Evaluation of Elastic Properties and Conductivity of Chitosan Acetate Films in Ammonia and Water Vapors Using Acoustic Resonators †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan Acetate Films
2.2. Topology and Profile of Chitosan Acetate Films
2.3. Determination of the Elastic and Viscosity Coefficients of Chitosan Films
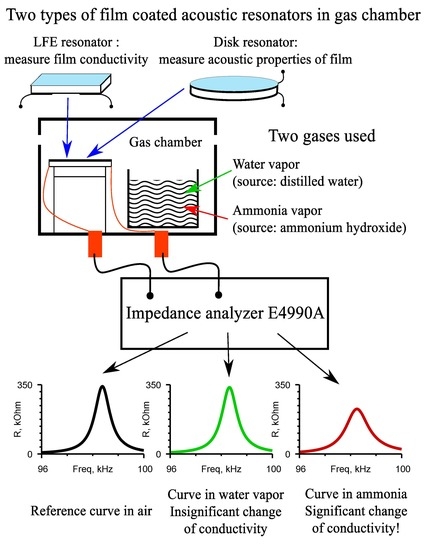
2.4. Measurement of the Electrical Conductivity of the Film
2.5. The study of the Structure “Resonator with Lateral Electric Field—Film of Chitosan”
3. Results & Discussion
3.1. Topology Studies
3.2. Determination of the Time Dependences of Ammonia Concentration and Air Humidity in a Closed Chamber
3.3. Determination of Elasticity and Viscosity Coefficients of Films in the Vapor of Volatile Liquids
3.4. Measurement of the Conductivity of Films in Vapor of Volatile Liquids
3.5. Measurement of the Resonance Properties of the Structure “Resonator with a Lateral Electric Field-Chitosan Film” in the Vapor of Volatile Liquids
3.6. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Su, Y.; Xie, G.; Wang, S.; Tai, H.; Zhang, Q.; Du, H. Novel high-performance self poweredhumidity detectionenabled by triboelectric effect. Sens. Actuators B Chem. 2017, 251, 144–152. [Google Scholar] [CrossRef]
- Su, P.; Yang, L. NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Sens. Actuators B Chem. 2016, 223, 202–208. [Google Scholar] [CrossRef]
- Hu, Y.; French, L., Jr.; Radecsky, K.; Pereira da Cunha, M.; Millard, P.; Vetelino, J. A lateral field excited liquid acoustic wave sensor. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.; McCann, J.; Parks, J.; Frankel, D.; Pereira da Cunha, M.; Vetelino, J. A lateral-field-excited LiTaO3 high frequency bulk acoustic wave sensor. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 779–787. [Google Scholar] [CrossRef]
- Vetelino, J. A lateral field excited acoustic wave sensor platform. In Proceedings of the IEEE Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; pp. 2269–2272. [Google Scholar] [CrossRef]
- Zaitsev, B.; Kuznetsova, I.; Shikhabudinov, A.; Ignatov, O.; Guliy, O. Biological Sensor Based on a Lateral Electric Field Excited Resonator. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 963–969. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Du, J.; Yuan, L.; Qian, Z.; Zhang, Z.; Zhang, C. Lateral-field-excited bulk acoustic wave sensors on langasite working on different modes. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 864–867. [Google Scholar] [CrossRef]
- Zaitsev, B.; Shikhabudinov, A.; Teplykh, A.; Kuznetsova, I. Liquid sensor based on a piezoelectric lateral electric field-excited resonator. Ultrasonics 2016, 68, 179–183. [Google Scholar] [CrossRef]
- Wang, M.; Shi, H.; Ma, T.; Qian, Z.; Kuznetsova, I.; Yuan, L.; Wang, J.; Du, J.; Zhang, C. High frequency vibration analysis of LiTaO3 piezoelectric plates excited by lateral electric fields produced by surface electrodes under viscous liquid loadings for sensing. Smart Mater. Struct. 2020. [Google Scholar] [CrossRef]
- Zaitsev, B.; Teplykh, A.; Shikhabudinov, A.; Borodina, I.; Kisin, V.; Sinev, I. The influence of the conducting film on the characteristics of the lateral electric field excited piezoelectric resonator. Ultrasonics 2018, 84, 96–100. [Google Scholar] [CrossRef]
- Zaitsev, B.; Semyonov, A.; Teplykh, A.; Borodina, I. The effect of the conductivity of a film located near a piezoelectric resonator with a lateral electric field based on the PZT ceramics on its characteristics. Ultrasonics 2019, 94, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, L.; Luo, J.; Song, Y.; Liu, J.; Zhang, S.; Cai, X. Selective Recognition of 5-Hydroxytryptamine and Dopamine on a Multi-Walled Carbon Nanotube-Chitosan Hybrid Film-Modified Microelectrode Array. Sensors 2015, 15, 1008–1021. [Google Scholar] [CrossRef] [Green Version]
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z.A. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuators B Chem. 2013, 177, 522–528. [Google Scholar] [CrossRef]
- Li, W.; Jang, D.M.; An, S.Y.; Kim, D.; Hong, S.K.; Kim, H. Polyaniline–chitosan nanocomposite: High performance hydrogen sensor from new principle. Sens. Actuators B Chem. 2011, 160, 1020–1025. [Google Scholar] [CrossRef]
- Triyana, K.; Sembiring, A.; Rianjanu, A.; Hidayat, S.N.; Riowirawan, R.; Julian, T.; Kusumaatmaja, A.; Santoso, I.; Roto, R. Chitosan-Based Quartz Crystal Microbalance for Alcohol Sensing. Electronics 2018, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Bouvree, A.; Feller, J.F.; Castro, M.; Grohens, Y.; Rinaudo, M. Conductive polymer nano-biocomposites (CPC): Chitosan-carbon nanoparticle a good candidate to design polar vapour sensors. Sens. Actuators B Chem. 2009, 138, 138–147. [Google Scholar] [CrossRef]
- Zaitsev, B.; Fedorov, F.; Semyonov, A.; Teplykh, A.; Borodina, I.; Nasibulin, A. Gas Sensor Based on the Piezoelectric Resonator with Lateral Electric Field and Films of Chitosan Salts. In Proceedings of the IEEE Ultrasonics Symposium, Glasgow, UK, 6–9 October 2019; pp. 607–610. [Google Scholar] [CrossRef]
- Bairi, V.G.; Bourdo, S.E.; Sacre, N.; Nair, D.; Berry, B.C.; Biris, A.S.; Viswanathan, T. Ammonia gas sensing behavior of tanninsulfonic acid doped polyaniline-TiO2 composite. Sensors 2015, 15, 26415–26429. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Izquierdo, J.E.; Braga, G.S.; Dirani, E.A.; Pereira-da-Silva, M.A.; Rodríguez, E.F.; Fonseca, F.J. Enhanced sensitivity of gas sensor based on poly (3-hexylthiophene) thin-film transistors for disease diagnosis and environment monitoring. Sensors 2015, 15, 9592–96093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannov, A.G.; Prášek, J.; Jašek, O.; Zajíčková, L. Investigation of pristine graphite oxide as room-temperature chemiresistive ammonia gas sensing material. Sensors 2017, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Šetka, M.; Drbohlavová, J.; Hubálek, J. Nanostructured polypyrrole-based ammonia and volatile organic compound sensors. Sensors 2017, 17, 562. [Google Scholar] [CrossRef]
- Teplykh, A.; Zaitsev, B.; Borodina, I.; Semyonov, A.; Ziangirova, M.; Almyasheva, N.; Golyshkin, A.; Krasnopolskaya, L.; Kuznetsova, I. The study of the mechanical properties of thin films using piezoceramic acoustic resonator. ITM Web Conf. 2019, 30, 07002. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Zudin Y., B. Non-equilibrium Evaporation and Condensation Processes. Analytical Solutions; Springer: Berlin, Germany, 2018; p. 217. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.; Peppley, B.; Bui, V. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]









| Fitted Material Constants of Piezoceramics (Ba0.24Pb0.75Sr0.01(Ti0.47Zr0.53)O3) | |
|---|---|
| Constant Name | Constant Value |
| c11, 1010 Pa | 14.96 |
| c12, 1010 Pa | 8.173 |
| c13, 1010 Pa | 8.318 |
| c33, 1010 Pa | 12.84 |
| c44, 1010 Pa | 2.649 |
| e15, C/m2 | 15.93 |
| e31, C/m2 | −5.42 |
| e33, C/m2 | 17.15 |
| ε11 | 1717 |
| ε 33 | 893 |
| ρ, kg/m3 | 7238 |
| Chitosan acetate film in air mechanical characteristics | |
| c11, 1010 Pa | 1.709 |
| c44, 1010 Pa | 1.147 |
| η, 10−8 rad/s | 8.64 |
| ρ, kg/m3 | 860 |
| Materail Constants of Piezoceramics (Pb0.95Sr0.05(Ti0.47Zr0.53)O3) (Were Determined Previously by the Authors) | |
|---|---|
| Constant Name | Constant Value |
| c11, ×1010 Pa | 10.9 |
| c12, ×1010 Pa | 6.1 |
| c13, ×1010 Pa | 5.4 |
| c33, ×1010 Pa | 9.3 |
| c44, ×1010 Pa | 2.4 |
| e15, C/m2 | 10.6 |
| e31, C/m2 | −4.9 |
| e33, C/m2 | 14.9 |
| ε11 | 820 |
| ε 33 | 840 |
| ρ, kg/m3 | 7330 |
| Chitosan acetate film in air electrical characteristics | |
| ε11 | 1.1 |
| σ, μS/m | 56 |
| Chitosan acetate film in ammonia 1600 ppm electrical characteristics | |
| ε | 1.1 |
| σ, μS/m | 5600 |
| Chitosan acetate film in water vapor, relative humidity 45% electrical characteristics | |
| ε | 1.1 |
| σ, μS/m | 700 |
| Sensor Type | Range or Value of Concentration, ppm | LOD, ppm | Sensor Response, % | Sensitivity Coefficient, %/ppm | Response Time– Reset Time | Transducing Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Single PPy nanowire | 40–300 | 40 | N/A | N/A | 15–10 min–15 min | Chemiresistive | [25] |
| PPy nanowires | 1.5–73 | 1.5 | N/A | N/A | 60 s for 73 ppm | Chemiresistive | [25] |
| Single crystal PPy nanotube | 0.00005–1 | 0.00005 | N/A | N/A | ~16 s–~16 s for 1 ppm | Chemiresistive | [25] |
| PPy/graphene with TiO2 nanoparticles | 1–50 | 1 | N/A | N/A | 36 s–~16 s for 50 ppm | Chemiresistive | [25] |
| Graphite oxide | 100–500 | 100 | 22–30 | 0.0185 | 10 min–<10 min | Chemiresistive | [24] |
| Single-wall carbon nanotubes | 62.5–100 | N/A | 3-6 | N/A | N/A | Chemiresistive | [24] |
| TANIPANI/TiO2 | 20–60 | N/A | 150–382 | 5.8 | ~42 s–150 s | Chemiresistive | [22] |
| TANIPANI | 20–60 | N/A | 83–256 | 4.33 | ~42 s–~172 s | Chemiresistive | [22] |
| Organic thin-film transistor | 0–50 | N/A | 65 | 0.227 ± 0.008 | N/A–N/A | Chemiresistive | [23] |
| Chitosan acetate on disk | 0–1600 | 50 | 105 | 0.065 | N/A–<600 s | Change of the film viscosity | Current work |
| Chitosan acetate on glass | 0–1600 | 100 | 98 | 0.062 | N/A–<180 s | Chemiresistive | Current work |
| Chitosan acetate on resonator with lateral electric field | 0–1600 | 50 | −33.2 | −0.021 | N/A–<300 s | Change of the maximum of the real part of electric impedance | Current work |
| Sensor Type | Range of Relative Humidity, % | LOD, % | Sensor Response, % | Sensitivity Coefficient, %/% | Response Time–Reset Time | Transducing Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Organic film transistor | 40–300 | 40 | N/A | N/A | 15–10 min–15 min | Chemiresistive | [25] |
| RGO-PVR film on THS | 5–95 | N/A | –600% | 6.7 | 2.8 s–3.5 s | Chemiresistive | [3] |
| Chitosan acetate on disk PZT | 20–60 | 1.5 | 54 | 1.35 | N/A–<300 s | Change of film viscosity | Current work |
| Chitosan acetate on glass | 20–60 | 5 | 96 | 2.4 | N/A–<180 s | Chemiresistive | Current work |
| Chitosan acetate on resonator with lateral electric field | 20–60 | 5 | –4.9 | –0.12 | N/A–<300 s | Change of the maximum of the real part of electric impedance | Current work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, B.D.; Teplykh, A.A.; Fedorov, F.S.; Grebenko, A.K.; Nasibulin, A.G.; Semyonov, A.P.; Borodina, I.A. Evaluation of Elastic Properties and Conductivity of Chitosan Acetate Films in Ammonia and Water Vapors Using Acoustic Resonators. Sensors 2020, 20, 2236. https://doi.org/10.3390/s20082236
Zaitsev BD, Teplykh AA, Fedorov FS, Grebenko AK, Nasibulin AG, Semyonov AP, Borodina IA. Evaluation of Elastic Properties and Conductivity of Chitosan Acetate Films in Ammonia and Water Vapors Using Acoustic Resonators. Sensors. 2020; 20(8):2236. https://doi.org/10.3390/s20082236
Chicago/Turabian StyleZaitsev, Boris D., Andrey A. Teplykh, Fedor S. Fedorov, Artem K. Grebenko, Albert G. Nasibulin, Alexander P. Semyonov, and Irina A. Borodina. 2020. "Evaluation of Elastic Properties and Conductivity of Chitosan Acetate Films in Ammonia and Water Vapors Using Acoustic Resonators" Sensors 20, no. 8: 2236. https://doi.org/10.3390/s20082236