Synthesis and Characterization of N-Isopropylacrylamide Microspheres as pH Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Buffer Solutions
2.3. Synthesis of pH-Sensitive N-Isopropylacrylamide Copolymers
2.4. Membrane Preparation
2.5. Turbidity Measurements
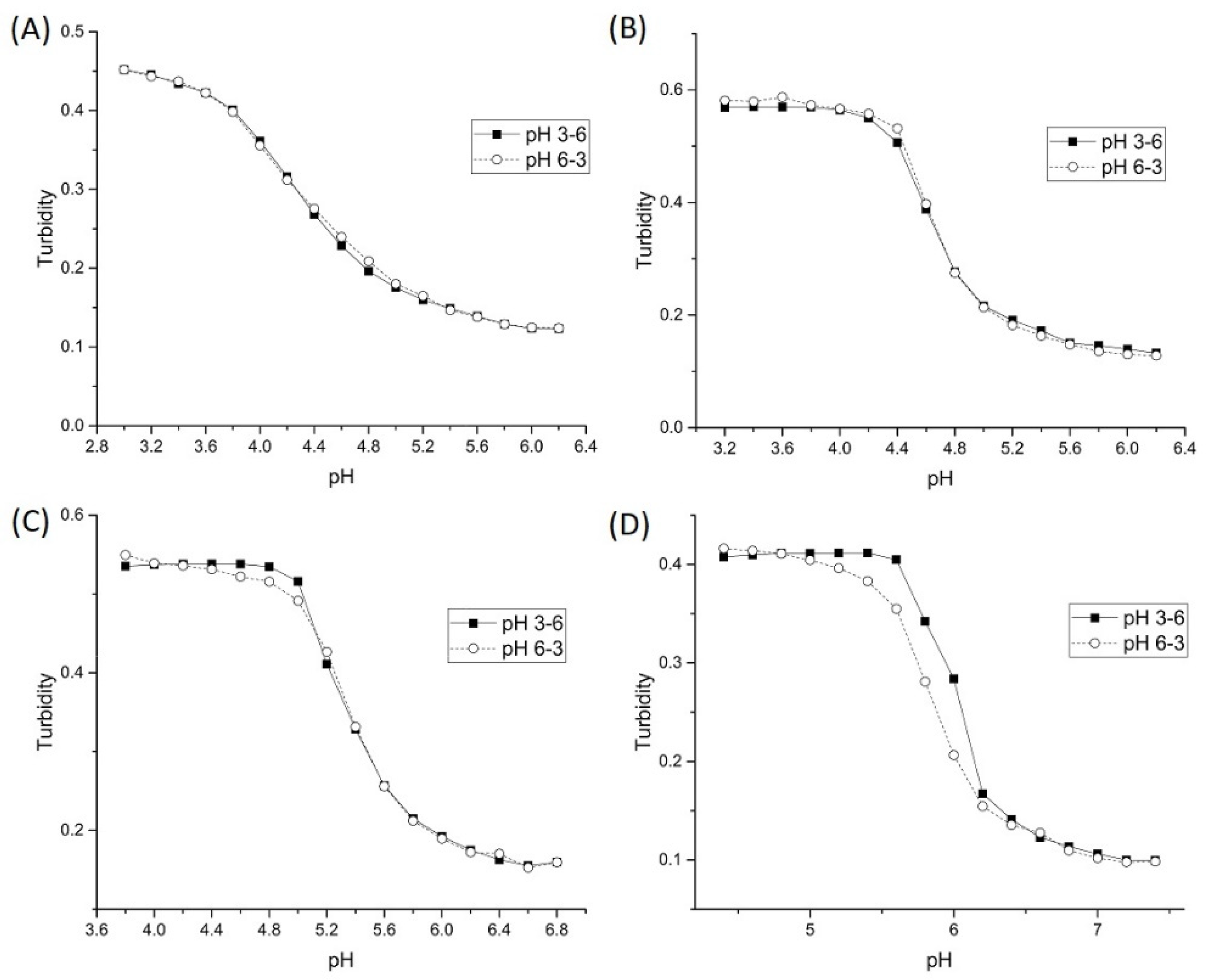
3. Results and Discussion
3.1. Copolymers of NIPA and MAA
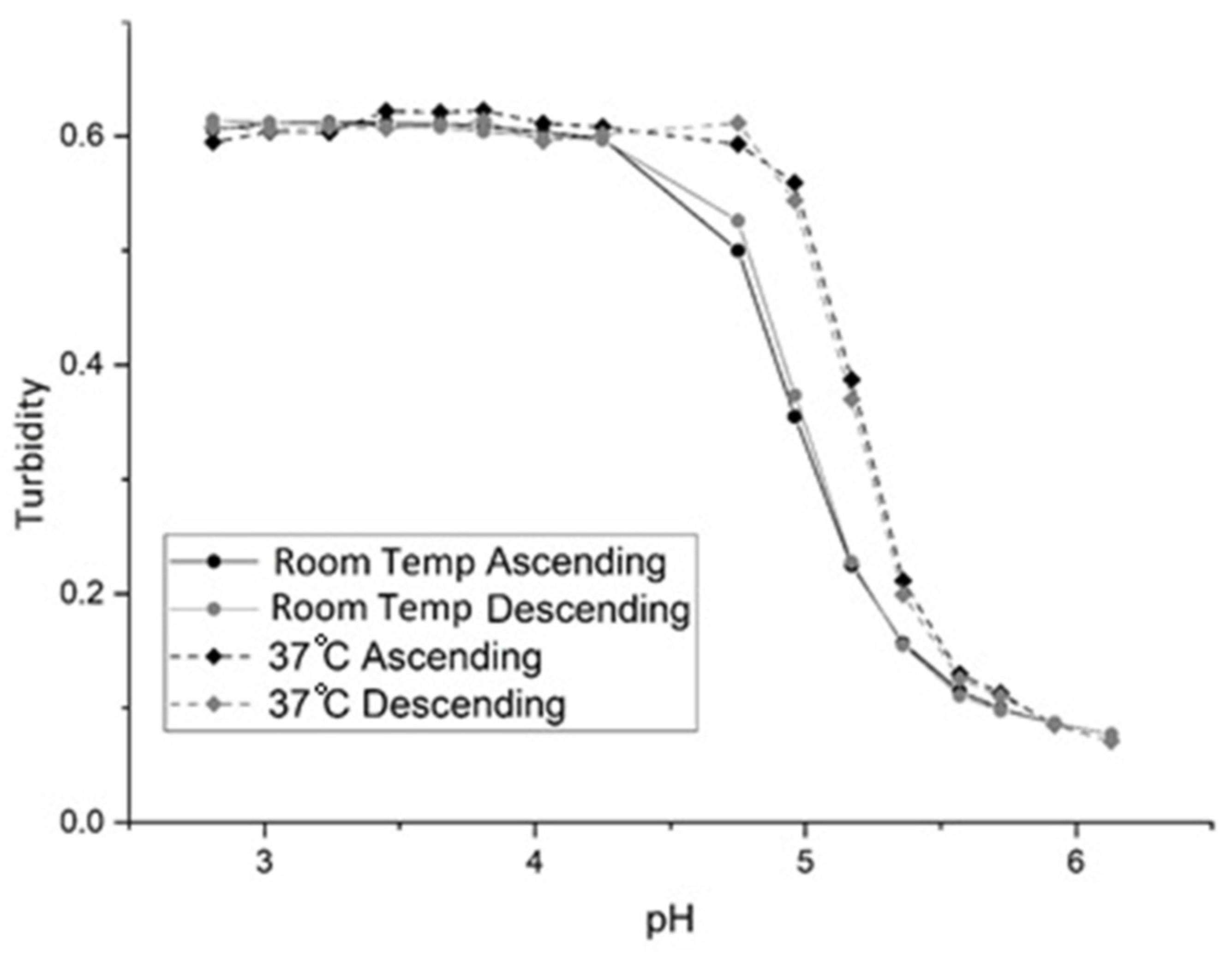
3.2. Batch Variability
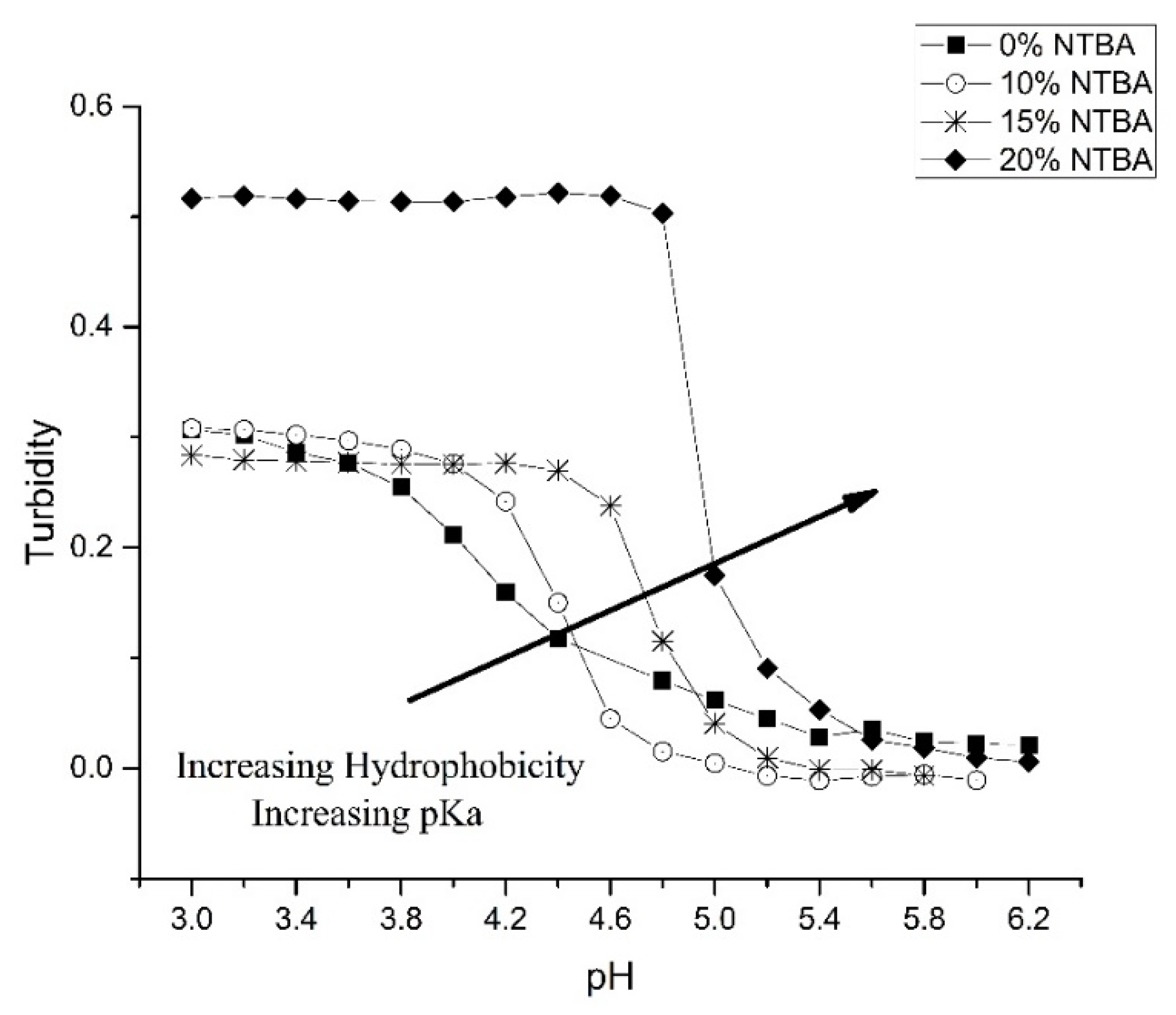
3.3. Alkyl Acrylic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, Y.; Chen, J.; Fang, K. New Technology for the Detection of pH. J. Biochem. Biphys. Methods 2005, 63, 1–9. [Google Scholar]
- Service, R.F. Rising Acidity Levels Brings an Ocean of Trouble. Science 2016, 337, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Jeevarajan, A.S.; Vani, S.; Taylor, T.D.; Anderson, M.M. Continuous pH Monitoring in a Perfused Bioreactor System Using an Optical pH Sensor. Biotech. Bioeng. 2002, 78, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Soller, B.R.; Micheels, R.H. Partial Least Squares Modeling of Near Infrared Reflectance Data for Noninvasive In Vivo Determination of Deep Tissue. Appl. Spect. 1998, 52, 393–399. [Google Scholar] [CrossRef]
- Netto, E.; Peterson, J.; McShane, M.; Hampshire, V. A Fiber Optic Broad-Range pH Sensor System for Gastric Measurements. Sens. Actuators B 1995, 29, 157–163. [Google Scholar] [CrossRef]
- Salerno, M.; Ajimo, J.J.; Dudley, J.A.; Binzel, K.; Urayama, P. Characterization of Dual Wavelength Seminapthofluorescein and Seminaphorhodaflor Dyes for pH Sensing Under High Hydrostatic Pressures. Anal. Biochem. 2007, 362, 258–267. [Google Scholar] [CrossRef]
- Medlock, K.; Harmer, H.; Worsley, G.; Hogan, A.; Pritchard, J. pH-Sensitive Holograms for Continuous Monitoring in Plasma. Anal. Bional. Chem. 2007, 389, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Schroder, C.R.; Weidgans, B.M.; Klimant, I. pH Fluorescence for Use in Marine Systems. Analyst 2005, 130, 907–916. [Google Scholar] [CrossRef]
- Miled, O.B.; Grasso, D.; Sanchez, C.; Livage, J. An Optical Fiber pH Sensor Based on Dye Doped Mesostructured Silica. J. Phys. Chem. Solids 2004, 65, 1751–1755. [Google Scholar] [CrossRef]
- Gao, X.; Yang, W.; Pang, P.; Liao, S.; Cai, Q.; Zeng, K.; Grimes, C.A. A Wireless Magnetoelastic Biosensor for Rapid Detection of Glucose Concentration in Urine Samples. Sens. Actuators B 2007, 128, 161–167. [Google Scholar] [CrossRef]
- Cai, Q.; Zeng, K.; Ruan, C.; Desai, T.A.; Grimes, C. A Wireless Remote Query Glucose Biosensor Based on pH-Sensitive Polymer. Anal. Chem. 2004, 76, 4038–4043. [Google Scholar] [CrossRef]
- Zhang, L.; Seitz, W.R. A pH Sensor Based on Force Generated by pH-Dependent Polymer Swelling. Anal. Bioanal. Chem. 2002, 373, 555–559. [Google Scholar] [CrossRef]
- Zhang, L.; Langmuir, M.E.; Bai, M.; Seitz, W.R. A Sensor for pH Based on an Optical Reflective Device Coupled to the Swelling of an Animated Polystyrene Membrane. Talanta 1997, 44, 1691–1698. [Google Scholar] [CrossRef]
- Lesho, M.J.; Sheppard, N.F. Adhesion of Polymer Films to Oxidized Silicon and Its Effect on Performance of a Conductometric pH Sensor. Sens. Actuators B 1996, 37, 61–66. [Google Scholar] [CrossRef]
- Sheppard, N.F.; Lesho, M.J.; McNally, P.; Francomacaro, S. Microfabricated Conductometric pH Sensor. Sens. Actuators B 1995, 28, 95–102. [Google Scholar] [CrossRef]
- Michie, W.C.; Culshaw, B.; McKenzie, I.; Konstankis, M.; Graham, N.B.; Moran, C.; Santos, F.; Bergquist, E.; Carlstrom, B. Water and pH Measurements Using Fiber Optics and Swellable Polymeric Systems. Optics Lett. 1995, 20, 103–105. [Google Scholar] [CrossRef]
- Rooney, M.T.V.; Seitz, W.R. An Optically Sensitive Membrane for pH Based on Swellable Polymer Microspheres in a Hydrogel. Anal. Commun. 1999, 36, 267–270. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.J.P. Review on Hydrogel-Based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [Green Version]
- Lavine, B.K.; Westover, D.J.; Kaval, N.; Oxenford, L. New Approaches to Chemical Sensing: Sensors Based on Polymer Swelling. Anal. Letters. 2006, 39, 1773–1783. [Google Scholar] [CrossRef]
- Seitz, W.R.; Rooney, M.T.V.; Miele, E.W.; Wang, H.; Kaval, N.; Zhang, L.; Doherty, S.; Milde, S.; Lenda, J. Derivatized, Swellable Polymer Microspheres for Chemical Transduction. Anal. Chim. Acta. 1999, 400, 55–64. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymers in Sensor Applications. Prog. Poly Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Fernando-Nieves, A.; Wyss, H.M.; Mattson, J.; Weitz, D.A. Microgel Suspensions—Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2011; pp. 73–116. [Google Scholar]
- Hunter, A.; Xu, X.; Mauer, G. Swelling of Poly(N-Isopropyl Acrylamide) Hydrogels in Aqueous Solutions of Sodium Chloride. Fluid Phase Equil. 2006, 240, 186–196. [Google Scholar]
- Lavine, B.K.; Pampati, S.R.; Dahal, K.S.; Kim, M.; Perera, U.D.N.; Benjamin, M.; Bunce, R.A. Swellable Copolymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing. Molecules 2020, 25, 1408. [Google Scholar] [CrossRef] [Green Version]
- Ferritto, M.; Tirrell, D.A. Poly (2-ethacrylic acid). Macro. Synth. 1997, 11, 59–62. [Google Scholar]
- Buffer Calculator, Centre for Proteomic Research, Liverpool. Available online: www.liv.ac.uk/buffers/buffercalc.html (accessed on 23 September 2021).
- Lavine, B.K.; Oxenford, L.; Kim, M.; Kaval, N.; Benjamin, M.; Seitz, W.R. Novel Turbidimetric Method to Study Polymer Swelling. Microchem. J. 2012, 103, 97–104. [Google Scholar] [CrossRef]
- Seker, F.; Ellis, A.B.J. Correlation of Chemical Structure and Swelling Behavior in N-Alkylacrylamide Hydrogels. Polym. Sci. Part. A Polym. Chem. 1998, 36, 2005–2012. [Google Scholar] [CrossRef]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef]
- Elias, H.G. An Introduction to Polymer Science, 1st ed.; VCH: Weinheim, Germany, 1997. [Google Scholar]
- Murthy, N.; Robichaud, J.R.; Tirrell, D.A.; Slayton, P.S.; Hoffman, A.S. The Design and Synthesis of Polymers for Eukaryotic Membrane Disruption. J. Control. Release 1999, 61, 137–143. [Google Scholar] [CrossRef]
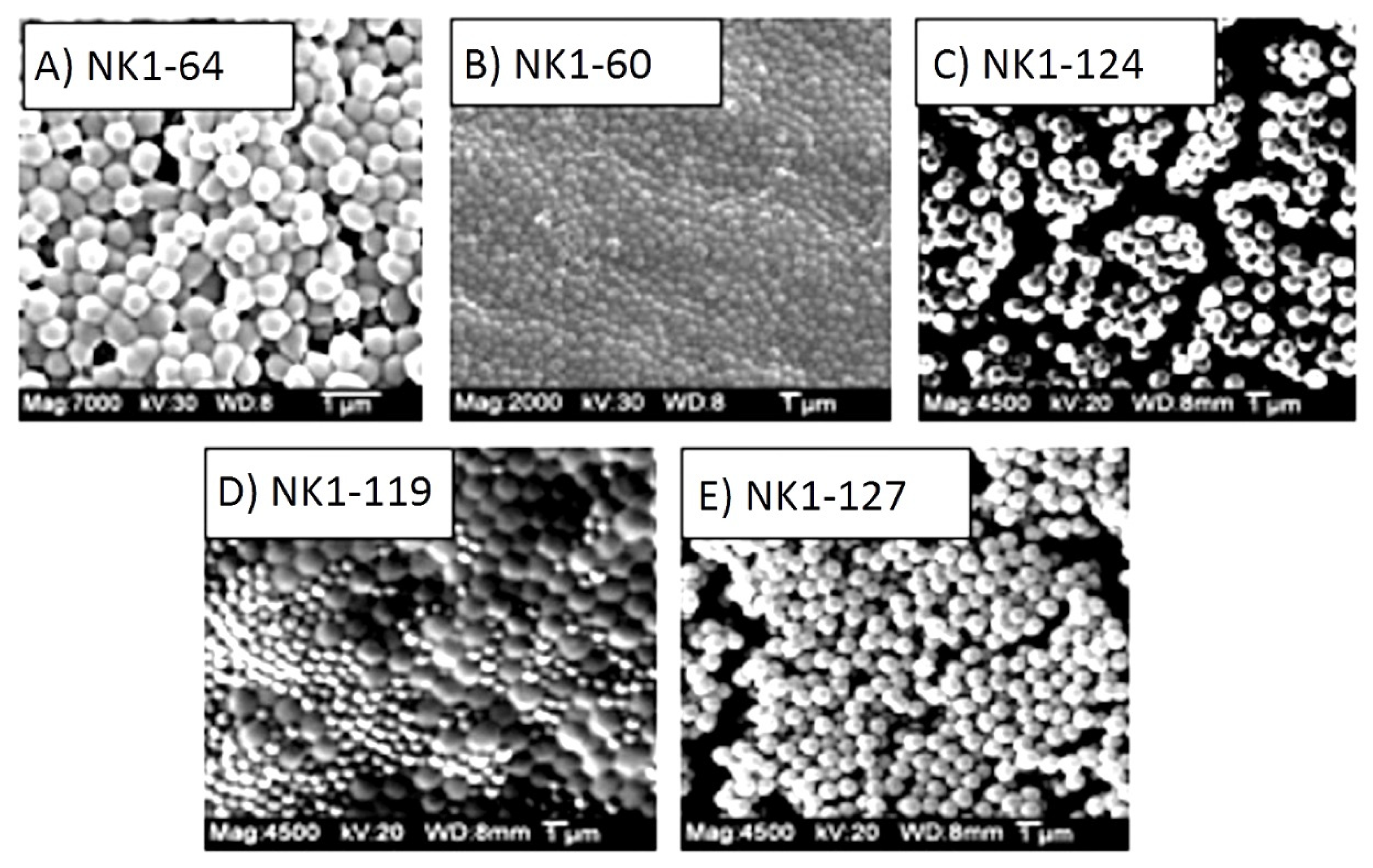




| NIPA Copolymer | *pKa (0.1 M IS) | *pKa (1.0 M IS) | NIPA | MAA | NTBA | MBA |
|---|---|---|---|---|---|---|
| NK 1-60 (10% crosslinking) | 4.8 | 4.9 | 14 mmoles | 2 mmoles | 2 mmoles | 2 mmoles |
| NK 1-28 (5% crosslinking) | 3.9 | 4.5 | 17 mmoles | 2 mmoles | 0 mmoles | 1 mmoles |
| NK 1-77 (5% crosslinking) | 3.9 | 4.7 | 16 mmoles | 1 mmoles | 2 mmoles | 1 mmoles |
| NIPA Copolymer | *pKa | NIPA | MAA | NTBA | MBA |
|---|---|---|---|---|---|
| NK 1-72 0% NTBA | 4.10 | 16 mmoles | 1 mmoles | 0 mmoles | 1 mmoles |
| NK 1-77 10% NTBA | 4.43 | 15 mmoles | 1 mmoles | 2 mmoles | 1 mmoles |
| NK 1-56 15% NTBA | 4.73 | 14 mmoles | 1 mmoles | 3 mmoles | 1 mmoles |
| NK 1-50 20% NTBA | 4.91 | 14 mmoles | 1 mmoles | 4 mmoles | 1 mmoles |
| NIPA Copolymer | pKa | NIPA | MAA | NTBA | MBA |
|---|---|---|---|---|---|
| NK 1-64 5% MAA | 4.90 | 15 mmoles | 1 mmoles | 2 mmoles | 2 mmoles |
| NK 1-60 10% MAA | 4.57 | 14 mmoles | 2 mmoles | 2 mmoles | 2 mmoles |
| NK 1-124 15% MAA | 4.47 | 13 mmoles | 3 mmoles | 2 mmoles | 2 mmoles |
| NK 1-119 20% MAA | 4.66 | 12 mmoles | 4 mmoles | 2 mmoles | 2 mmoles |
| NK 1-127 25% MAA | 4.90 | 11 mmoles | 5 mmoles | 2 mmoles | 2 mmoles |
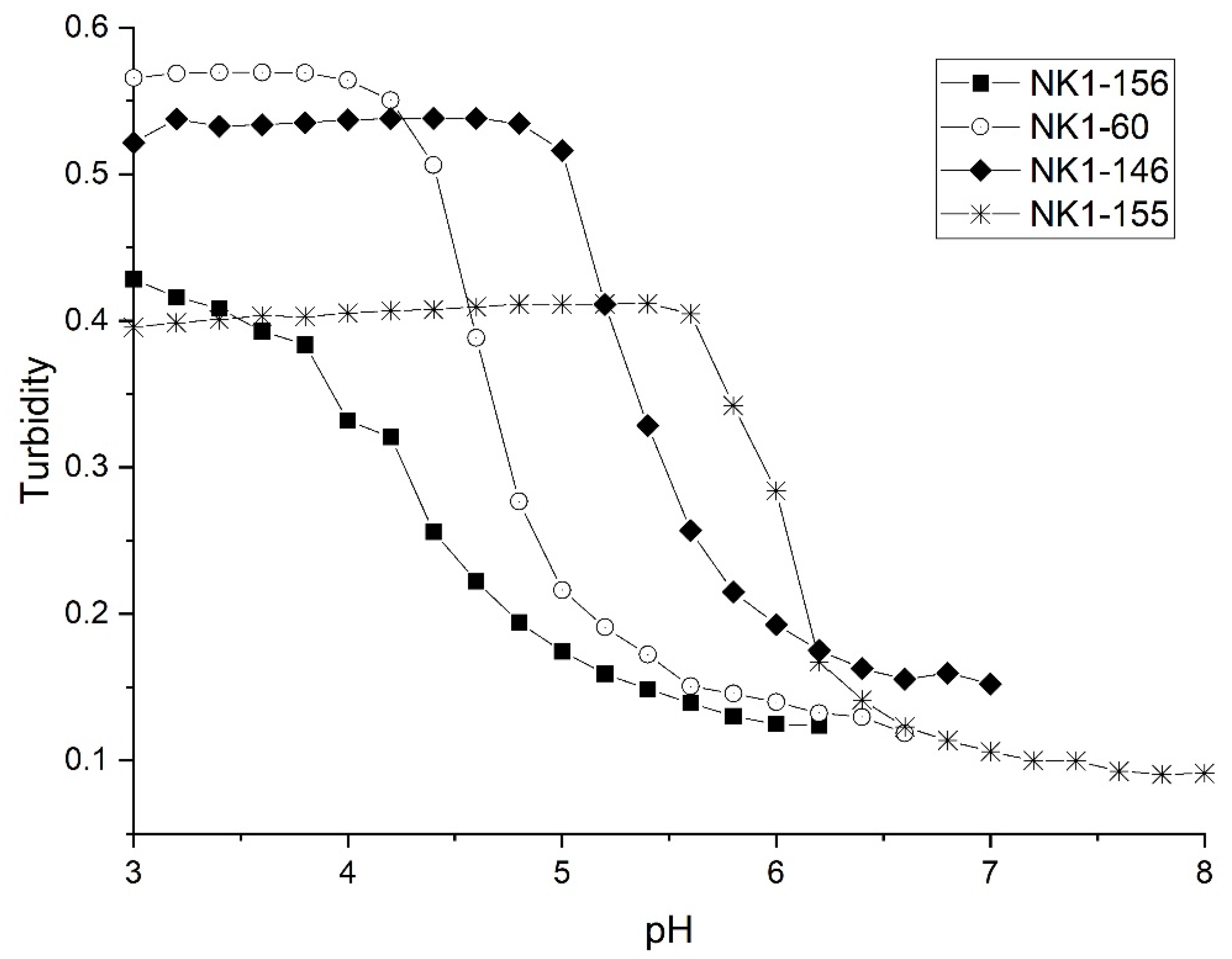
| NIPA Copolymer | Apparent pKa | pKa of Functional Comonomer | NIPA | Functional Comonomer | NTBA | MBA |
|---|---|---|---|---|---|---|
| NK 1-156 | 4.37 | 4.2 (AA) | 14 mmoles | 2 mmoles of AA 1 | 2 mmoles | 2 mmoles |
| NK 1-60 | 4.70 | 4.7 (MAA) | 14 mmoles | 2 mmoles of MAA 2 | 2 mmoles | 2 mmoles |
| NK 1-146 | 5.39 | 4.7 (EAA) | 14 mmoles | 2 mmoles of EAA 3 | 2 mmoles | 2 mmoles |
| NK 1-155 | 6.07 | 4.8 (PAA) | 14 mmoles | 2 mmoles of PAA 4 | 2 mmoles | 2 mmoles |
| Functional Comonomer | ΔH 2 (J/mol) | ΔS 2 (J/mol·K) |
|---|---|---|
| NK 1-156 (AA) | −47,000 ± 2300 | −242 ± 7 |
| NK 1-60 (MAA) | −74,900 ± 4400 | −336 ± 15 |
| NK 1-146 (EAA) | −100,000 ± 3300 | −435 ± 11 |
| NK 1-155 (PAA) | −60,000 ± 2400 | −312 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavine, B.K.; Kaval, N.; Oxenford, L.; Kim, M.; Dahal, K.S.; Perera, N.; Seitz, R.; Moulton, J.T.; Bunce, R.A. Synthesis and Characterization of N-Isopropylacrylamide Microspheres as pH Sensors. Sensors 2021, 21, 6493. https://doi.org/10.3390/s21196493
Lavine BK, Kaval N, Oxenford L, Kim M, Dahal KS, Perera N, Seitz R, Moulton JT, Bunce RA. Synthesis and Characterization of N-Isopropylacrylamide Microspheres as pH Sensors. Sensors. 2021; 21(19):6493. https://doi.org/10.3390/s21196493
Chicago/Turabian StyleLavine, Barry K., Necati Kaval, Leah Oxenford, Mariya Kim, Kaushalya Sharma Dahal, Nuwan Perera, Rudolf Seitz, James T. Moulton, and Richard A. Bunce. 2021. "Synthesis and Characterization of N-Isopropylacrylamide Microspheres as pH Sensors" Sensors 21, no. 19: 6493. https://doi.org/10.3390/s21196493






