Simplified Optimal Estimation of Time-Varying Electromyogram Standard Deviation (EMGσ): Evaluation on Two Datasets
Abstract
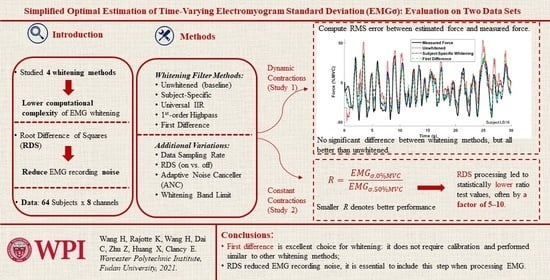
:1. Introduction
2. Experimental Data and Universal Whitening Filters
2.1. Experimental Data
2.2. Development of Universal Whitening Filters
3. Study 1: Force-Varying Contractions
3.1. Methods of Analysis—Study 1
- Sampling rate: We used the original 4096 Hz sampling rate and rates of 2048 and 1024 Hz, representing a range of rates used in practice. Sampling rates are important to study, since high sampling rates are particularly sensitive to whitening-induced noise at higher frequencies [27,36]. When decimated, the signal was low-pass filtered at 80% of the new Nyquist rate (seventh-order Chebyshev Type I filter, 0.05 dB peak-to-peak passband ripple) and then down-sampled.
- Stage 1–3 whitening filters: We studied six variations: (1, 2) our traditional subject-specific 60th-order FIR Stage 1 whitening filter with and without Stages 2–3, (3, 4) second-order universal IIR Stage 1 whitening with and without Stages 2–3, (5) high-pass filter whitening without Stages 2–3, and (6) first difference whitening without Stages 2–3. The high-pass and first difference methods were studied without Stages 2–3, since these stages are not part of these methods. For high-pass filter whitening, the cut-off frequency was varied upwards from 100 Hz in increments of 30 Hz. The maximum cut-off frequency was the lesser of 1930 Hz or the value prior to exceeding the Nyquist frequency.
- Stage 4 whitening bandwidth: At sampling rates of 2048 and 4096 Hz, we varied the whitening bandwidth to 1000 and 600 Hz via ninth-order, Chebyshev Type I, 0.05 dB peak-to-peak ripple low-pass filters that were causally implemented. Additionally, all sampling rates studied the full Nyquist frequency (no bandwidth limit).
- Noise correction: This was done with and without RDS processing.
3.2. Results—Study 1
4. Study 2: Constant-Force Contractions
4.1. Methods of Analysis—Study 2
4.2. Results—Study 2
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample Rate (Hz) | Universal IIR Whitening Filter, Defined as a Linear, Constant-Coefficient Difference Equation: | ||||
|---|---|---|---|---|---|
| 1000 | –5.10427 | 6.82006 | –4.09619 | 0.742714 | –0.128509 |
| 1024 | –3.90799 | 5.90018 | –4.23552 | 0.800134 | –0.0871683 |
| 2000 | –6.81618 | 12.9140 | –7.89417 | 0.632655 | –0.136978 |
| 2048 | –7.20675 | 13.2972 | –7.80079 | 0.760178 | –0.00269560 |
| 4000 | –17.5275 | 32.1657 | –15.3385 | 0.452029 | 0.0876669 |
| 4096 | –17.5038 | 31.2572 | –14.6111 | 0.371506 | 0.0980280 |
References
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inman, V.T.; Ralston, H.J.; Saunders, J.B.; Feinstein, B.; Wright, E.W. Relation of human electromyogram to musculuar tension. EEG Clin. Neurophysiol. 1952, 4, 187–194. [Google Scholar] [CrossRef]
- McManus, L.; De Vito, G.; Lowery, M.M. Analysis and biophysics of surface EMG for physiotherapists and kinesiologists: Toward a common language with rehabilitation engineers. Front. Neurol. 2020, 11, 576729. [Google Scholar] [CrossRef]
- Dai, C.; Bardizbanian, B.; Clancy, E.A. Comparison of constant-posture force-varying EMG-force dynamic models about the elbow. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1529–1538. [Google Scholar] [CrossRef]
- Dai, C.; Zhu, Z.; Martinez-Luna, C.; Hunt, T.R.; Farrell, T.R.; Clancy, E.A. Two degrees of freedom, dynamic, hand-wrist EMG-force using a minimum number of electrodes. J. Electromyogr. Kinesiol. 2019, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Staudenmann, D.; Roeleveld, K.; Stegeman, D.F.; van Dieen, J.H. Methodological aspects of EMG recordings for force estimation—A tutorial and review. J. Electromyogr. Kinesiol. 2010, 20, 375–387. [Google Scholar] [CrossRef]
- Hashemi, J.; Morin, E.; Mousavi, P.; Mountjoy, K.; Hashtrudi-Zaad, K. EMG-force modeling using parallel cascade identification. J. Electromyogr. Kinesiol. 2012, 22, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Morin, E.; Mousavi, P.; Hashtrudi-Zaad, K. Enhanced dynamic EMG-force estimation through calibration and PCI modeling. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Doheny, E.P.; Lowery, M.M.; FitzPatrick, D.P.; O’Malley, M.J. Effect of elbow joint angle on force-EMG relationships in human elbow flexor and extensor muscles. J. Electromyogr. Kinesiol. 2008, 18, 760–770. [Google Scholar] [CrossRef]
- Dai, C.; Martel, S.; Martel, F.; Rancourt, D.; Clancy, E.A. Single-trial estimation of quasi-static EMG-to-joint-mechanical-impedance relationship over a range of joint torques. J. Electromyogr. Kinesiol. 2019, 45, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Abul-Haj, C.J.; Hogan, N. Functional assessment of control systems for cybernetic elbow prosthesis—Part I: Description of the technique. IEEE Trans. Biomed. Eng. 1990, 37, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, J.; Koike, Y. A myokinetic arm model for estimating joint torque and stiffness from EMG signals during maintained posture. J. Neurophysiol. 2009, 101, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Kawase, T.; Kambara, H.; Koike, Y. A power assist device based on joint equilibrium point estimation from EMG signals. J. Robot. Mechatron. 2012, 24, 205–218. [Google Scholar] [CrossRef]
- Pfeifer, S.; Vallery, H.; Hardegger, M.; Riener, R.; Perreault, E.J. Model-based estimation of knee stiffness. IEEE Trans. Biomed. Eng. 2012, 59, 2604–2612. [Google Scholar] [CrossRef]
- Golkar, M.A.; Tehrani, E.S.; Kearney, R.E. Linear parametric varying identification of dynamic joint stiffness during time-varying voluntary contractions. Front. Comput. Neurosci. 2017, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Ostry, D.J.; Feldman, A.G. A critical evaluation of the force control hypothesis in motor control. Exp. Brain Res. 2003, 153, 275–288. [Google Scholar] [CrossRef]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in clinical assessment and neurorehabilitation: Barriers limiting its use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Parker, P.; Englehart, K.; Hudgins, B. Myoelectric signal processing for control of powered limb prostheses. J. Electromyogr. Kinesiol. 2006, 16, 541–548. [Google Scholar] [CrossRef]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef]
- Popat, R.A.; Drebs, D.E.; Mansfield, J.; Russell, D.; Clancy, E.; Gill-Body, K.M.; Hogan, N. Quantitative assessment of four men using above-elbow prosthetic control. Arch. Phys. Med. Rehabil. 1993, 74, 720–729. [Google Scholar] [CrossRef]
- Hagg, G.M.; Melin, B.; Kadefors, R. Applications in ergonomics. In Electromyography: Physiology, Engineering, and Noninvasive Applications; Merletti, R., Parker, P.A., Eds.; IEEE Press/Wiley-Interscience: Hoboken, NJ, USA, 2004; pp. 343–363. [Google Scholar]
- Kumar, S.; Mital, A. Electromyography in Ergonomics; Taylor & Francis: Briston, PA, USA, 1996. [Google Scholar]
- Disselhorst-Klug, C.; Schmitz-Rode, T.; Rau, G. Surface electromyography and muscle force: Limits in sEMG-force relationship and new approaches for applications. Clin. Biomech. 2009, 24, 225–235. [Google Scholar] [CrossRef]
- Doorenbosch, C.A.M.; Harlaar, J. A clinically applicable EMG-force model to quantify active stabilization of the knee after a lesion of the anterior cruciate ligament. Clin. Biomech. 2003, 18, 142–149. [Google Scholar] [CrossRef]
- Clancy, E.A.; Morin, E.L.; Merletti, R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J. Electromyogr. Kinesiol. 2002, 12, 1–16. [Google Scholar] [CrossRef]
- Wang, H.; Rajotte, K.J.; Wang, H.; Dai, C.; Zhu, Z.; Bhuiyan, M.; Huang, X.; Clancy, E.A. Optimal estimation of EMG standard deviation (EMGσ) in additive measurement noise: Model-based derivations and their implications. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Farry, K.A. Adaptive whitening of the electromyogram to improve amplitude estimation. IEEE Trans. Biomed. Eng. 2000, 47, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.; Mann, R.W. Myoelectric signal processing: Optimal estimation applied to electromyography—Part I: Derivation of the optimal myoprocessor. IEEE Trans. Biomed. Eng. 1980, 27, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.; Mann, R.W. Myoelectric signal processing: Optimal estimation applied to electromyography—Part II: Experimental demonstration of optimal myoprocessor performance. IEEE Trans. Biomed. Eng. 1980, 27, 396–410. [Google Scholar] [CrossRef]
- St-Amant, Y.; Rancourt, D.; Clancy, E.A. Influence of smoothing window length on electromyogram amplitude estimates. IEEE Trans. Biomed. Eng. 1998, 45, 795–799. [Google Scholar] [CrossRef]
- Clancy, E.A.; Hogan, N. Single site electromyograph amplitude estimation. IEEE Trans. Biomed. Eng. 1994, 41, 159–167. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P.; Clancy, E.A.; Scheme, E.; Englehart, K.B. Electromyogram whitening for improved classification accuracy in upper limb prosthesis control. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Hogan, N. Relating agonist-antagonist electromyograms to joint torque during isometric, quasi-isotonic, non-fatiguing contractions. IEEE Trans. Biomed. Eng. 1997, 44, 1024–1028. [Google Scholar] [CrossRef]
- Kaiser, E.; Petersen, I. Adaptive Filter for EMG Control Signals. In The Control of Upper-Extremity Prostheses and Orthoses; Herberts, P., Kadefors, R., Magnusson, R., Petersen, I., Eds.; Charles C. Thomas: Springfield, IL, USA, 1974; pp. 54–57. [Google Scholar]
- Clancy, E.A. Stochastic Modeling of the Relationship Between the Surface Electromygram and Muscle Torque. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1991; pp. 303–352, 449–469. [Google Scholar]
- Dasog, M.; Koirala, K.; Liu, P.; Clancy, E.A. Electromyogram bandwidth requirements when the signal is whitened. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Potvin, J.R.; Brown, S.H.M. Less is more: High pass filtering, to remove up to 99% of the surface EMG signal power, improves EMG-based biceps brachii muscle force estimates. J. Electromyogr. Kinesiol. 2004, 14, 389–399. [Google Scholar] [CrossRef]
- Harba, M.I.A.; Lynn, P.A. Optimizing the acquisition and processing of surface electromyographic signals. J. Biomech. Eng. 1981, 3, 100–106. [Google Scholar] [CrossRef]
- Phinyomark, A.; Quaine, F.; Charbonnier, S.; Serviere, C.; Tarpin-Bernard, F.; Laurillau, Y. Feature extraction of the first difference of EMG time series for EMG pattern recognition. Comp. Meth. Prog. Biomed. 2014, 117, 247–256. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Y.; Xu, D.; Kang, S.H.; Zhang, L.-Q. EMG-based real-time linear-nonlinear cascade regression decoding of shoulder, elbow, and wrist movements in able-bodied persons and stroke survivors. IEEE Trans. Biomed. Eng. 2020, 67, 1272–1281. [Google Scholar] [CrossRef]
- Williams III, T.W. Practical methods for controlling powered upper-extremity prostheses. Assist. Technol. 1990, 2, 3–18. [Google Scholar] [CrossRef]
- Hansson, G.-A. Letter to the editor. J. Biomech. 2011, 44, 1637. [Google Scholar] [CrossRef] [PubMed]
- Rajotte, K.J.; Wang, H.; Wang, H.; Dai, C.; Zhu, Z.; Huang, X.; Clancy, E.A. Simplified optimal estimation of time-varying electromyogram standard deviation (EMGσ). In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 42. [Google Scholar]
- Clancy, E.A. Electromyogram amplitude estimation with adaptive smoothing window length. IEEE Trans. Biomed. Eng. 1999, 46, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, L.; Clancy, E.A. Influence of joint angle on EMG-torque model during constant-posture, torque-varying contractions. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 1039–1046. [Google Scholar] [CrossRef]
- Clancy, E.A.; Hogan, N. Multiple site electromyograph amplitude estimation. IEEE Trans. Biomed. Eng. 1995, 42, 203–211. [Google Scholar] [CrossRef]
- Storn, R. Differential evolution design of an IIR-filter. In Proceedings of the IEEE International Conference on Evolutionary Computation, Nagoya, Japan, 20–22 May 1996. [Google Scholar]
- Karaboga, N. Digital IIR filter design using differential evolution algorithm. EURASIP J. Appl. Sig. Proc. 2005, 8, 1269–1276. [Google Scholar] [CrossRef] [Green Version]
- Wang, H. Advanced Electromyogram Signal Processing with an Emphasis on Simplified, Near-Optimal Whitening; Worcester Polytechnic Institute: Worcester, MA, USA, 2019; pp. 19–34. [Google Scholar]
- Koirala, K.; Dasog, M.; Liu, P.; Clancy, E.A. Using the electromyogram to anticipate torques about the elbow. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Belter, J.T.; Segil, J.L.; Dollar, A.M.; Weir, R.F. Mechanical design and performance specifications of anthropomorphic prosthetic hands: A review. J. Rehabil. Res. Devel. 2013, 50, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Frey Law, L.; Avin, K.; Krishnan, C. Response to letter to the editor. J. Biomech. 2011, 44, 1637–1638. [Google Scholar] [CrossRef]






| Fixed Whiten Filter | Adaptive Noise Cancel? | White Band Limit | 4096 Hz Sampling Rate | 2048 Hz Sampling Rate | 1024 Hz Sampling Rate |
|---|---|---|---|---|---|
| 600 Hz | 4.90 ± 2.15 | 4.93 ± 2.18 | — | ||
| No | 1000 Hz | 5.00 ± 2.29 | 4.97 ± 2.30 | — | |
| Subject | Nyquist | 6.03 ± 3.33 | 4.98 ± 2.30 | 5.05 ± 2.20 | |
| Specific | 600 Hz | 4.86 ± 2.06 | 4.93 ± 2.09 | — | |
| Yes | 1000 Hz | 4.78 ± 2.10 | 4.85 ± 2.09 | — | |
| Nyquist | 4.74 ± 2.07 | 4.88 ± 2.09 | 5.08 ± 2.10 | ||
| 600 Hz | 4.95 ± 2.20 | 5.03 ± 2.18 | — | ||
| No | 1000 Hz | 4.84 ± 2.10 | 4.90 ± 2.10 | — | |
| IIR | Nyquist | 5.57 ± 2.76 | 4.90 ± 2.09 | 5.08 ± 2.20 | |
| 600 Hz | 4.95 ± 2.20 | 5.04 ± 2.17 | — | ||
| Yes | 1000 Hz | 4.79 ± 2.06 | 4.90 ± 2.09 | — | |
| Nyquist | 4.83 ± 2.07 | 4.91 ± 2.10 | 5.09 ± 2.22 | ||
| 600 Hz | 4.98 ± 2.15 (2047 Hz) | 4.95 ± 2.15 (1023 Hz) | — | ||
| High-pass | N/A | 1000 Hz | 4.92 ± 2.10 (2047 Hz) | 4.88 ± 2.10 (970 Hz) | — |
| Nyquist | 4.89 ± 2.06 (1300 Hz) | 4.90 ± 2.11 (880 Hz) | 4.98 ± 2.04 (490 Hz) | ||
| 600 Hz | 5.00 ± 2.16 | 5.03 ± 2.22 | — | ||
| First | N/A | 1000 Hz | 4.95 ± 2.12 | 5.00 ± 2.19 | — |
| Diff. | Nyquist | 4.91 ± 2.09 | 5.00 ± 2.18 | 5.12 ± 2.25 |
| Fixed Whiten Filter | Adaptive Noise Cancel? | White Band Limit | 4096 Hz Sampling Rate | 2048 Hz Sampling Rate | 1024 Hz Sampling Rate | |||
|---|---|---|---|---|---|---|---|---|
| No RDS | Yes RDS | No RDS | Yes RDS | No RDS | Yes RDS | |||
| 600 Hz | 0.13 ± 0.11 | 0.048 ± 0.11 | 0.13 ± 0.12 | 0.047 ± 0.11 | — | — | ||
| No | 1000 Hz | 0.26 ± 0.17 | 0.024 ± 0.097 | 0.23 ± 0.16 | 0.027 ± 0.099 | — | — | |
| Subject | Nyquist | 0.55 ± 0.23 | 0.0073 ± 0.078 | 0.23 ± 0.16 | 0.027 ± 0.099 | 0.11 ± 0.11 | 0.056 ± 0.11 | |
| Specific | 600 Hz | 0.074 ± 0.076 | 0.048 ± 0.096 | 0.076 ± 0.087 | 0.048 ± 0.10 | — | — | |
| Yes | 1000 Hz | 0.13 ± 0.11 | 0.041 ± 0.099 | 0.12 ± 0.11 | 0.042 ± 0.096 | — | — | |
| Nyquist | 0.34 ± 0.21 | 0.017 ± 0.090 | 0.12 ± 0.11 | 0.042 ± 0.095 | 0.076 ± 0.087 | 0.054 ± 0.098 | ||
| 600 Hz | 0.14 ± 0.12 | 0.054 ± 0.13 | 0.14 ± 0.14 | 0.059 ± 0.16 | — | — | ||
| No | 1000 Hz | 0.30 ± 0.19 | 0.024 ± 0.11 | 0.24 ± 0.19 | 0.033 ± 0.14 | — | — | |
| IIR | Nyquist | 0.57 ± 0.22 | 0.0076 ± 0.082 | 0.24 ± 0.19 | 0.033 ± 0.14 | 0.12 ± 0.22 | 0.083 ± 0.34 | |
| 600 Hz | 0.089 ± 0.082 | 0.066 ± 0.10 | 0.076 ± 0.093 | 0.060 ± 0.12 | — | — | ||
| Yes | 1000 Hz | 0.080 ± 0.083 | 0.059 ± 0.10 | 0.071 ± 0.091 | 0.055 ± 0.11 | — | — | |
| Nyquist | 0.070 ± 0.082 | 0.053 ± 0.096 | 0.071 ± 0.091 | 0.055 ± 0.11 | 0.078 ± 0.15 | 0.070 ± 0.24 | ||
| 600 Hz | 0.098 ± 0.092 | 0.051 ± 0.096 | 0.10 ± 0.095 | 0.051 ± 0.097 | — | — | ||
| High-pass | N/A | 1000 Hz | 0.14 ± 0.11 | 0.043 ± 0.096 | 0.15 ± 0.12 | 0.041 ± 0.097 | — | — |
| Nyquist | 0.23 ± 0.15 | 0.026 ± 0.089 | 0.14 ± 0.11 | 0.043 ± 0.097 | 0.098 ± 0.094 | 0.055 ± 0.10 | ||
| 600 Hz | 0.096 ± 0.091 | 0.051 ± 0.095 | 0.093 ± 0.090 | 0.052 ± 0.095 | — | — | ||
| First | N/A | 1000 Hz | 0.13 ± 0.11 | 0.046 ± 0.096 | 0.11 ± 0.096 | 0.049 ± 0.095 | — | — |
| Diff. | Nyquist | 0.19 ± 0.13 | 0.032 ± 0.091 | 0.11 ± 0.096 | 0.049 ± 0.095 | 0.080 ± 0.084 | 0.052 ± 0.092 | |
| fc (Hz) | Whitening Band (Hz) | Non-Significant Paired Comparisons |
|---|---|---|
| 4096 | 600 | IIR with ANC vs. (high-pass, first difference) |
| 4096 | 1000 | Subject-specific without ANC vs. IIR without ANC; IIR with ANC vs. high-pass |
| 4096 | Nyquist | — |
| 2048 | 600 | Subject-specific without ANC vs. (IIR with ANC, high-pass); IIR with ANC vs. (high-pass, first difference) |
| 2048 | 1000 | Subject-specific without ANC vs. IIR without ANC |
| 2048 | Nyquist | Subject-specific without ANC vs. IIR without ANC |
| 1024 | Nyquist | Subject-specific without ANC vs. IIR with ANC; high-pass vs. first difference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Rajotte, K.J.; Wang, H.; Dai, C.; Zhu, Z.; Huang, X.; Clancy, E.A. Simplified Optimal Estimation of Time-Varying Electromyogram Standard Deviation (EMGσ): Evaluation on Two Datasets. Sensors 2021, 21, 5165. https://doi.org/10.3390/s21155165
Wang H, Rajotte KJ, Wang H, Dai C, Zhu Z, Huang X, Clancy EA. Simplified Optimal Estimation of Time-Varying Electromyogram Standard Deviation (EMGσ): Evaluation on Two Datasets. Sensors. 2021; 21(15):5165. https://doi.org/10.3390/s21155165
Chicago/Turabian StyleWang, He, Kiriaki J. Rajotte, Haopeng Wang, Chenyun Dai, Ziling Zhu, Xinming Huang, and Edward A. Clancy. 2021. "Simplified Optimal Estimation of Time-Varying Electromyogram Standard Deviation (EMGσ): Evaluation on Two Datasets" Sensors 21, no. 15: 5165. https://doi.org/10.3390/s21155165