Carbon Nanotube Yarn Microelectrodes Promote High Temporal Measurements of Serotonin Using Fast Scan Cyclic Voltammetry
Abstract
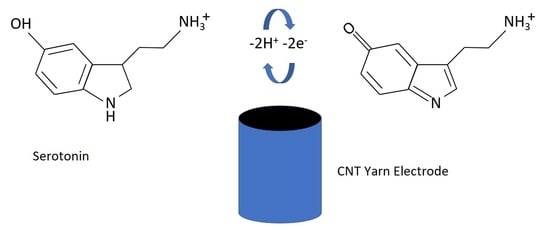
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Electrochemical Measurements
3.2. Adsorption Control
3.3. Anti-Fouling Properties
3.4. High Temporal Measurements (Wave Application Frequency Independence)
4. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zestos, A.; Carpenter, C.A.; Kim, Y.; Low, M.J.; Kennedy, R.T.; Gnegy, M.E. Ruboxistaurin Reduces Cocaine-Stimulated Increases in Extracellular Dopamine by Modifying Dopamine-Autoreceptor Activity. ACS Chem. Neurosci. 2019, 10, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, P.; Dankoski, E.; Petrovic, J.; Keithley, R.; Wightman, R.M. Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Anal. Chem. 2009, 81, 9462–9471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zestos, A.; Luna-Munguia, H.; Stacey, W.C.; Kennedy, R.T. Use and Future Prospects of in Vivo Microdialysis for Epilepsy Studies. ACS Chem. Neurosci. 2018, 10, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Luna-Munguia, H.; Zestos, A.; Gliske, S.V.; Kennedy, R.T.; Stacey, W.C. Chemical biomarkers of epileptogenesis and ictogenesis in experimental epilepsy. Neurobiol. Dis. 2019, 121, 177–186. [Google Scholar] [CrossRef]
- Jun, H.; Yu, H.; Gong, J.; Jiang, J.; Qiao, X.; Perkey, E.; Kim, N.-I.; Emont, M.; Zestos, A.; Cho, J.-S.; et al. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat. Med. 2018, 24, 814–822. [Google Scholar] [CrossRef]
- Zestos, A.; Kennedy, R.T. Microdialysis Coupled with LC-MS/MS for In Vivo Neurochemical Monitoring. AAPS J. 2017, 19, 1284–1293. [Google Scholar] [CrossRef]
- Yokoi, F.; Gründer, G.; Biziere, K.; Stephane, M.; Dogan, A.S.; Dannals, R.F.; Ravert, H.; Suri, A.; Bramer, S.; Wong, D.F. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): A study using positron emission tomography and [11C] raclopride. Neuropsychopharmacology 2002, 27, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.; Fernandes, P.V.; Pereira, C.; Silva, F. Electrochemical sensors and biosensors for determination of catecholamine neurotransmitters: A review. Talanta 2016, 160, 653–679. [Google Scholar] [CrossRef]
- Raju, D.; Mendoza, A.; Wonnenberg, P.; Mohanaraj, S.; Sarbanes, M.; Truong, C.; Zestos, A. Polymer modified carbon fiber-microelectrodes and waveform modifications enhance neurotransmitter metabolite detection. Anal. Methods 2019, 11, 1620–1630. [Google Scholar] [CrossRef]
- Zestos, A.; Nguyen, M.D.; Poe, B.L.; Jacobs, C.; Venton, B.J. Epoxy insulated carbon fiber and carbon nanotube fiber microelectrodes. Sens. Actuators B Chem. 2013, 182, 652–658. [Google Scholar] [CrossRef]
- Wang, J.; Deo, R.P.; Poulin, P.; Mangey, M. Carbon nanotube fiber microelectrodes. J. Am. Chem. Soc. 2003, 125, 14706–14707. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Jacobs, C.; Vickrey, T.L.; Venton, B.J. Functional groups modulate the sensitivity and electron transfer kinetics of neurochemicals at carbon nanotube modified microelectrodes. Analyst 2011, 136, 3557–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, N.; Venton, B.J. Rapid, sensitive detection of neurotransmitters at microelectrodes modified with self-assembled SWCNT forests. Anal. Chem. 2012, 84, 7816–7822. [Google Scholar] [CrossRef] [PubMed]
- Zestos, A. Carbon Nanoelectrodes for the Electrochemical Detection of Neurotransmitters. Int. J. Electrochem. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Peairs, M.J.; Ross, A.E.; Venton, B.J. Comparison of Nafion- and overoxidized polypyrrole-carbon nanotube electrodes for neurotransmitter detection. Anal. Methods 2011, 3, 2379–2386. [Google Scholar] [CrossRef]
- Mohanaraj, S.; Wonnenberg, P.; Cohen, B.; Zhao, H.; Hartings, M.R.; Zou, S.; Fox, U.M.; Zestos, A. Gold Nanoparticle Modified Carbon Fiber Microelectrodes for Enhanced Neurochemical Detection. J. Vis. Exp. 2019, e59552. [Google Scholar] [CrossRef]
- Zestos, A.; Yang, C.; Jacobs, C.; Hensley, D.; Venton, B.J. Carbon nanospikes grown on metal wires as microelectrode sensors for dopamine. Analyst 2015, 140, 7283–7292. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Jacobs, C.; Nguyen, M.D.; Ganesana, M.; Zestos, A.; Ivanov, I.N.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Venton, B.J. Carbon Nanotubes Grown on Metal Microelectrodes for the Detection of Dopamine. Anal. Chem. 2015, 88, 645–652. [Google Scholar] [CrossRef]
- Vigolo, B. Macroscopic Fibers and Ribbons of Oriented Carbon Nanotubes. Science 2000, 290, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, E.; Suh, D.-S.; Collins, S.; Selvidge, M.; Dalton, A.; Kim, B.G.; Razal, J.M.; Ussery, G.; Rinzler, A.G.; Martínez, M.T.; et al. Highly Conducting Carbon Nanotube/Polyethyleneimine Composite Fibers. Adv. Mater. 2005, 17, 1064–1067. [Google Scholar] [CrossRef]
- Zestos, A.; Jacobs, C.; Trikantzopoulos, E.; Ross, A.E.; Venton, B.J. Polyethylenimine Carbon Nanotube Fiber Electrodes for Enhanced Detection of Neurotransmitters. Anal. Chem. 2014, 86, 8568–8575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harreither, W.; Trouillon, R.; Poulin, P.; Neri, W.; Ewing, A.G.; Safina, G. Carbon Nanotube Fiber Microelectrodes Show a Higher Resistance to Dopamine Fouling. Anal. Chem. 2013, 85, 7447–7453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zestos, A. Novel Carbon-Based Microelectrodes for Neurotransmitter Detection; University of Virginia: Charlottesville, VA, USA, 2014. [Google Scholar]
- Zestos, A.; Venton, B.J. Carbon Nanotube-Based Microelectrodes for Enhanced Neurochemical Detection. ECS Trans. 2017, 80, 1497–1509. [Google Scholar] [CrossRef]
- Jacobs, C.; Ivanov, I.N.; Nguyen, M.D.; Zestos, A.; Venton, B.J. High Temporal Resolution Measurements of Dopamine with Carbon Nanotube Yarn Microelectrodes. Anal. Chem. 2014, 86, 5721–5727. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Trikantzopoulos, E.; Jacobs, C.B.; Venton, B.J. Evaluation of carbon nanotube fiber microelectrodes for neurotransmitter detection: Correlation of electrochemical performance and surface properties. Anal. Chim. Acta 2017, 965, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wang, Y.; Jacobs, C.B.; Ivanov, I.N.; Venton, B.J. O2 Plasma Etching and Antistatic Gun Surface Modifications for CNT Yarn Microelectrode Improve Sensitivity and Antifouling Properties. Anal. Chem. 2017, 89, 5605–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zestos, A.; Venton, B.J. Communication—Carbon Nanotube Fiber Microelectrodes for High Temporal Measurements of Dopamine. J. Electrochem. Soc. 2018, 165, 3071–3073. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Wang, X.; Zhu, Y.; Sombers, L.A. Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue. ACS Nano 2013, 7, 7864–7873. [Google Scholar] [CrossRef]
- Weese, M.E.; Krevh, R.A.; Li, Y.; Alvarez, N.T.; Ross, A.E. Defect Sites Modulate Fouling Resistance on Carbon-Nanotube Fiber Electrodes. ACS Sensors 2019, 4, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Jakubinek, M.B.; Johnson, M.B.; White, M.A.; Jayasinghe, C.; Li, G.; Cho, W.; Schulz, M.J.; Shanov, V. Thermal and electrical conductivity of array-spun multi-walled carbon nanotube yarns. Carbon 2012, 50, 244–248. [Google Scholar] [CrossRef]
- Abot, J.L.; Song, Y.; Vatsavaya, M.S.; Medikonda, S.; Kier, Z.; Jayasinghe, C.; Rooy, N.; Shanov, V.; Schulz, M. Delamination detection with carbon nanotube thread in self-sensing composite materials. Compos. Sci. Technol. 2010, 70, 1113–1119. [Google Scholar] [CrossRef]
- Patel, A.; Tan, S.-Y.; Miller, T.; MacPherson, J.; Unwin, P.R. Comparison and Reappraisal of Carbon Electrodes for the Voltammetric Detection of Dopamine. Anal. Chem. 2013, 85, 11755–11764. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Wen, J.; Sennett, M.; Gibson, H.; Ren, Z. Effect of nickel, iron and cobalt on growth of aligned carbon nanotubes. Appl. Phys. A 2002, 74, 387–391. [Google Scholar] [CrossRef]
- Deck, C.P.; Vecchio, K. Prediction of carbon nanotube growth success by the analysis of carbon–catalyst binary phase diagrams. Carbon 2006, 44, 267–275. [Google Scholar] [CrossRef]
- Heien, M.L.A.V.; Phillips, P.E.M.; Stuber, G.D.; Seipel, A.T.; Wightman, R.M. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 2003, 128, 1413–1419. [Google Scholar] [CrossRef]
- Takmakov, P.; Zachek, M.K.; Keithley, R.; Walsh, P.L.; Donley, C.; Mccarty, G.S.; Wightman, R.M. Carbon Microelectrodes with a Renewable Surface. Anal. Chem. 2010, 82, 2020–2028. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Miller, G.W.; Alter, S.; Strong, R.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J. Neurochem. 2013, 126, 591–603. [Google Scholar] [CrossRef]
- Carpenter, C.; Zestos, A.; Altshuler, R.; Sorenson, R.J.; Guptaroy, B.; Showalter, H.D.; Kennedy, R.T.; Jutkiewicz, E.; Gnegy, M.E. Direct and Systemic Administration of a CNS-Permeant Tamoxifen Analog Reduces Amphetamine-Induced Dopamine Release and Reinforcing Effects. Neuropsychopharmacology 2017, 42, 1940–1949. [Google Scholar] [CrossRef] [Green Version]
- Zestos, A.G.; Mikelman, S.R.; Kennedy, R.T.; Gnegy, M.E. PKCβ inhibitors attenuate amphetamine-stimulated dopamine efflux. ACS Chem. Neurosci. 2016, 7, 757–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, M.J.; Nemeroff, C.B. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clin. Chem. 1994, 40, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, A.; Atcherley, C.W.; Pathirathna, P.; Samaranayake, S.; Qiang, B.; Peña, E.; Morgan, S.L.; Heien, M.L.; Hashemi, P. In Vivo Ambient Serotonin Measurements at Carbon-Fiber Microelectrodes. Anal. Chem. 2017, 89, 9703–9711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, K.M.; Hashemi, P. Fast-Scan Cyclic Voltammetry Analysis of Dynamic Serotonin Reponses to Acute Escitalopram. ACS Chem. Neurosci. 2013, 4, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.M.; Zeqja, A.; Nijhout, H.F.; Reed, M.C.; Best, J.; Hashemi, P. Voltammetric and mathematical evidence for dual transport mediation of serotonin clearance in vivo. J. Neurochem. 2014, 130, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Guell, A.; Meadows, K.E.; Unwin, P.R.; MacPherson, J. Trace voltammetric detection of serotonin at carbon electrodes: Comparison of glassy carbon, boron doped diamond and carbon nanotube network electrodes. Phys. Chem. Chem. Phys. 2010, 12, 10108–10114. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, A.; Asrat, T.; Liu, F.; Wonnenberg, P.; Zestos, A.G. Carbon Nanotube Yarn Microelectrodes Promote High Temporal Measurements of Serotonin Using Fast Scan Cyclic Voltammetry. Sensors 2020, 20, 1173. https://doi.org/10.3390/s20041173
Mendoza A, Asrat T, Liu F, Wonnenberg P, Zestos AG. Carbon Nanotube Yarn Microelectrodes Promote High Temporal Measurements of Serotonin Using Fast Scan Cyclic Voltammetry. Sensors. 2020; 20(4):1173. https://doi.org/10.3390/s20041173
Chicago/Turabian StyleMendoza, Alexander, Thomas Asrat, Favian Liu, Pauline Wonnenberg, and Alexander G. Zestos. 2020. "Carbon Nanotube Yarn Microelectrodes Promote High Temporal Measurements of Serotonin Using Fast Scan Cyclic Voltammetry" Sensors 20, no. 4: 1173. https://doi.org/10.3390/s20041173