Real-Time Optical Detection of Isoleucine in Living Cells through a Genetically-Encoded Nanosensor
Abstract
:1. Introduction
2. Materials and Methods
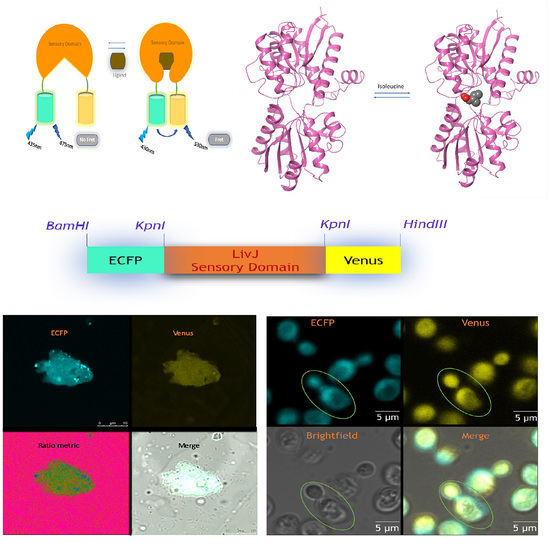
2.1. Molecular Modeling
2.2. Desiging and Construction of Nanosensor
2.3. Expression and Purification of the Nanosensor
2.4. Characterization of the Nanosensor
2.5. Affinity Mutants
2.6. Monitoring of Isoleucine in Living Bacterial Cells
2.7. Real-Time Monitoring of Isoleucine in Yeast Cells
3. Results and Discussion
3.1. In Silico Docking Studies
3.2. Spectral Analysis of Nanosensor
3.3. Analysis of Specificity of the Nanosensor
3.4. Affinity of the GEII Nanosensor and its Mutants
3.5. Analysis of pH Stability of the Nanosensor
3.6. Measurement of Isoleucine in Live Bacterial Cells Using GEII Nanosensor
3.7. Live cell of Imaging of Isoleucine in Yeast Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doi, M.; Yamaoka, I.; Nakayama, M.; Sugahara, K.; Yoshizawa, F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1683–E1693. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2012, 8, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurtz, P.; Soininen, P.; Kangas, A.J.; Ronnemaa, T.; Lehtimaki, T.; Kahonen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-Chain and Aromatic Amino Acids Are Predictors of Insulin Resistance in Young Adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, K.K.; Seeley, R.J. Physiology. Food as a hormone. Science 2013, 339, 918–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozengurt, E.; Sternini, C. Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol. 2007, 7, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, F.; Wang, G.; Liu, Y.; Liu, D. Partial deficiency of isoleucine impairs root development and alters transcript levels of the genes involved in branched-chain amino acid and glucosinolate metabolism in Arabidopsis. J. Exp. Bot. 2012, 64, 599–612. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, S.; Chico, J.M.; Solano, R. The jasmonate pathway: The ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xu, L.; Shi, J.; Xu, Q.; Chen, N. Effect of transport proteins on l-isoleucine production with the l-isoleucine-producing strain Corynebacterium glutamicum YILW. J. Ind. Microbiol. Biotechnol. 2012, 39, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Beckett, P.R.; Hardin, D.S.; Davis, T.A.; Nguyen, H.V.; Wray-Cahen, D.; Copeland, K.C. Spectrophometric Assay for Measuring Branched-Chain Amino Acid Concentrations: Application for Measuring the Sensitivity of Protein Metabolism to Insulin. Anal. Biochem. 1996, 240, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.; Kramer, R. Mechanism and Regulation of Isoleucine Excretion in Corynebacterium glutamicum. Appl. Environ. Microbiol. 1996, 62, 3238–3244. [Google Scholar]
- Martin, R.E.; Kirk, K. Transport of the essential nutrient isoleucine in human erythrocytes infected with the malaria parasite Plasmodium falciparum. Blood 2007, 109, 2217–2224. [Google Scholar] [CrossRef]
- Bishop, M.J.; Crow, B.; Norton, D.; Kovalcik, K.; George, J.; Bralley, J.A. A simple and selective method for the measurement of leucine and isoleucine from plasma using electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1920–1924. [Google Scholar] [CrossRef]
- Chino, S.; Sakaguchi, A.; Yamoto, R.; Ferri, S.; Sode, K. Branched-chain Amino Acid Biosensing Using Fluorescent Modified Engineered Leucine/Isoleucine/Valine Binding Protein. Int. J. Mol. Sci. 2007, 8, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Mustafi, N.; Grünberger, A.; Kohlheyer, D.; Bott, M.; Frunzke, J. The development and application of a single-cell biosensor for the detection of l-methionine and branched-chain amino acids. Metab. Eng. 2012, 14, 449–457. [Google Scholar] [CrossRef]
- Mohsin, M.; Abdin, M.Z.; Nischal, L.; Kardam, H.; Ahmad, A. Genetically encoded FRET-based nanosensor for in vivo measurement of leucine. Biosens. Bioelectron. 2013, 50, 72–77. [Google Scholar] [CrossRef]
- Ahmad, M.; Ameen, S.; Siddiqi, T.O.; Khan, P.; Ahmad, A. Live cell monitoring of glycine betaine by FRET-based genetically encoded nanosensor. Biosens. Bioelectron. 2016, 86, 169–175. [Google Scholar] [CrossRef]
- Bischof, H.; Rehberg, M.; Stryeck, S.; Artinger, K.; Eroglu, E.; Waldeck-Weiermair, M.; Gottschalk, B.; Rost, R.; Deak, A.T.; Niedrist, T.; et al. Novel genetically encoded fluorescent probes enable real-time detection of potassium in vitro and in vivo. Nat. Commun. 2017, 8, 1422. [Google Scholar] [CrossRef] [PubMed]
- Fehr, M.; Lalonde, S.; Lager, I.; Wolff, M.W.; Frommer, W.B. In Vivo Imaging of the Dynamics of Glucose Uptake in the Cytosol of COS-7 Cells by Fluorescent Nanosensors. J. Biol. Chem. 2003, 278, 19127–19133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Fehr, M.; Frommer, W.B.; Lalonde, S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc. Natl. Acad. Sci. USA 2002, 99, 9846–9851. [Google Scholar] [CrossRef] [Green Version]
- Okumoto, S.; Looger, L.L.; Micheva, K.D.; Reimer, R.J.; Smith, S.J.; Frommer, W.B. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. USA 2005, 102, 8740–8745. [Google Scholar] [CrossRef] [Green Version]
- Ameen, S.; Ahmad, M.; Mohsin, M.; Qureshi, M.I.; Ibrahim, M.M.; Abdin, M.Z.; Ahmad, A. Designing, construction and characterization of genetically encoded FRET-based nanosensor for real time monitoring of lysine flux in living cells. J. Nanobiotechnol. 2016, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Trakhanov, S.; Vyas, N.K.; Luecke, H.; Kristensen, D.M.; Ma, J.; Quiocho, F.A. Ligand-Free and -Bound Structures of the Binding Protein (LivJ) of the Escherichia coli ABC Leucine/Isoleucine/Valine Transport System: Trajectory and Dynamics of the Interdomain Rotation and Ligand Specificity. Biochemistry 2005, 44, 6597–6608. [Google Scholar] [CrossRef]
- Whitfield, J.H.; Zhang, W.H.; Herde, M.K.; Clifton, B.E.; Radziejewski, J.; Janovjak, H.; Henneberger, C.; Jackson, C.J. Construction of a robust and sensitive arginine biosensor through ancestral protein reconstruction. Protein Sci. 2015, 24, 1412–1422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.H.; Herde, M.K.; Mitchell, J.A.; Whitfield, J.H.; Wulff, A.B.; Vongsouthi, V.; Sanchez-Romero, I.; Gulakova, P.E.; Minge, D.; Breithausen, B.; et al. Monitoring hippocampal glycine with the computationally designed optical sensor GlyFS. Nat. Chem. Biol. 2018, 14, 861–869. [Google Scholar] [CrossRef]
- Deuschle, K.; Okumoto, S.; Fehr, M.; Looger, L.L.; Kozhukh, L.; Frommer, W.B. Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci. 2005, 14, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Molden, D.P.; Seegmiller, J.E. Maple syrup urine disease: Branched-chain amino acid concentrations and metabolism in cultured human lymphoblasts. Biochem. Genet. 1976, 14, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, R.; Lütkemeyer, D.; Büntemeyer, H.; Lehmann, J. Effects of dissolved oxygen levels and the role of extra- and intracellular amino acid concentrations upon the metabolism of mammalian cell lines during batch and continuous cultures. Cytotechnology 1998, 26, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Ahmad, A. Genetically-encoded nanosensor for quantitative monitoring of methionine in bacterial and yeast cells. Biosens. Bioelectron. 2014, 59, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, O.; Soleja, N.; Khan, P.; Imtaiyaz Hassan, M.; Mohsin, M. Visualization of thiamine in living cells using genetically encoded fluorescent nanosensor. Biochem. Eng. J. 2019, 146, 170–178. [Google Scholar] [CrossRef]







| Sensor | Mutation | Kd * | Operating Range † | ΔRmax § |
|---|---|---|---|---|
| GEII | Wild-Type | 63 μM ± 6 μM | 3 μM–382 μM | 0.49 |
| GEII-F276R | F276R | 518 nM ± 18 nM | 96 nM–4228 nM | 0.56 |
| GEII-D121M | D121M | 5.3 μM ± 0.5 μM | 0.8 μM–69 μM | 0.53 |
| GEII-S80R | S80R | 411 μM ± 17 μM | 73 μM–1493 μM | 0.50 |
| GEII-Control ¶ | Y202D | >100 mM | Not defined | Not defined |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sharma, M.P.; Alqarawi, A.A.; Hashem, A.; Abd_Allah, E.F.; Ahmad, A. Real-Time Optical Detection of Isoleucine in Living Cells through a Genetically-Encoded Nanosensor. Sensors 2020, 20, 146. https://doi.org/10.3390/s20010146
Singh S, Sharma MP, Alqarawi AA, Hashem A, Abd_Allah EF, Ahmad A. Real-Time Optical Detection of Isoleucine in Living Cells through a Genetically-Encoded Nanosensor. Sensors. 2020; 20(1):146. https://doi.org/10.3390/s20010146
Chicago/Turabian StyleSingh, Shruti, Maheshwar Prasad Sharma, Abdulaziz A. Alqarawi, Abeer Hashem, Elsayed Fathi Abd_Allah, and Altaf Ahmad. 2020. "Real-Time Optical Detection of Isoleucine in Living Cells through a Genetically-Encoded Nanosensor" Sensors 20, no. 1: 146. https://doi.org/10.3390/s20010146