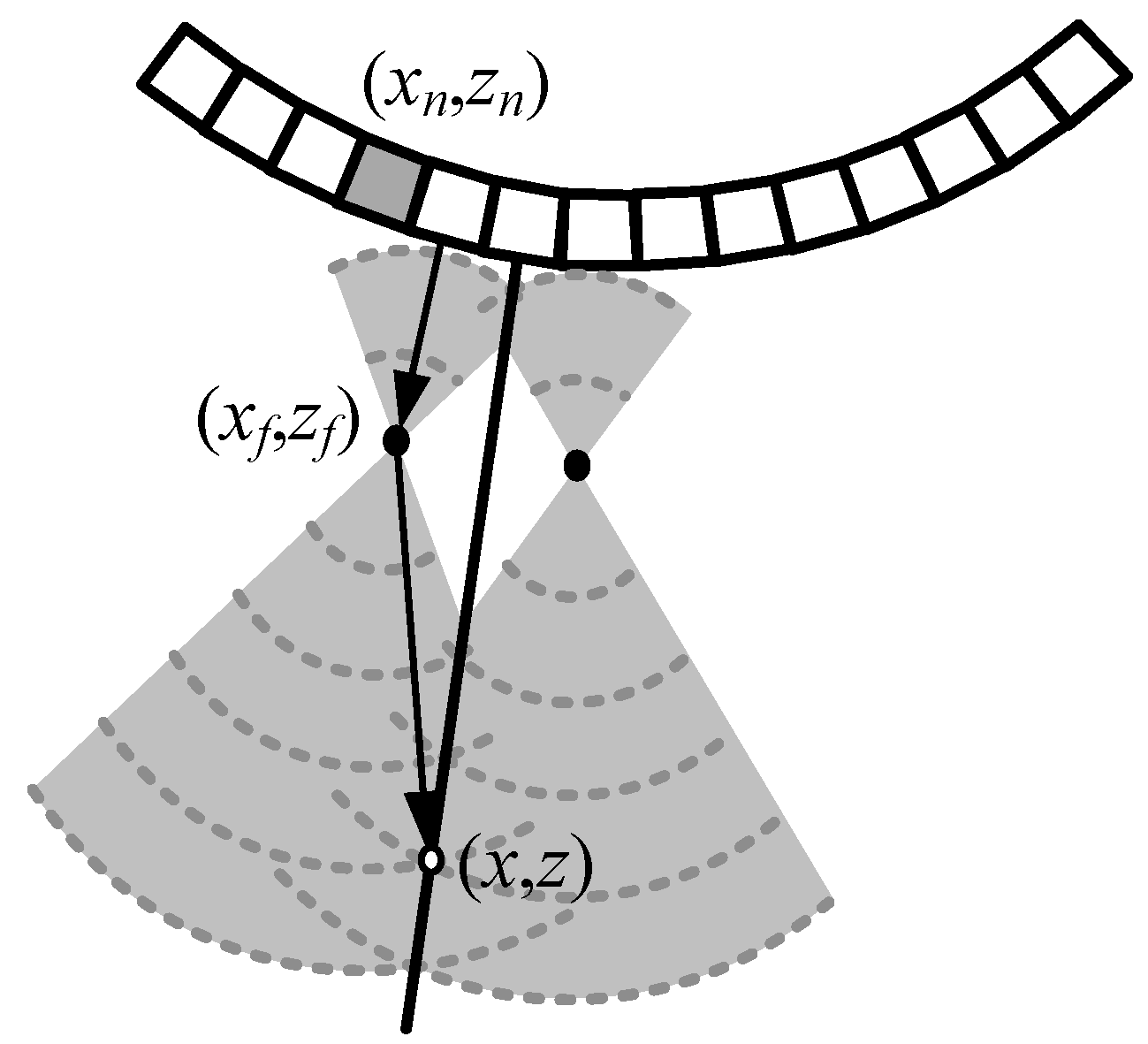
2.1. Principle of Synthetic Aperture Imaging with a Virtual Source
2.2. Experimental Setup and Evaluation Metrics
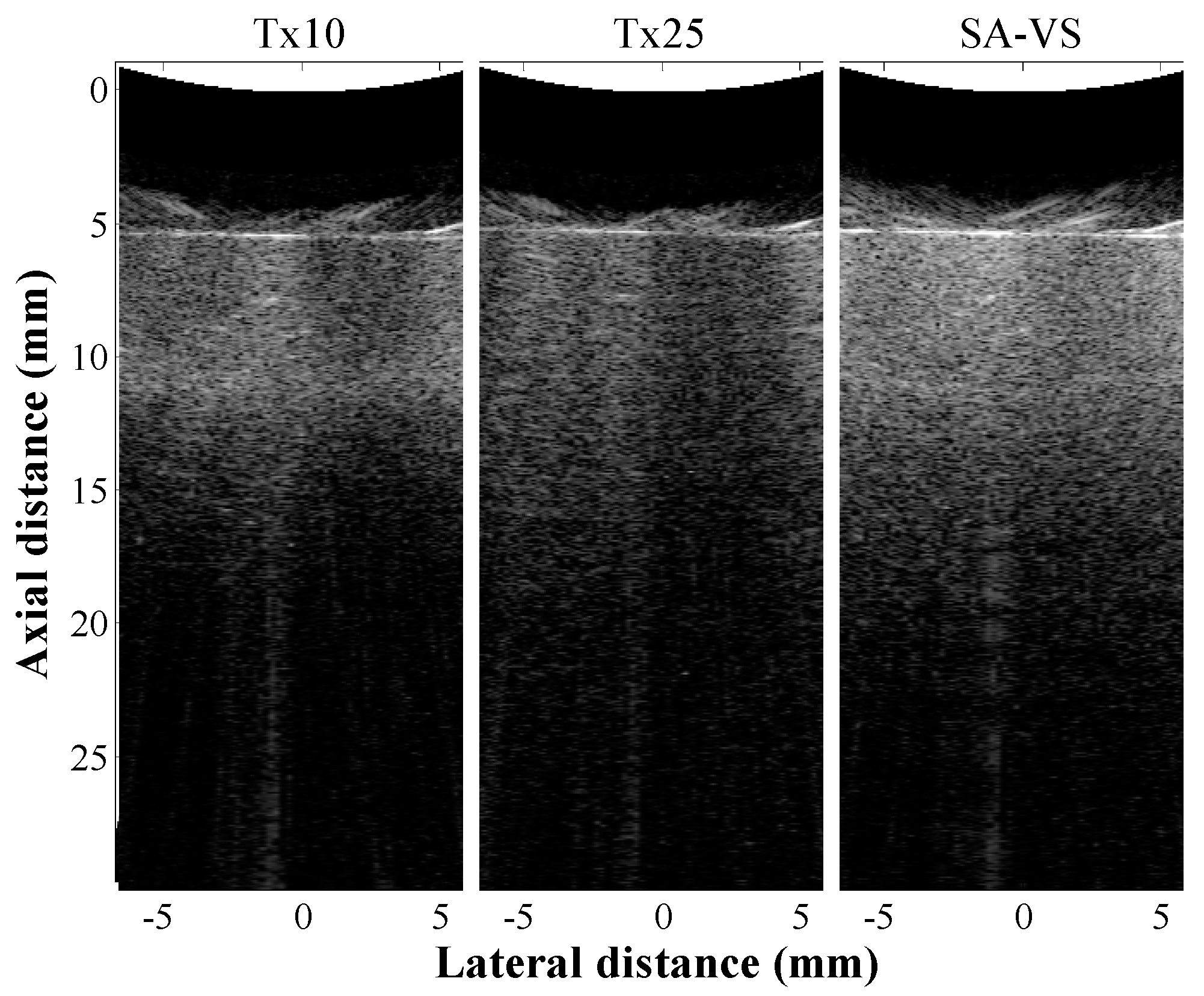
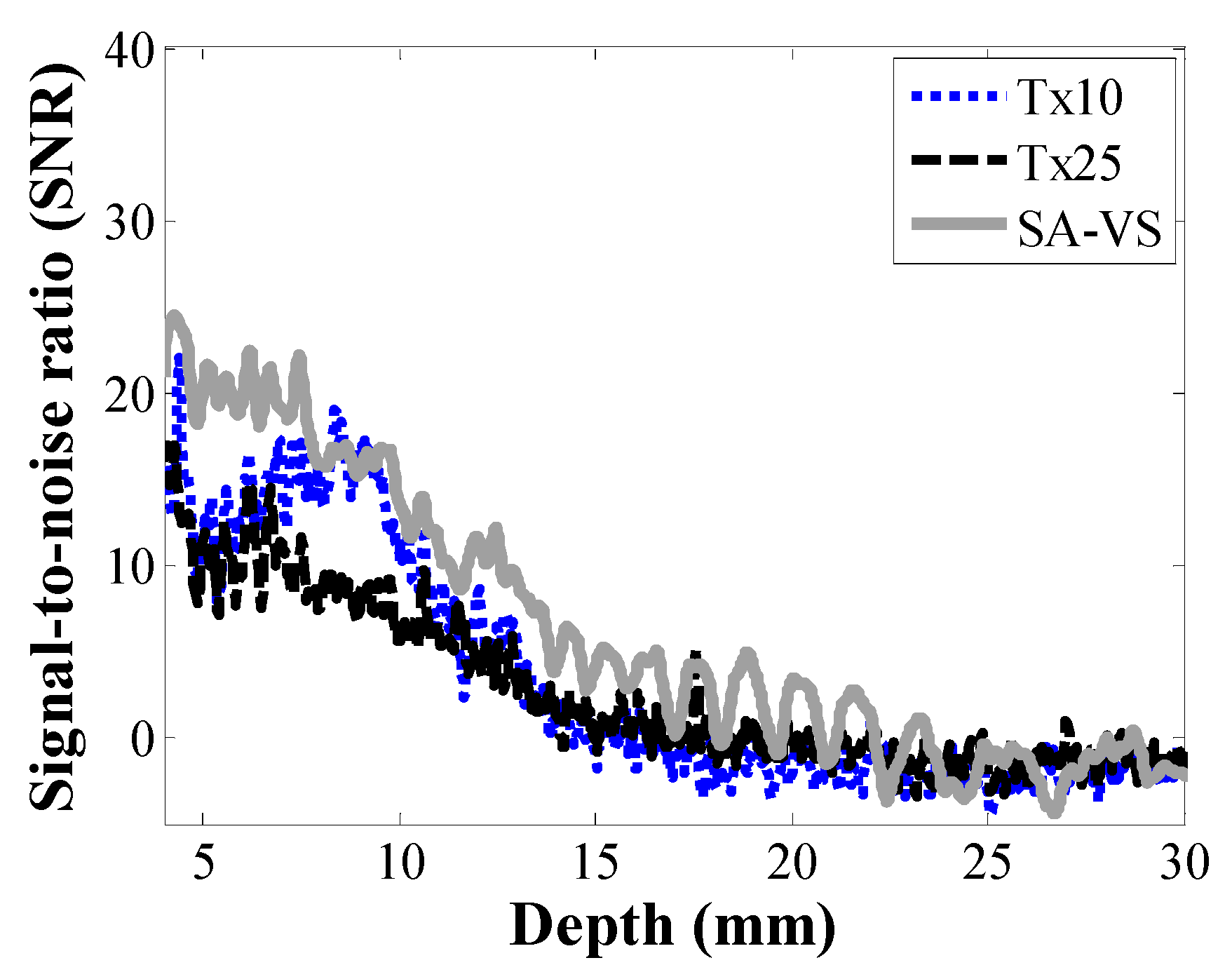
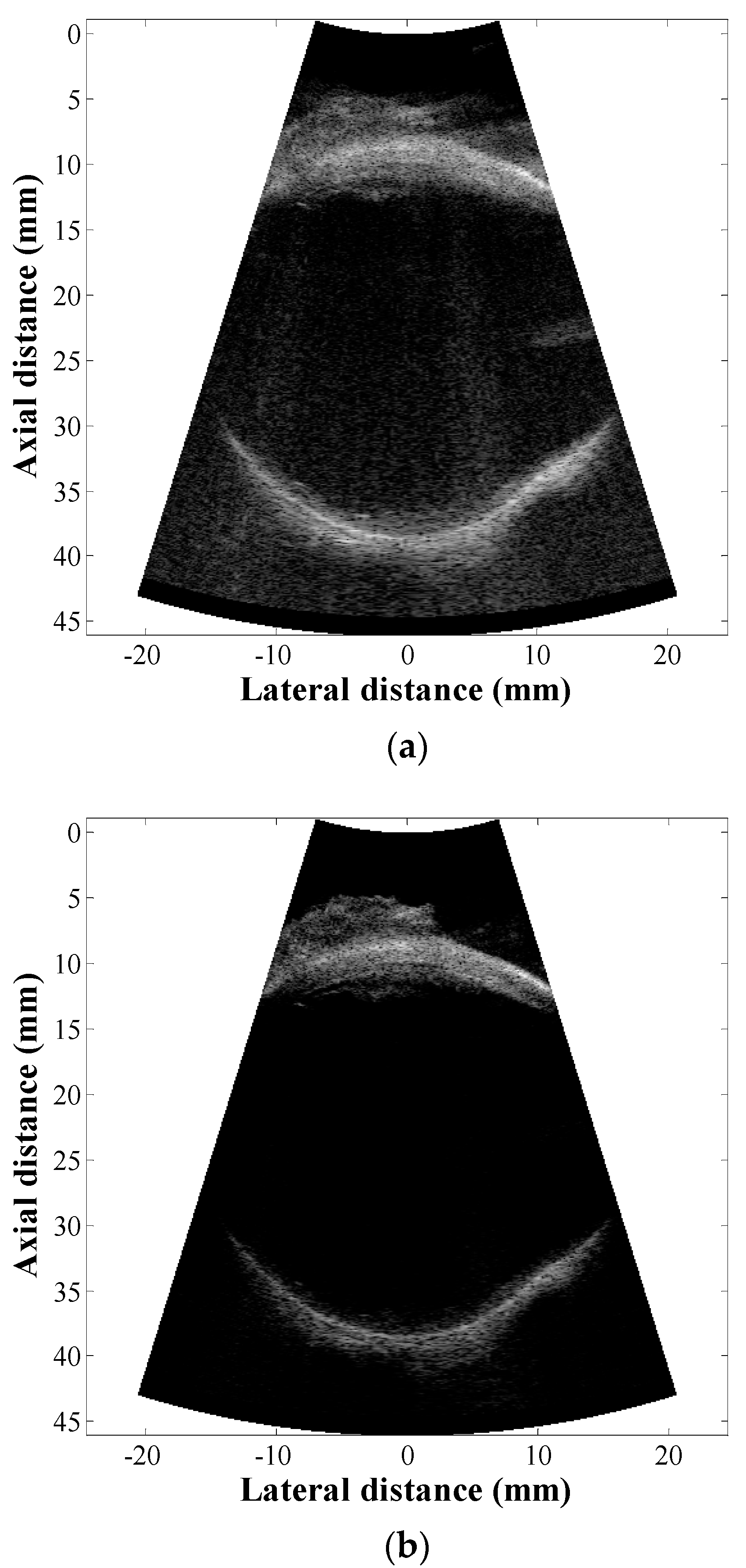
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbull, D.; Starkoski, B.G.; Harasiewicz, K.A.; Semple, J.L.; Gupta, A.K.; Sauder, D.N.; Foster, F.S. A 40–100 MHz B-scan ultrasound backscatter microscope for skin imaging. Ultrasound Med. Biol. 1995, 21, 79–88. [Google Scholar] [CrossRef]
- Lockwood, G.R.; Turnbull, D.H.; Christopher, D.A.; Foster, F.S. Beyond 30 MHz: Applications of high frequency ultrasonic imaging. IEEE Eng. Med. Biol. 1996, 15, 60–71. [Google Scholar] [CrossRef]
- Passman, C.; Ermert, H. A 100 MHz ultrasound imaging system for dermatologic and ophthalmologic diagnostics. IEEE Trans. Ultrason. Ferroelect. Freq. Control 1996, 43, 545–552. [Google Scholar] [CrossRef]
- Cannata, J.M.; Ritter, T.A.; Chen, W.-H.; Silverman, R.H.; Shung, K.K. Design of efficient, broadband single element (20–80 MHz) ultrasonic transducers for medical imaging applications. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2003, 50, 1548–1557. [Google Scholar] [CrossRef]
- Shung, K.K. High frequency ultrasonic imaging. J. Med. Ultrasound 2009, 17, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, W.; Chung, Y.; Shih, W.Y.; Shih, W.-H.; Zhou, W.; Shung, K.K. 80-MHz Intravascular ultrasound transducer using PMN-PT free-standing film. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2011, 58, 2281–2288. [Google Scholar]
- Yeo, S.; Yoon, C.; Lien, C.-L.; Song, T.K.; Shung, K.K. Monitoring of Adult Zebrafish Heart Regeneration Using High-Frequency Ultrasound Spectral Doppler and Nakagami Imaging. Sensors 2019, 19, 4094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-H.; Jeng, G.-S.; Wu, T.-K.; Li, P.-C. ECG triggering and gating for ultrasound small animal imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1590–1596. [Google Scholar]
- Foster, F.S.; Hossack, J.; Adamson, S.L. Micro-ultrasound for preclinical imaging. Interface Focus 2011, 1, 576–601. [Google Scholar] [CrossRef]
- Qiu, W.; Yu, Y.; Tsang, K.; Sun, L. An FPGA-based open platform for ultrasound biomicroscopy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1432–1442. [Google Scholar]
- Li, M.-L.; Guan, W.-J.; Li, P.-C. Improved Synthetic Aperture Focusing Technique with Applications in High-Frequency Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 2004, 51, 63–70. [Google Scholar]
- Hu, C.-H.; Snook, K.A.; Cao, P.-J.; Shung, K.K. High-frequency ultrasound annular array imaging. Part II: Digital beamformer design and imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 309–316. [Google Scholar]
- Yoon, C.; Kim, H.; Shung, K.K. Development of a low complexity, cost effective digital beamformer architecture for high-frequency ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Yoo, Y.; Song, T.-K.; Chang, J.H. Orthogonal quadrature chirp signals for simultaneous multi-zone focusing in medical ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Kim, G.-D.; Yoo, Y.; Song, T.-K.; Chang, J.H. Frequency equalized compounding for effective speckle reduction inmedical ultrasound imaging. Biomed Signal Process. Control 2013, 8, 876–887. [Google Scholar] [CrossRef]
- Mamou, J.; Ketterling, J.A.; Silverman, R.H. Chirp-Coded Excitation Imaging With a High-Frequency Ultrasound Annular Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 508–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.; Lee, W.; Chang, J.H.; Song, T.-K.; Yoo, Y. An Efficient Pulse Compression Method of Chirp-Coded Excitation in Medical Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2225–2229. [Google Scholar] [CrossRef]
- Karaman, M.; Li, P.-C.; O’Donnell, M. Synthetic aperture imaging for small scale systems. IEEE Trans. Ultrason. Ferroelect. Freq. Control 1995, 42, 429–442. [Google Scholar] [CrossRef]
- Frazier, C.H.; O’Brien, W.D., Jr. Synthetic aperture techniques with a virtual source element. IEEE Trans. Ultrason. Ferroelect. Freq. Control 1998, 45, 196–207. [Google Scholar] [CrossRef]
- Bae, M.-H.; Jeong, M.-K. A study of synthetic-aperture imaging with virtual source elements in B-mode ultrasound imaging systems. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2000, 47, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Kortbek, J.; Jensen, J.A.; Gammelmark, K.L. Sequential beamforming for synthetic aperture imaging. Ultrasonics 2013, 53, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Yoon, C.; Park, J.-H.; Lee, Y.; Kim, W.H.; Chang, J.M.; Choi, B.I.; Song, T.-K.; Yoo, Y. Evaluation of Ultrasound Synthetic Aperture Imaging Using Bidirectional Pixel-Based Focusing: Preliminary Phantom and In Vivo Breast Study. IEEE Trans. Biomed. Eng. 2013, 60, 2716–2724. [Google Scholar]
- Hansen, P.M.; Hemmsen, M.; Brandt, A.; Rasmussen, J.; Lange, T.; Krohn, P.S.; Lonn, L.; Jensen, J.A.; Nielsen, M.B. Clinical evaluation of synthetic aperture sequential beamforming ultrasound in patients with liver tumors. Ultrasound Med. Biol. 2014, 40, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Yoon, C.; Chang, J.H.; Yoo, Y.; Song, T.-K. A real-time synthetic aperture beamformer for medical ultrasound imaging. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010. [Google Scholar]
- So, H.K.H.; Junying, C.; Yu, A.C.H. Medical ultrasound imaging: To GPU or not to GPU? IEEE Micro 2011, 31, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-F.; Li, P.-C. Software beamforming: Comparison between a phased array and synthetic transmit aperture. Ultrason. Imaging 2011, 33, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Hu, C.; Park, J.; Kang, B.J.; Wiliams, J.A.; Cannata, J.M.; Shung, K.K. Characterization and evaluation of high frequency convex array transducers. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010. [Google Scholar]
- Yoon, C.; Lee, Y.; Chang, J.H.; Song, T.-K.; Yoo, Y. In vitro estimation of mean sound speed based on minimum average phase variance in medical ultrasound imaging. Ultrasonics 2011, 51, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Carman, J.C. Classroom measurements of sound speed in fresh/saline water. J. Acoust. Soc. Am. 2012, 131, 2455. [Google Scholar] [CrossRef]
- Dekorte, C.L.; Vandersteen, A.F.W.; Thijssen, J.M. Acoustic velocity and attenuation of eye tissues at 20 MHz. Ultrasound Med. Biol. 1994, 20, 471–480. [Google Scholar] [CrossRef]
- Silverman, R.H.; Ketterling, J.A.; Mamou, J.; Lloyd, H.O.; Filoux, E.; Coleman, D.J. Pulse-Encoded Ultrasound Imaging of the Vitreous With an Annular Array. Ophthalmic Surg. Lasers Imaging 2012, 43, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Karaman, M.; Bilge, H.S.; O’Donnell, M. Adaptive Multi-element Synthetic Aperture Imaging with Motion and Phase Aberration Correction. IEEE Trans. Ultrason. Ferroelect. Freq. Control 1998, 45, 1077–1087. [Google Scholar] [CrossRef]

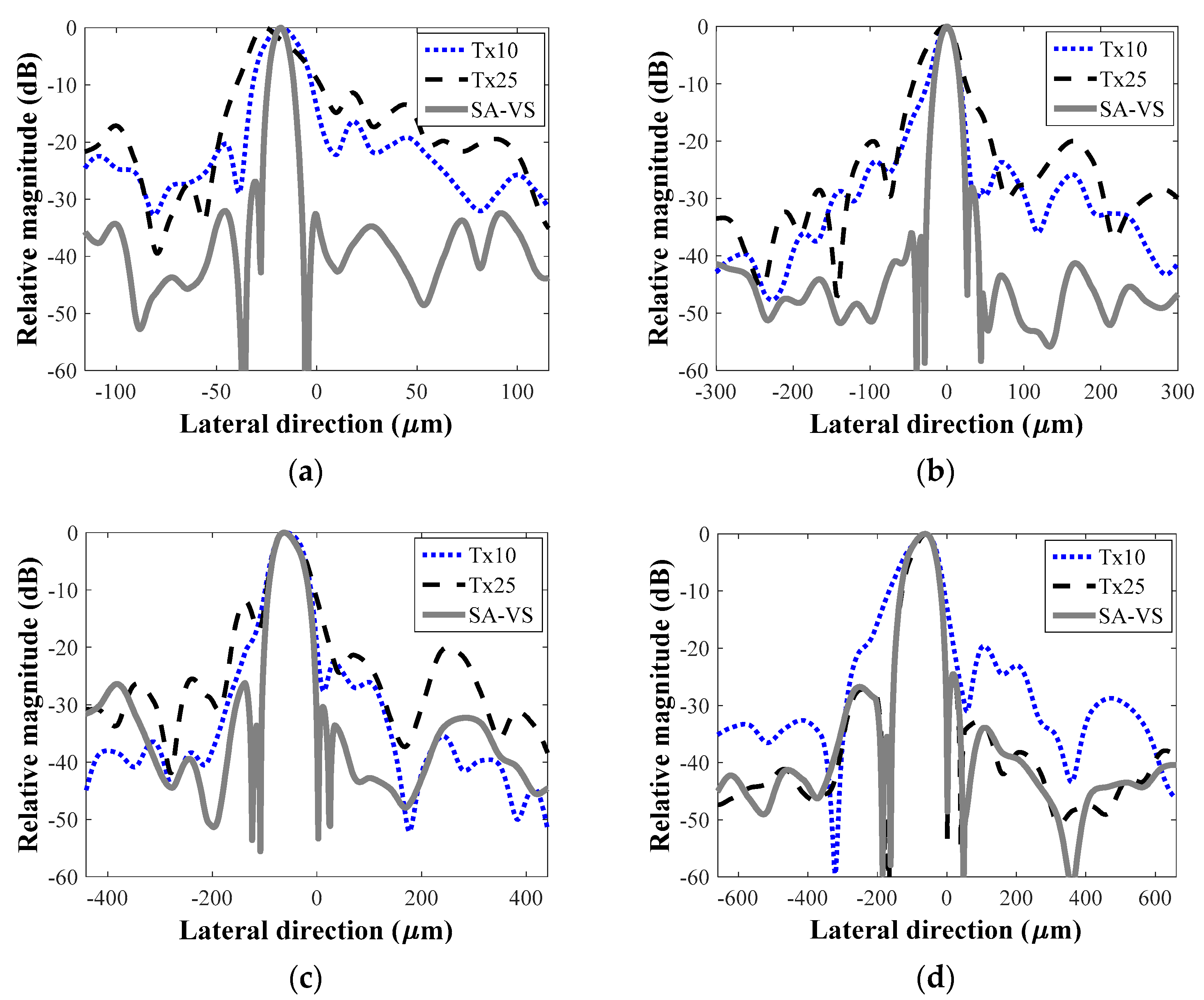
| −6 dB Lateral Resolution (μm)/First Sidelobe Level (dB) | ||||
|---|---|---|---|---|
| 5 mm | 14 mm | 18 mm | 24 mm | |
| Conventional Tx10 | 24.0/−16.4 | 32.2/−23.7 | 71/−22.5 | 119.3/−19.6 |
| Conventional Tx25 | 29.6/−11.4 | 51.3/−20.0 | 72.6/−12 | 106.0/−24.6 |
| SA-VS | 10.2/−27.0 | 26.9/−28.1 | 64.3/−26.2 | 105.5/−24.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.G.; Kim, H.H.; Yoon, C. Synthetic Aperture Imaging Using High-Frequency Convex Array for Ophthalmic Ultrasound Applications. Sensors 2021, 21, 2275. https://doi.org/10.3390/s21072275
Lim HG, Kim HH, Yoon C. Synthetic Aperture Imaging Using High-Frequency Convex Array for Ophthalmic Ultrasound Applications. Sensors. 2021; 21(7):2275. https://doi.org/10.3390/s21072275
Chicago/Turabian StyleLim, Hae Gyun, Hyung Ham Kim, and Changhan Yoon. 2021. "Synthetic Aperture Imaging Using High-Frequency Convex Array for Ophthalmic Ultrasound Applications" Sensors 21, no. 7: 2275. https://doi.org/10.3390/s21072275







