Adaptive Echolocation and Flight Behaviors in Bats Can Inspire Technology Innovations for Sonar Tracking and Interception
Abstract
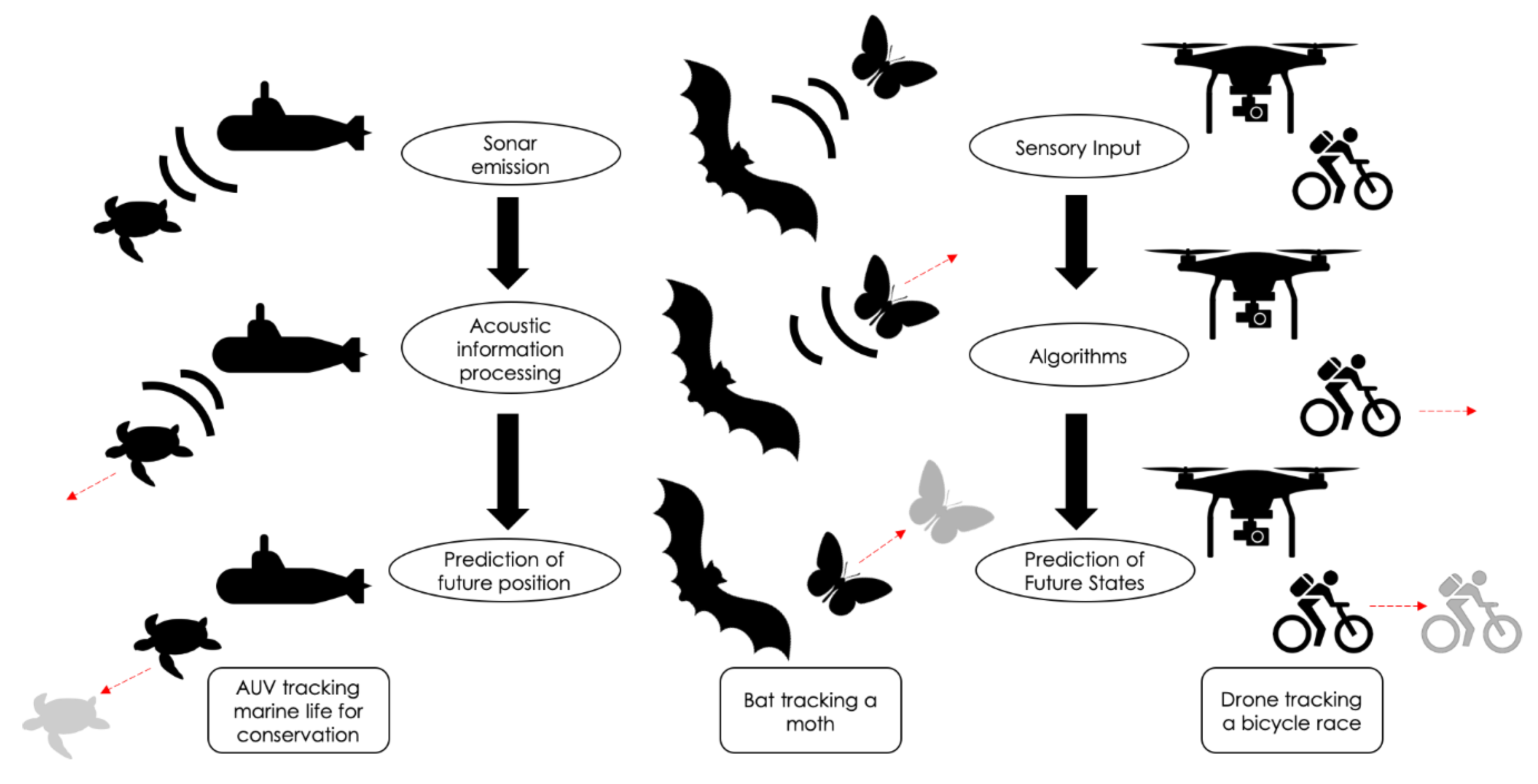
1. Introduction
2. Echolocation in Bats
3. Target Tracking by Echolocating Bats
3.1. Sonar Tracking Behaviors
3.2. Tracking Evasive Prey
4. Adaptive Bat Echolocation Behaviors Inspire Artificial Sonar Tracking Systems
- Bats use echolocation to sense distance and can identify and categorize targets relative to background barriers.
- Bats fly randomly, varying the frequency and intensity of narrowband echolocation signals to detect prey, and can adjust the parameters of their sonar sounds relative to their distance to the target.
- Call intensity varies, from a large positive value to a minimum constant value.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thrun, S. Robotic Mapping: A Survey. In Exploring Artificial Intelligence in the New Millennium; Morgan Kaufmann Publishers Inc.: San Francisco, CA, USA, 2003; pp. 1–35. [Google Scholar]
- Steckel, J.; Peremans, H. BatSLAM: Simultaneous Localization and Mapping Using Biomimetic Sonar. PLoS ONE 2013, 8, e54076. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.M.; Krauchunas, S.M.; Eddy, M.; McBeath, M.K. How Dogs Navigate to Catch Frisbees. Am. Psychol. Soc. 2003, 15, 437–441. [Google Scholar]
- McBeath, M.K.; Shaffer, D.M.; Kaiser, M.K. How Baseball Outfielders Determine Where to Run to Catch Fly Balls. Science 1995, 268, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Olberg, R.M.; Worthington, A.H.; Venator, K.R. Prey Pursuit and Interception in Dragonflies. J. Comp. Physiol. A 2000, 186, 155–162. [Google Scholar] [CrossRef]
- Mischiati, M.; Lin, H.-T.; Herold, P.; Imler, E.; Olberg, R.; Leonardo, A. Internal Models Direct Dragonfly Interception Steering. Nature 2014, 517, 333–338. [Google Scholar] [CrossRef]
- Yang, X.-S.; Deb, S. Engineering Optimisation by Cuckoo Search. Int. J. Math. Model. Numer. Optim. 2010, 1, 330–343. [Google Scholar] [CrossRef]
- Dorigo, M.; Maniezzo, V.; Colorni, A. Ant System: Optimization by a Colony of Cooperating Agents. IEEE Trans. Syst. Man Cybern. Part B Cybern. 1996, 26, 29–41. [Google Scholar] [CrossRef]
- Yang, X.-S. Firefly Algorithm, Stochastic Test Functions and Design Optimisation. Int. J. Bio-Inspired Comput. 2010, 2, 78–84. [Google Scholar] [CrossRef]
- Norberg, U.M. Evolution of Vertebrate Flight: An Aerodynamic Model for the Transition from Gliding to Active Flight. Am. Nat. 1985, 126, 303–327. [Google Scholar] [CrossRef]
- Chiu, C.; Xian, W.; Moss, C.F. Adaptive echolocation behavior in bats for the analysis of auditory scenes. J. Exp. Boil. 2009, 212, 1392–1404. [Google Scholar] [CrossRef]
- Chiu, C.; Reddy, P.V.; Xian, W.; Krishnaprasad, P.S.; Moss, C.F. Effects of competitive prey capture on flight behavior and sonar beam pattern in paired big brown bats, Eptesicus fuscus. J. Exp. Boil. 2010, 213, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Jakobsen, L.; Surlykke, A.; Moss, C.F. Bats coordinate sonar and flight behavior as they forage in open and cluttered environments. J. Exp. Boil. 2014, 217, 4356–4364. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.B.; Simmons, N.B. Bats; University of Chicago Press: Chicago, IL, USA, 2015; Available online: https://www.press.uchicago.edu/ucp/books/book/chicago/B/bo17089187.html (accessed on 13 March 2020).
- Fenton, M.B.; Grinnell, A.; Popper, A.N.; Fay, R.R.; Acoustical Society of America (Eds.) Bat Bioacoustics. In Springer Handbook of Auditory Research; ASA Press/Springer: New York, NY, USA, 2016; Volume 54. [Google Scholar]
- Webster, F.A.; Brazier, O.G. Experimental Studies on Target Detection, Evaluation and Interception by Echolocating Bats. In Biological Information Handling Systems and Their Functional Analogs; Aerospace Medical Res. Lab.: Wright-Patterson Airforce Base, OH, USA, 1965. [Google Scholar]
- Schnitzler, H.-U.; Kalko, E.K.V. Echolocation by Insect-Eating Bats: We Define Four Distinct Functional Groups of Bats and Find Differences in Signal Structure That Correlate with the Typical Echolocation Tasks Faced by Each Group. BioScience 2001, 51, 557–569. [Google Scholar] [CrossRef]
- Simmons, J.A. The Resolution of Target Range by Echolocating bats. J. Acoust. Soc. Am. 1973, 54, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.; Moss, C.F.; Ferragamo, M. Convergence of Temporal and Spectral Information into Acoustic Images of Complex Sonar Targets Perceived by the Echolocating Bat, Eptesicus Fuscus. J. Comp. Physiol. A 1990, 166. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.F.; Surlykke, A. Auditory Scene Analysis by Echolocation in Bats. J. Acoust. Soc. Am. 2001, 110, 2207–2226. [Google Scholar] [CrossRef]
- Müller, R. A Numerical Study of the Role of the Tragus in the Big Brown Bat. J. Acoust. Soc. Am. 2004, 116, 3701–3712. [Google Scholar] [CrossRef]
- Chiu, C.; Moss, C.F. The Role of the External Ear in Vertical Sound Localization in the Free Flying Bat, Eptesicus Fuscus. J. Acoust. Soc. Am. 2007, 121, 2227–2235. [Google Scholar] [CrossRef]
- Simmons, J.A.; Kick, S.A.; Lawrence, B.D.; Hale, C.; Bard, C. Acuity of Horizontal Angle Discrimination by the Echolocating Bat, Eptesicus fuscus. J. Comp. Physiol. A 1983, 153, 321–330. [Google Scholar] [CrossRef]
- Wohlgemuth, M.; Luo, J.; Moss, C.F. Three-Dimensional Auditory Localization in the Echolocating Bat. Curr. Opin. Neurobiol. 2016, 41, 78–86. [Google Scholar] [CrossRef]
- Wohlgemuth, M.; Kothari, N.B.; Moss, C.F. Action Enhances Acoustic Cues for 3-D Target Localization by Echolocating Bats. PLoS Boil. 2016, 14, e1002544. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.F.; Schnitzler, H.-U. Behavioral Studies of Auditory Information Processing. In Hearing by Bats; Springer Handbook of Auditory Research; Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 1995; pp. 87–145. [Google Scholar] [CrossRef]
- Moss, C.F.; Chiu, C.; Surlykke, A. Adaptive Vocal Behavior Drives Perception by Echolocation in Bats. Curr. Opin. Neurobiol. 2011, 21, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Von Der Emde, G.; Schnitzler, H.-U. Fluttering Target Detection in Hipposiderid Bats. J. Comp. Physiol. A 1986, 159, 765–772. [Google Scholar] [CrossRef]
- Schnitzler, H.-U.; Flieger, E. Detection of Oscillating Target Movements by Echolocation in the Greater Horseshoe Bat. J. Comp. Physiol. A 1983, 153, 385–391. [Google Scholar] [CrossRef]
- Schnitzler, H.-U.; Moss, C.F.; Denzinger, A. From Spatial Orientation to Food Acquisition in Echolocating Bats. Trends Ecol. Evol. 2003, 18, 386–394. [Google Scholar] [CrossRef]
- Schnitzler, H.-U. The Ultrasonic Locating Sounds of the Horseshoe Bats (Chiroptera Rhinolophidae) in Various Orientation Situations. Z. Vergl. Physiol. 1968, 57, 376–408. [Google Scholar] [CrossRef]
- Neuweiler, G.; Bruns, V.; Schüller, G. Ears Adapted for the Detection of motion, or how echolocating bats have exploited the capacities of the mammalian auditory system. J. Acoust. Soc. Am. 1980, 68, 741–753. [Google Scholar] [CrossRef]
- Suga, N. Biosonar and Neural Computation in Bats. Sci. Am. 1990, 262, 60–68. [Google Scholar] [CrossRef]
- Kalko, E.K.V.; Schnitzler, H.-U. The Echolocation and Hunting Behavior of Daubenton’s Bat, Myotis Daubentoni. Behav. Ecol. Sociobiol. 1989, 24, 225–238. [Google Scholar] [CrossRef]
- Sleep, D.J.H.; Brigham, R.M. An Experimental Test of Clutter Tolerance in Bats. J. Mammal. 2003, 84, 216–224. [Google Scholar] [CrossRef]
- Bates, M.; Simmons, J.A.; Zorikov, T.V. Bats Use Echo Harmonic Structure to Distinguish Their Targets from Background Clutter. Science 2011, 333, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.F.; Bohn, K.; Gilkenson, H.; Surlykke, A. Active Listening for Spatial Orientation in a Complex Auditory Scene. PLoS Boil. 2006, 4, e79. [Google Scholar] [CrossRef] [PubMed]
- Yovel, Y.; Falk, B.; Moss, C.F.; Ulanovsky, N. Optimal Localization by Pointing Off Axis. Science 2010, 327, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Ghose, K.; Moss, C.F. The Sonar Beam Pattern of a Flying Bat as it Tracks Tethered Insects. J. Acoust. Soc. Am. 2003, 114, 1120–1131. [Google Scholar] [CrossRef]
- Ghose, K.; Moss, C.F. Steering by Hearing: A Bat’s Acoustic Gaze is Linked to Its Flight Motor Output by a Delayed, Adaptive Linear Law. J. Neurosci. 2006, 26, 1704–1710. [Google Scholar] [CrossRef]
- Petrites, A.E.; Eng, O.S.; Mowlds, D.S.; Simmons, J.A.; Delong, C.M. Interpulse Interval Modulation by Echolocating Big Brown Bats (Eptesicus fuscus) in Different Densities of Obstacle Clutter. J. Comp. Physiol. A 2009, 195, 603–617. [Google Scholar] [CrossRef]
- Sändig, S.; Schnitzler, H.-U.; Denzinger, A. Echolocation Behaviour of the Big Brown Bat (Eptesicus fuscus) in an Obstacle Avoidance Task of Increasing Difficulty. J. Exp. Boil. 2014, 217, 2876–2884. [Google Scholar] [CrossRef]
- Kothari, N.; Wohlgemuth, M.; Hulgard, K.; Surlykke, A.; Moss, C.F. Timing Matters: Sonar Call Groups Facilitate Target Localization in Bats. Front. Physiol. 2014, 5, 168. [Google Scholar] [CrossRef]
- Hiryu, S.; Bates, M.E.; Simmons, J.A.; Riquimaroux, H. FM Echolocating Bats Shift Frequencies to Avoid Broadcast–Echo Ambiguity in Clutter. Proc. Natl. Acad. Sci. USA 2010, 107, 7048–7053. [Google Scholar] [CrossRef]
- Wright, G.S.; Chiu, C.; Xian, W.; Wilkinson, G.S.; Moss, C.F. Social Calls Predict Foraging Success in Big Brown Bats. Curr. Boil. 2014, 24, 885–889. [Google Scholar] [CrossRef]
- Warnecke, M.; Chiu, C.; Engelberg, J.; Moss, C.F. Active Listening in a Bat Cocktail Party: Adaptive Echolocation and Flight Behaviors of Big Brown Bats, Eptesicus fuscus, Foraging in a Cluttered Acoustic Environment. Brain Behav. Evol. 2015, 86, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.J.; Moss, C.F. Sensing in a Noisy World: Lessons from Auditory Specialists, Echolocating Bats. J. Exp. Boil. 2017, 220, 4554–4566. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.J.; Conner, W.E. Bats Jamming Bats: Food competition through Sonar Interference. Science 2014, 346, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.M.R. Interindividual Use of Echolocation Calls: Eavesdropping by Bats. Behav. Ecol. Sociobiol. 1982, 10, 271–275. [Google Scholar] [CrossRef]
- Surlykke, A.; Moss, C.F. Echolocation Behavior of Big Brown Bats, Eptesicus fuscus, in the Field and the Laboratory. J. Acoust. Soc. Am. 2000, 108, 2419–2429. [Google Scholar] [CrossRef]
- Simmons, J.; Fenton, M.; O’Farrell, M. Echolocation and Pursuit of Prey by Bats. Science 1979, 203, 16–21. [Google Scholar] [CrossRef]
- Matsuta, N.; Hiryu, S.; Fujioka, E.; Yamada, Y.; Riquimaroux, H.; Watanabe, Y. Adaptive Beam-Width Control of Echolocation Sounds by CF-FM Bats, Rhinolophus ferrumequinum Nippon, during Prey-Capture Flight. J. Exp. Boil. 2013, 216, 1210–1218. [Google Scholar] [CrossRef]
- Fawcett, K.; Jacobs, D.S.; Surlykke, A.; Ratcliffe, J.M. Echolocation in the Bat, Rhinolophus capensis: The Influence of Clutter, Conspecifics and Prey on Call Design and Intensity. Boil. Open 2015, 4, 693–701. [Google Scholar] [CrossRef]
- Kane, S.A.; Zamani, M. Falcons Pursue Prey Using Visual Motion Cues: New Perspectives from Animal-Borne Cameras. J. Exp. Boil. 2014, 217, 225–234. [Google Scholar] [CrossRef]
- Lanchester, B.S.; Mark, R.F. Pursuit and Prediction in the Tracking of Moving Food by a Teleost Fish (Acanthaluteres spilomelanurus). J. Exp. Boil. 1975, 63, 627–645. [Google Scholar]
- Yager, D.D. Structure, Development, and Evolution of Insect Auditory Systems. Microsc. Res. Tech. 1999, 47, 380–400. [Google Scholar] [CrossRef]
- Miller, L.A.; Surlykke, A. How Some Insects Detect and Avoid Being Eaten by Bats: Tactics and Countertactics of Prey and Predator. BioScience 2001, 51, 570. [Google Scholar] [CrossRef]
- Hoy, R.; Nolen, T.; Brodfuehrer, P. The Neuroethology of Acoustic Startle and Escape in Flying Insects. J. Exp. Boil. 1989, 146, 287–306. [Google Scholar]
- Ter Hofstede, H.M.; Ratcliffe, J.M. Evolutionary Escalation: The Bat–Moth Arms Race. J. Exp. Boil. 2016, 219, 1589–1602. [Google Scholar] [CrossRef]
- Forrest, T.G.; E Farris, H.; Hoy, R.R. Ultrasound Acoustic Startle Response in Scarab Beetles. J. Exp. Boil. 1995, 198, 2593–2598. [Google Scholar]
- Yager, D.D.; Spangler, H.G. Behavioral Response to Ultrasound by the Tiger Beetle Cicindela Marutha Dow Combines Aerodynamic Changes and Sound Production. J. Exp. Boil. 1997, 200, 11. [Google Scholar]
- Miller, L.A.; Olesen, J. Avoidance Behavior in Green Lacewings. J. Comp. Physiol. A 1979, 131, 113–120. [Google Scholar] [CrossRef]
- Miller, L.A. The Orientation and Evasive Behavior of Insects to Bat Cries. In Exogenous and Endogenous Influences on Metabolic and Neural Control, vol 1.; Addink, D.F., Spronk, N., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 393–405. [Google Scholar]
- Miller, L.A. How Insects Detect and Avoid Bats. In Neuroethology and Behavioral Physiology; Huber, F., Markl, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 251–266. [Google Scholar] [CrossRef]
- Corcoran, A.J.; Barber, J.; Conner, W.E. Tiger Moth Jams Bat Sonar. Science 2009, 325, 325–327. [Google Scholar] [CrossRef]
- Corcoran, A.J.; Barber, J.; Hristov, N.I.; Conner, W.E.; Lin, H.-T.; Trimmer, B.A. How Do Tiger Moths Jam Bat Sonar? J. Exp. Boil. 2011, 214, 2416–2425. [Google Scholar] [CrossRef]
- Ghose, K.; Horiuchi, T.K.; Krishnaprasad, P.S.; Moss, C.F. Echolocating Bats Use a Nearly Time-Optimal Strategy to Intercept Prey. PLoS Boil. 2006, 4, e108. [Google Scholar] [CrossRef]
- Rafie-Rad, M. Time-Optimal Solutions of Parallel Navigation and Finsler Geodesics. Nonlinear Anal. Real World Appl. 2010, 11, 3809–3814. [Google Scholar] [CrossRef]
- Fabian, S.T.; Sumner, M.E.; Wardill, T.J.; Rossoni, S.; Gonzalez-Bellido, P.T. Interception by Two Predatory Fly Species is Explained by a Proportional Navigation Feedback Controller. J. R. Soc. Interface 2018, 15, 20180466. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, A.; Chahl, J.; Srinivasan, M.V. Motion Camouflage in Dragonflies. Nature 2003, 423, 604. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.; Justh, E.W.; Krishnaprasad, P.S. Motion Camouflage with Sensorimotor Delay. In Proceedings of the 2007 46th IEEE Conference on Decision and Control, New Orleans, LA, USA, 12–14 December 2007; pp. 1660–1665. [Google Scholar]
- Masters, W.; Moffat, A.; Simmons, J. Sonar tracking of horizontally moving targets by the big brown bat Eptesicus fuscus. Science 1985, 228, 1331–1333. [Google Scholar] [CrossRef]
- Campbell, K.A.; Suthers, R.A. Predictive Tracking of Horizontally Moving Targets by the Fishing Bat, Noctilio leporinus. In Animal Sonar; Springer: Boston, MA, USA, 1988; pp. 501–506. [Google Scholar]
- Erwin, H.R. Algorithms for Sonar Tracking in Biomimetic Robotics. In School of Computing and Technology; University of Sunderland: Sunderland, UK, 2001. [Google Scholar]
- Fujioka, E.; Aihara, I.; Sumiya, M.; Aihara, K.; Hiryu, S. Echolocating bats use future-target information for optimal foraging. Proc. Natl. Acad. Sci. USA 2016, 113, 4848–4852. [Google Scholar] [CrossRef]
- Salles, A.; Diebold, C.; Moss, C.F. Prediction strategies for target tracking in the echolocating bat, Eptesicus fuscus. In Proceedings of the Society for Neuroscience Meeting, Chicago, IL, USA, 19–23 October 2019. [Google Scholar]
- Yang, X.-S. A New Metaheuristic Bat-Inspired Algorithm. In Nature Inspired Cooperative Strategies for Optimization (NICSO 2010); Studies in Computational Intelligence; González, J.R., Pelta, D.A., Cruz, C., Terrazas, G., Krasnogor, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 65–74. [Google Scholar] [CrossRef]
- Yang, X.-S.; Gandomi, A.H. Bat Algorithm: A Novel Approach for Global Engineering Optimization. Eng. Comput. 2012, 29, 464–483. [Google Scholar] [CrossRef]
- Gandomi, A.H.; Yang, X.-S.; Alavi, A.H.; Talatahari, S. Bat Algorithm for Constrained Optimization Tasks. Neural Comput. Appl. 2012, 22, 1239–1255. [Google Scholar] [CrossRef]
- Chakri, A.; Khelif, R.; Benouaret, M.; Yang, X.-S. New directional bat algorithm for continuous optimization problems. Expert Syst. Appl. 2017, 69, 159–175. [Google Scholar] [CrossRef]
- Srivastava, S.; Sahana, S. Application of Bat Algorithm for Transport Network Design Problem. Appl. Comput. Intell. Soft Comput. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Ma, X.-X.; Wang, J.-S. Optimized Parameter Settings of Binary Bat Algorithm for Solving Function Optimization Problems. J. Electr. Comput. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Mohsen, M.; Islam, M.N.; Karimi, R. Simultaneous Localization and Mapping: Issues and Approaches. Int. J. Comput. Sci. Telecommun. 2013, 4, 1–7. [Google Scholar]
- Milford, M.; Wyeth, G.; Prasser, D. RatSLAM: A Hippocampal Model for Simultaneous Localization and Mapping. In Proceedings of the IEEE International Conference on Robotics and Automation, 2004. Proceedings, ICRA ’04. 2004, New Orleans, LA, USA, 26 April–1 May 2004; Volume 1, pp. 403–408. [Google Scholar] [CrossRef]
- Steckel, J.; Peremans, H. Spatial Sampling Strategy for a 3D Sonar Sensor Supporting BatSLAM. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 723–728. [Google Scholar]
- Chen, M.; Hu, W. Research on BatSLAM Algorithm for UAV Based on Audio Perceptual Hash Closed-Loop Detection. Int. J. Pattern Recognit. Artif. Intell. 2018, 33, 1959002. [Google Scholar] [CrossRef]
- Siebel, N.T. Design and Implementation of People Tracking Algorithms for Visual Surveillance Applications. Ph.D. Thesis, University of Reading, Reading, UK, March 2003. [Google Scholar]
- Goobic, A.; Welser, M.; Acton, S.; Ley, K. Biomedical application of target tracking in clutter. In Proceedings of the Conference Record of Thirty-Fifth Asilomar Conference on Signals, Systems and Computers (Cat.No.01CH37256), Pacific Grove, CA, USA, 4–7 November 2001. [Google Scholar]
- Pan, J.; Hu, B.; Zhang, J.Q. An Efficient Object Tracking Algorithm with Adaptive Prediction of Initial Searching Point. In Advances in Image and Video Technology; Chang, L.-W., Lie, W.-N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4319, pp. 1113–1122. Available online: http://link.springer.com/10.1007/11949534_112 (accessed on 22 January 2020).
- Julier, S.J.; Uhlmann, J. A counter example to the theory of simultaneous localization and map building. In Proceedings of the 2001 ICRA. IEEE International Conference on Robotics and Automation (Cat. No.01CH37164), Seoul, Korea, 21–26 May 2001; Volume 4, pp. 4238–4243. [Google Scholar]
- Huang, G.P.; Mourikis, A.I.; Roumeliotis, S.I. Analysis and improvement of the consistency of extended Kalman filter based SLAM. In Proceedings of the 2008 IEEE International Conference on Robotics and Automation, Pasadena, CA, USA, 19–23 May 2008; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2008; pp. 473–479. [Google Scholar]
- Julier, S.J.; Uhlmann, J.K. A General Method for Approximating Nonlinear Transformations of Probability Distributions; Technical report for Robotics Research Group: Oxford, UK, 1996. [Google Scholar]
- Julier, S.J.; Uhlmann, J. New Extension of the Kalman Filter to Nonlinear Systems. In Signal Processing, Sensor Fusion, and Target Recognition VI; International Society for Optics and Photonics: Bellingham, WA, USA, 1997; Volume 3068, pp. 182–193. [Google Scholar] [CrossRef]
- Blackman, S. Multiple Hypothesis Tracking for Multiple Target Tracking. IEEE Aerosp. Electron. Syst. Mag. 2004, 19, 5–18. [Google Scholar] [CrossRef]
- Erwin, H.R.; Wilson, W.W.; Moss, C.F. A Computational Sensorimotor Model of Bat Echolocation. J. Acoust. Soc. Am. 2001, 110, 1176–1187. [Google Scholar] [CrossRef]
- Mao, B.; Aytekin, M.; Wilkinson, G.S.; Moss, C.F. Big Brown Bats (Eptesicus fuscus) Reveal Diverse Strategies for Sonar Target tracking in Cluttera. J. Acoust. Soc. Am. 2016, 140, 1839–1849. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diebold, C.A.; Salles, A.; Moss, C.F. Adaptive Echolocation and Flight Behaviors in Bats Can Inspire Technology Innovations for Sonar Tracking and Interception. Sensors 2020, 20, 2958. https://doi.org/10.3390/s20102958
Diebold CA, Salles A, Moss CF. Adaptive Echolocation and Flight Behaviors in Bats Can Inspire Technology Innovations for Sonar Tracking and Interception. Sensors. 2020; 20(10):2958. https://doi.org/10.3390/s20102958
Chicago/Turabian StyleDiebold, Clarice Anna, Angeles Salles, and Cynthia F. Moss. 2020. "Adaptive Echolocation and Flight Behaviors in Bats Can Inspire Technology Innovations for Sonar Tracking and Interception" Sensors 20, no. 10: 2958. https://doi.org/10.3390/s20102958
APA StyleDiebold, C. A., Salles, A., & Moss, C. F. (2020). Adaptive Echolocation and Flight Behaviors in Bats Can Inspire Technology Innovations for Sonar Tracking and Interception. Sensors, 20(10), 2958. https://doi.org/10.3390/s20102958





