Global Muscle Coactivation of the Sound Limb in Gait of People with Transfemoral and Transtibial Amputation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
2.3. Data Analysis
2.3.1. Matching Procedure
2.3.2. Electromyographic Data
2.3.3. Global Coactivation of Lower Limb Muscles
Full Width at Half Maximum and Center of Activity
Coefficient of Multiple Correlation
- for each group, the within-subject similarity for TMCf (CMCTMCf_IS) among all TMCf curves of all strides for each subject and then, we computed the mean and standard deviation of the CMCTMCf_IS of all subjects within each group;
- the between-subject similarity on the mean TMCf curves (CMCTMCf_BS) of all subjects of each group;
- the similarity among the mean TMCf curves of the three groups, evaluated among all the subjects (CMCTMCf_BG).
Deviation Phase
2.3.4. Time-Distance Parameters
Symmetry Index
2.3.5. Energy Expenditure Parameters
2.4. Statistical Analysis
- weak correlation for 0 < r < 0.3;
- moderate correlation for 0.3 < r < 0.7;
- strong correlation for r > 0.7.
3. Results
3.1. People with Amputation
3.2. Lower Limb Global Coactivation
3.2.1. People with Amputation versus Controls
3.2.2. Type of Prosthesis
3.3. Correlation Finding
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geurts, A.C.; Mulder, T.W.; Nienhuis, B.; Rijken, R.A. Dual-task assessment of reorganization of postural control in persons with lower limb amputation. Arch. Phys. Med. Rehabil. 1991, 72, 1059–1064. [Google Scholar] [PubMed]
- Chen, R.; Corwell, B.; Yaseen, Z.; Hallett, M.; Cohen, L.G. Mechanisms of cortical reorganization in lower-limb amputees. J. Neurosci. 1998, 18, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Hebenton, J.; Scott, H.; Seenan, C.; Davie-Smith, F. Relationship between models of care and key rehabilitation milestones following unilateral transtibial amputation: A national cross-sectional study. Physiotherapy 2019, 105, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Silver-Thorn, M.B.; Steege, J.W.; Childress, D.S. A review of prosthetic interface stress investigations. J. Rehabil. Res. Dev. 1996, 33, 253–266. [Google Scholar] [PubMed]
- Prinsen, E.C.; Nederhand, M.J.; Rietman, J.S. Adaptation strategies of the lower extremities of patients with a transtibial or transfemoral amputation during level walking: A systematic review. Arch. Phys. Med. Rehabil. 2011, 92, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Varrecchia, T.; Serrao, M.; Rinaldi, M.; Ranavolo, A.; Conforto, S.; De Marchis, C.; Simonetti, A.; Poni, I.; Castellano, S.; Silvetti, A.; et al. Common and specific gait patterns in people with varying anatomical levels of lower limb amputation and different prosthetic components. Hum. Mov. Sci. 2019, 66, 9–21. [Google Scholar] [CrossRef]
- De Marchis, C.; Ranaldi, S.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Lacquaniti, F.; Conforto, S. Modular motor control of the sound limbin gait of people with trans-femoral amputation. J. Neuroeng. Rehabil. 2019, 16, 132. [Google Scholar] [CrossRef]
- Bateni, H.; Olney, S.J. Kinematic and kinetic variations of below-knee amputee gait. J. Prosthet. Orthot. 2002, 14, 2–10. [Google Scholar] [CrossRef]
- Wentink, E.; Prinsen, E.C.; Rietman, J.S.; Veltink, P.H. Comparison of muscle activity patterns of transfemoral amputees and control subjects during walking. J. Neuroeng. Rehabil. 2013, 10, 87. [Google Scholar] [CrossRef]
- Brandt, A.; Wen, Y.; Liu, M.; Stallings, J.; Huang, H.H. Interactions between transfemoral amputees and a powered knee prosthesis during load carriage. Sci. Rep. 2017, 7, 14480. [Google Scholar] [CrossRef]
- Clemens, S.; Kim, K.J.; Gailey, R.; Kirk-Sanchez, N.; Kristal, A.; Gaunaurd, I. Inertial sensor-based measures of gait symmetry and repeatability in people with unilateral lower limb amputation. Clin. Biomech. 2020, 72, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Carse, B.; Scott, H.; Brady, L.; Colvin, J. A characterisation of established unilateral transfemoral amputee gait using 3D kinematics, kinetics and oxygen consumption measures. Gait Posture 2019, 75, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jaegers, S.M.; Arendzen, J.H.; de Jongh, H.J. Prosthetic gait of unilateral transfemoral amputees: A kinematic study. Arch. Phys. Med. Rehabil. 1995, 76, 736–743. [Google Scholar] [CrossRef]
- Esquenazi, A. Gait analysis in lower-limb amputation and prosthetic rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 153–167. [Google Scholar] [CrossRef]
- Bae, T.S.; Choi, K.; Hong, D.; Mun, M. Dynamic analysis of above-knee amputee gait. Clin. Biomech. 2007, 22, 557–566. [Google Scholar] [CrossRef]
- Nolan, L.; Wit, A.; Dudziñski, K.; Lees, A.; Lake, M.; Wychowañski, M. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 2003, 17, 142–151. [Google Scholar] [CrossRef]
- Rossi, S.A.; Doyle, W.; Skinner, H.B. Gait initiation of persons with below-knee amputation: The characterization and comparison of force profiles. J. Rehabil. Res. Dev. 1995, 32, 120–127. [Google Scholar]
- Bonnet, X.; Villa, C.; Fodé, P.; Lavaste, F.; Pillet, H. Mechanical work performed by individual limbs of trans-femoral amputees during step-to-step transitions: Effect of walking velocity. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 228, 60–66. [Google Scholar]
- Fey, N.; Silverman, A.K.; Neptune, R. The influence of increasing steady-state walking speed on muscle activity in below-knee amputees. J. Electromyogr. Kinesiol. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- Breakey, J. Gait of unilateral below-knee amputees. Orthot. Prosthet. 1976, 30, 17–24. [Google Scholar]
- Murray, M.P.; Mollinger, L.A.; Sepic, S.B.; Gardner, G.M.; Linder, M.T. Gait patterns in above-knee amputee patients: Hydraulic swing control vs constant-friction knee components. Arch. Phys. Med. Rehabil. 1983, 64, 339–345. [Google Scholar] [PubMed]
- Engsberg, J.R.; Lee, A.G.; Tedford, K.G.; Harder, J.A. Normative ground reaction force data for able-bodied and trans-tibial amputee children during running. Prosthet. Orthot. Int. 1993, 17, 83–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, K. Force plate study on the artificial limb gait. J. Jpn. Orthop. Assoc. 1972, 46, 503–516. [Google Scholar]
- Engsberg, J.R.; Aldridge, K.C.; Harder, J.A. Lower limb intersegmental forces for below-knee amputee children during standing. Prosthet. Orthot. Int. 1991, 15, 185–191. [Google Scholar] [CrossRef][Green Version]
- Hsu, M.-J.; Nielsen, D.H.; Lin, S.-J.; Shurr, D. The effects of prosthetic foot design on physiologic measurements, self-selected walking velocity, and physical activity in people with transtibial amputation. Arch. Phys. Med. Rehabil. 2006, 87, 123–129. [Google Scholar] [CrossRef]
- Schafer, Z.A.; Perry, J.L.; Vanicek, N. A personalised exercise programme for individuals with lower limb amputation reduces falls and improves gait biomechanics: A block randomised controlled trial. Gait Posture 2018, 63, 282–289. [Google Scholar] [CrossRef]
- Highsmith, M.J.; Kahle, J.T.; Miro, R.M.; Cress, M.E.; Lura, D.; Quillen, W.S.; Carey, S.L.; Dubey, R.V.; Mengelkoch, L.J. Functional performance differences between the Genium and C-Leg prosthetic knees and intact knees. J. Rehabil. Res. Dev. 2016, 53, 753–766. [Google Scholar] [CrossRef]
- Hamill, J.; Bates, B.; Knutzen, K.; Sawhill, J. Variations in ground reaction force parameters at different running speeds. Hum. Mov. Sci. 1983, 2, 47–56. [Google Scholar] [CrossRef]
- Powers, C.M.; Torburn, L.; Perry, J.; Ayyappa, E. Influence of prosthetic foot design on sound limb loading in adults with unilateral below-knee amputations. Arch. Phys. Med. Rehabil. 1994, 75, 825–829. [Google Scholar] [CrossRef]
- Mileusnic, M.P.; Rettinger, L.; Highsmith, M.J.; Hahn, A. Benefits of the Genium microprocessor controlled prosthetic knee on ambulation, mobility, activities of daily living and quality of life: A systematic literature review. Disabil. Rehabil. Assist. Technol. 2019, 30, 1–12. [Google Scholar] [CrossRef]
- Ranavolo, A.; Mari, S.; Conte, C.; Serrao, M.; Silvetti, A.; Iavicoli, S.; Draicchio, F. A new muscle co-activation index for biomechanical load evaluation in work activities. Ergonomics 2015, 58, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Varrecchia, T.; Rinaldi, M.; Serrao, M.; Draicchio, F.; Conte, C.; Conforto, S.; Schmid, M.; Ranavolo, A. Global lower limb muscle coactivation during walking at different speeds: Relationship between spatio-temporal, kinematic, kinetic, and energetic parameters. J. Electromyogr. Kinesiol. 2018, 43, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Naing, L.; Winn, T.; Rusli, B.N. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Wu, G.; van der Helm, F.C.; Veeger, D.H.E.J.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R.; Iii, R.B.D. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Mari, S.; Serrao, M.; Casali, C.; Conte, C.; Martino, G.; Ranavolo, A.; Coppola, G.; Draicchio, F.; Padua, L.; Sandrini, G.; et al. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum 2013, 13, 226–236. [Google Scholar] [CrossRef]
- Rinaldi, M.; Ranavolo, A.; Conforto, S.; Martino, G.; Draicchio, F.; Conte, C.; Varrecchia, T.; Bini, F.; Casali, C.; Pierelli, F.; et al. Increased lower limb muscle coactivation reduces gait performance and increases metabolic cost in patients with hereditary spastic paraparesis. Clin. Biomech. 2017, 48, 63–72. [Google Scholar] [CrossRef]
- Barbero, M.; Merletti, R.; Rainoldi, A. Atlas of Muscle Innervation Zones; Springer: Milan, Italy, 2012. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Serrao, M.; Rinaldi, M.; Ranavolo, A.; Lacquaniti, F.; Martino, G.; Leonardi, L.; Conte, C.; Varrecchia, T.; Draicchio, F.; Coppola, G.; et al. Gait patterns in patients with hereditary spastic paraparesis. PLoS ONE 2016, 11, e0164623. [Google Scholar] [CrossRef]
- Martino, G.; Ivanenko, Y.; Serrao, M.; Ranavolo, A.; D’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor patterns in cerebellar ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Ivanenko, Y.; D’Avella, A.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Cappellini, G.; Casali, C.; Lacquaniti, F. Neuromuscular adjustments of gait associated with unstable conditions. J. Neurophysiol. 2015, 114, 2867–2882. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; D’Anna, C.; Schmid, M.; Conforto, S. Assessing the influence of SNR and pre-processing filter bandwidth on the extraction of different muscle co-activation indexes from surface EMG data. J. Electromyogr. Kinesiol. 2018, 43, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Labini, F.S.; Ivanenko, Y.; Cappellini, G.; Gravano, S.; Lacquaniti, F. Smooth changes in the EMG patterns during gait transitions under body weight unloading. J. Neurophysiol. 2011, 106, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Ranavolo, A.; Donini, L.M.; Mari, S.; Serrao, M.; Silvetti, A.; Iavicoli, S.; Cava, E.; Asprino, R.; Pinto, A.; Draicchio, F. Lower-limb joint coordination pattern in obese subjects. BioMed Res. Int. 2012, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Steinwender, G.; Saraph, V.; Scheiber, S.; Zwick, E.; Uitz, C.; Hackl, K. Intrasubject repeatability of gait analysis data in normal and spastic children. Clin. Biomech. 2000, 15, 134–139. [Google Scholar] [CrossRef]
- Motta, C.; Palermo, E.; Studer, V.; Germanotta, M.; Germani, G.; Centonze, D.; Cappa, P.; Rossi, S.; Rossi, S. Disability and fatigue can be objectively measured in multiple sclerosis. PLoS ONE 2016, 11, e0148997. [Google Scholar] [CrossRef]
- Nigg, S.; Vienneau, J.; Maurer, C.; Nigg, B.M. Development of a symmetry index using discrete variables. Gait Posture 2013, 38, 115–119. [Google Scholar] [CrossRef]
- Don, R.; Ranavolo, A.; Cacchio, A.; Serrao, M.; Costabile, F.; Iachelli, M.; Camerota, F.; Frascarelli, M.; Santilli, V. Relationship between recovery of calf-muscle biomechanical properties and gait pattern following surgery for achilles tendon rupture. Clin. Biomech. 2007, 22, 211–220. [Google Scholar] [CrossRef]
- Cavagna, G.; Willems, P.A.; Legramandi, M.A.; Heglund, N.C. Pendular energy transduction within the step in human walking. J. Exp. Biol. 2002, 205, 3413–3422. [Google Scholar]
- Whittle, M.W. Three-dimensional motion of the center of gravity of the body during walking. Hum. Mov. Sci. 1997, 16, 347–355. [Google Scholar] [CrossRef]
- Cavagna, G.A.; Thys, H.; Zamboni, A. The sources of external work in level walking and running. J. Physiol. 1976, 262, 639–657. [Google Scholar] [CrossRef]
- Watson, G.S.; Williams, E.J. On the construction of significance tests on the circle and the sphere. Biometrika 1956, 43, 344. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Kannenberg, A.; Zacharias, B.; Mileusnic, M.; Seyr, M. Activities of daily living. J. Prosthet. Orthot. 2013, 25, 110–117. [Google Scholar] [CrossRef]
- Tura, A.; Rocchi, L.; Raggi, M.; Cutti, A.G.; Chiari, L. Recommended number of strides for automatic assessment of gait symmetry and regularity in above-knee amputees by means of accelerometry and autocorrelation analysis. J. Neuroeng. Rehabil. 2012, 9, 11. [Google Scholar] [CrossRef]
- Cutti, A.G.; Lettieri, E.; Del Maestro, M.; Radaelli, G.; Luchetti, M.; Verni, G.; Masella, C. Stratified cost-utility analysis of C-Leg versus mechanical knees: Findings from an Italian sample of transfemoral amputees. Prosthet. Orthot. Int. 2016, 41, 227–236. [Google Scholar] [CrossRef]
- Gailey, R. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J. Rehabil. Res. Dev. 2008, 45, 15–30. [Google Scholar] [CrossRef]
- Hafner, B.J.; Willingham, L.L.; Buell, N.C.; Allyn, K.J.; Smith, D.G. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch. Phys. Med. Rehabil. 2007, 88, 207–217. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Levine, J.A.; Brey, R.H.; McCrady, S.K.; Padgett, D.J.; Joyner, M.J. Energy expenditure and activity of transfemoral amputees using mechanical and microprocessor-controlled prosthetic knees. Arch. Phys. Med. Rehabil. 2008, 89, 1380–1385. [Google Scholar] [CrossRef]
- Lura, D.J.; Wernke, M.M.; Carey, S.L.; Kahle, J.T.; Miro, R.M.; Highsmith, M.J. Differences in knee flexion between the Genium and C-Leg microprocessor knees while walking on level ground and ramps. Clin. Biomech. 2015, 30, 175–181. [Google Scholar] [CrossRef] [PubMed]

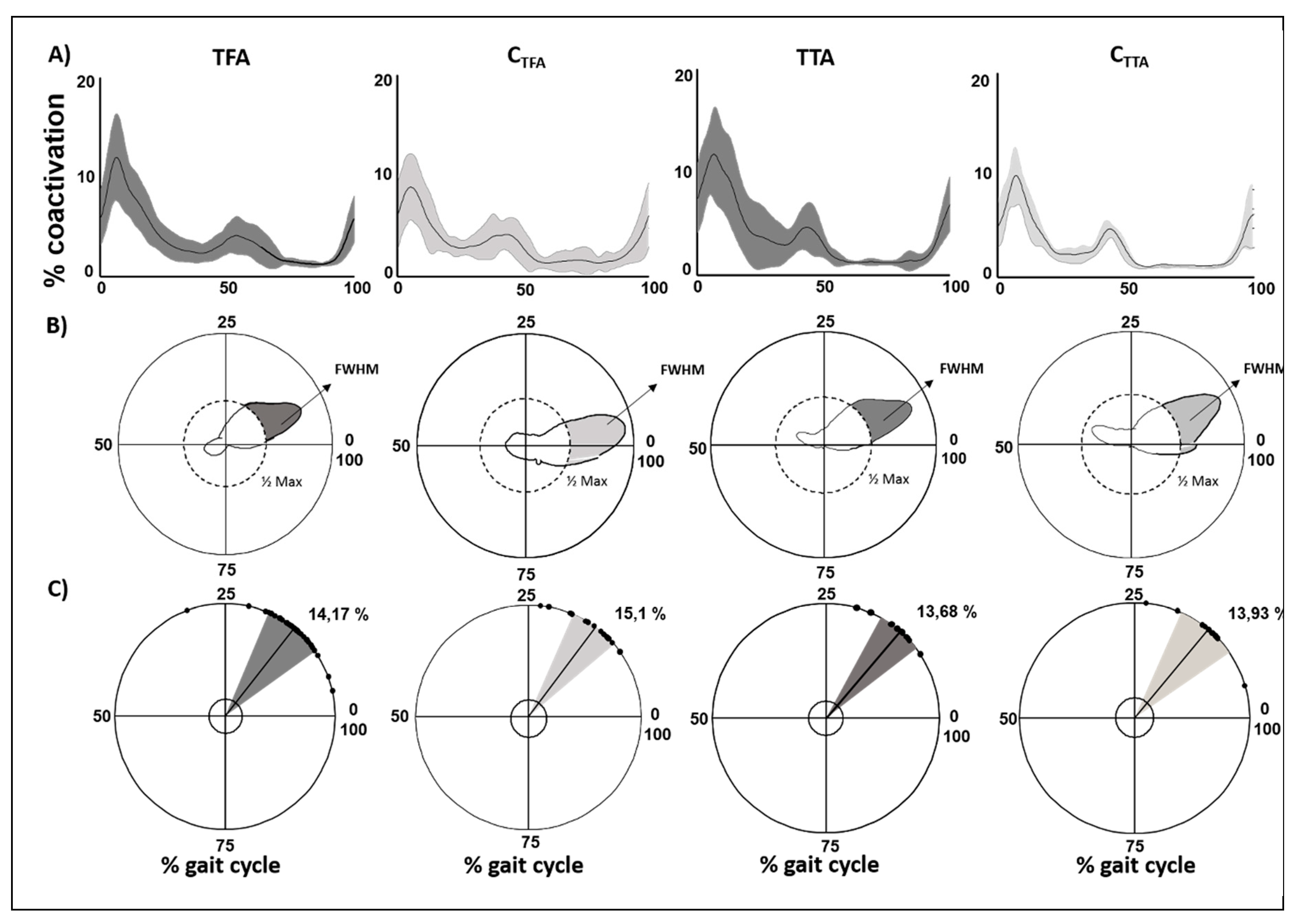
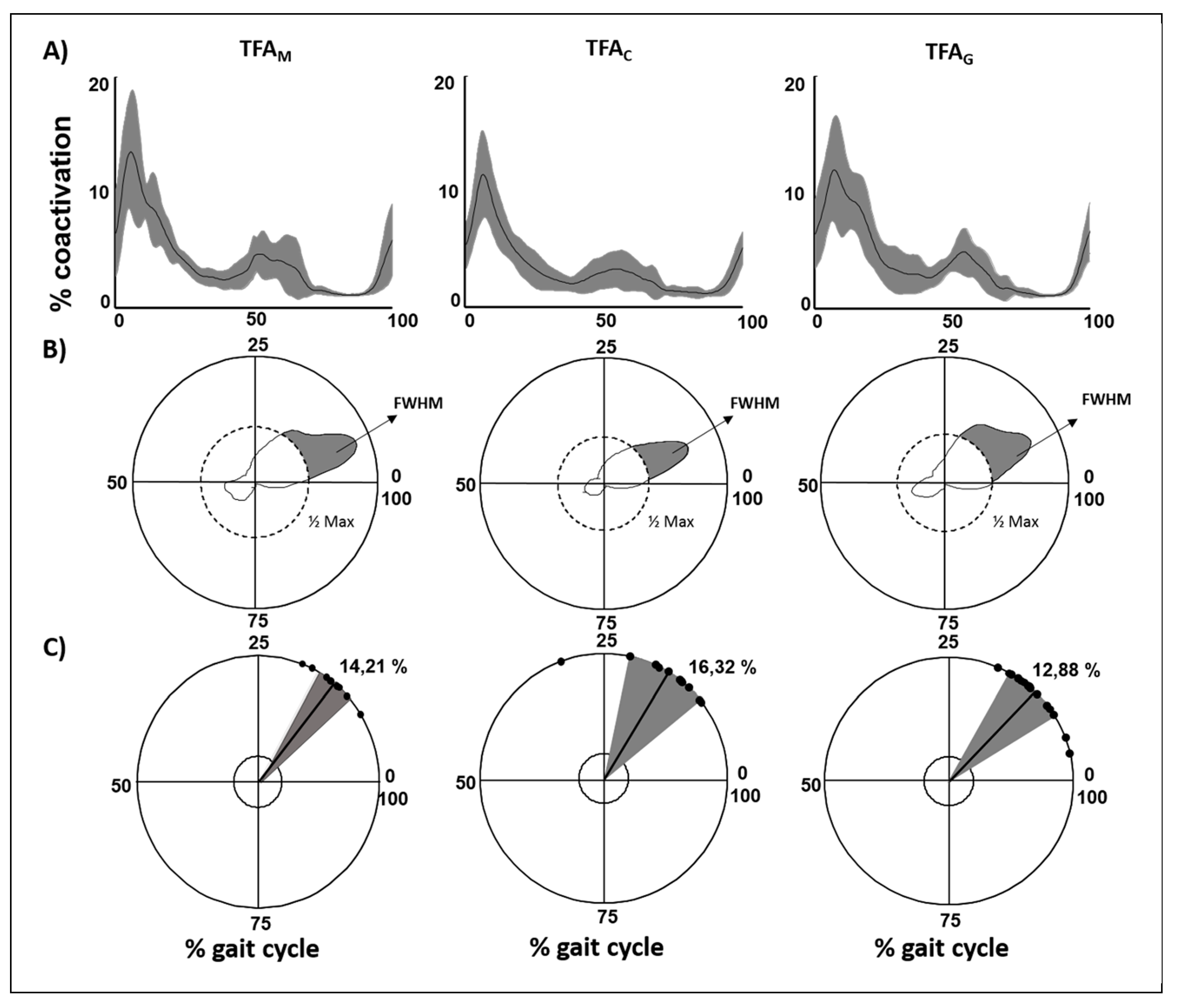
| People with Amputation versus Controls | Type of Prosthesis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TFA | CTFA | pgroup | TTA | CTTA | pgroup | TFAM | TFAC | TFAG | pgroup | |
| CI | 3.06 ± 0.58 | 2.43 ± 0.57 | <0.01 | 3.11 ± 1.07 | 2.25 ± 0.31 | <0.01 | 3.36 ± 0.55 | 2.78 ± 0.58 | 3.24 ± 0.48 | 0.02 |
| CMCIS | 0.84 ± 0.06 | 0.85 ± 0.05 | >0.05 | 0.88 ± 0.04 | 0.88 ± 0.04 | >0.05 | 0.86 ± 0.06 | 0.84 ± 0.05 | 0.84 ± 0.06 | >0.05 |
| DP | 1.36 ± 0.31 | 1.07 ± 0.3 | <0.01 | 1.28 ± 0.41 | 0.9 ± 0.2 | 0.01 | 1.43 ± 0.28 | 1.21 ± 0.26 | 1.41 ± 0.21 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarelli, A.; Serrao, M.; Varrecchia, T.; Fiori, L.; Draicchio, F.; Silvetti, A.; Conforto, S.; De Marchis, C.; Ranavolo, A. Global Muscle Coactivation of the Sound Limb in Gait of People with Transfemoral and Transtibial Amputation. Sensors 2020, 20, 2543. https://doi.org/10.3390/s20092543
Tatarelli A, Serrao M, Varrecchia T, Fiori L, Draicchio F, Silvetti A, Conforto S, De Marchis C, Ranavolo A. Global Muscle Coactivation of the Sound Limb in Gait of People with Transfemoral and Transtibial Amputation. Sensors. 2020; 20(9):2543. https://doi.org/10.3390/s20092543
Chicago/Turabian StyleTatarelli, Antonella, Mariano Serrao, Tiwana Varrecchia, Lorenzo Fiori, Francesco Draicchio, Alessio Silvetti, Silvia Conforto, Cristiano De Marchis, and Alberto Ranavolo. 2020. "Global Muscle Coactivation of the Sound Limb in Gait of People with Transfemoral and Transtibial Amputation" Sensors 20, no. 9: 2543. https://doi.org/10.3390/s20092543
APA StyleTatarelli, A., Serrao, M., Varrecchia, T., Fiori, L., Draicchio, F., Silvetti, A., Conforto, S., De Marchis, C., & Ranavolo, A. (2020). Global Muscle Coactivation of the Sound Limb in Gait of People with Transfemoral and Transtibial Amputation. Sensors, 20(9), 2543. https://doi.org/10.3390/s20092543






