Infrared Spectroscopy
The incorporation of phthalocyanine into the polyaniline chain was confirmed by IR studies.
Figure 2 shows the IR absorption spectrum for PANI-CuPc powder samples. The curve A correspond to the sample of pure polyaniline while curves B and C correspond to the polyaniline containing 5% and 20% CuPc respectively. The characteristic IR absorption intensity due to quinoid ring at 1590 cm
−1 and benzoid ring at 1500 cm
−1 are clearly indicative of these two states in the polymer chain. The C-N stretching vibrations are appeared at 1380 cm
−1 and 1305 cm
−1 in each sample. The benzene ring C-C vibration for stretching is observed at 1463 cm
−1. The IR spectra of polyaniline samples, in presence of phthalocyanine exhibit new absorption peaks distinctly at 1210, 1150, 1095, 950, 900, 830, 770, 740 and 510 cm
−1 which are assignable to phthalocyanine skeletal vibration modes. The absence of peak at 650 cm
−1 assignable to C-Cl vibrations, in the functionalized polymeric samples clearly shows that polymer (polyaniline) gets attached to the phthalocyanine ring via C-Cl termination. The data represented for these polymers is well matched with the reported literature IR data for polyaniline [
10] and phthalocyanine [
11]. Thus, IR studies on functionalized polymer clearly gives an evidence of incorporation of phthalocyanine moieties into the polymer chain.
UV-VIS Spectroscopy
The UV-VIS spectrum of polyaniline depends strongly on its oxidation states.
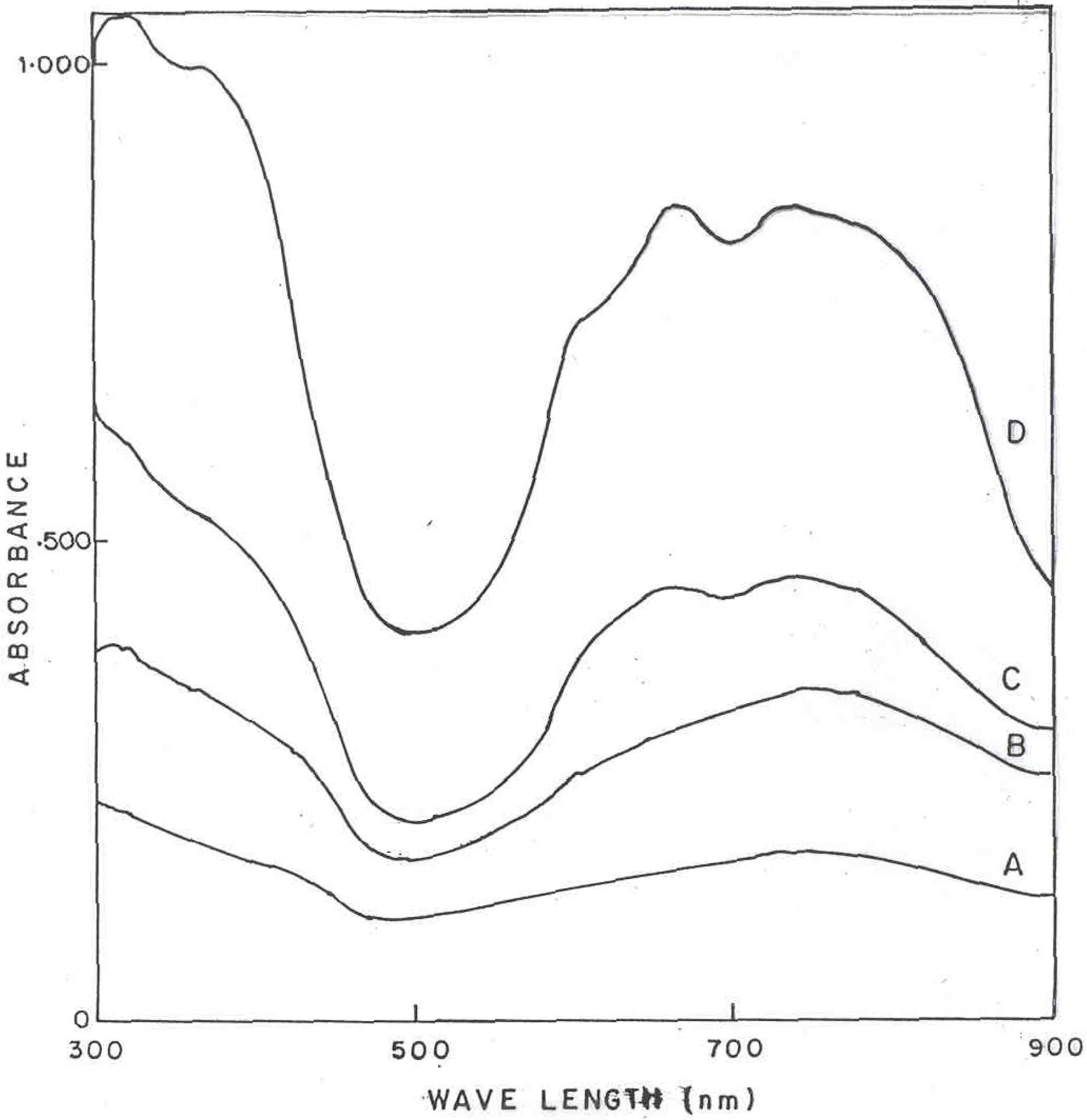
Figure 3 shows the optical absorption spectra (in sulfuric acid solution) of polyaniline and polyaniline functionalized with CuPc samples in the wavelength range 300-900 nm. Curves A to D corresponds to the polyaniline powder samples with CuPc content of 0, 3, 10 and 20 mole percent respectively. It can be seen that there are two new broad absorption bands occurring at 580 nm and 650 nm, which are associated with the chlorinated phthalocyanine (green) moieties [
12]. Pure PANI has a broad absorption band centered at 750 nm, which is associated with the oxidation/doping of the polymer, giving rise to polaronic states within the band gap [
13]. It is interesting to note that the main absorption band at 320 nm and 420 nm of PANI are also affected by the presence of CuPc in the polymer. This can be due to higher degree of conjugation in PANI-CuPc due to the highly conjugated planar CuPc moieties attached to the main chain as well as high level of dopant present in these polymers as compared to pure PANI.
Figure 2.
FT-IR spectrum of PANI-CuPc polymers (A) Pure PANI (B) PANI + 5% CuPc (C) PANI + 20% CuPc.
Figure 2.
FT-IR spectrum of PANI-CuPc polymers (A) Pure PANI (B) PANI + 5% CuPc (C) PANI + 20% CuPc.
Figure 3.
UV-VIS spectrum of PANI-CuPc polymers in dil. H2SO4 (A) Pure PANI (B) PANI + 3% CuPc (C) PANI + 10% CuPc and (D) PANI + 20% CuPc.
Figure 3.
UV-VIS spectrum of PANI-CuPc polymers in dil. H2SO4 (A) Pure PANI (B) PANI + 3% CuPc (C) PANI + 10% CuPc and (D) PANI + 20% CuPc.
The gas sensing ability of pure polyaniline as well as polyaniline functionalized with phthalocyanine was checked. The pure polyaniline does not dissolve in solvents from which it can be cast as a film. Hence, it was mixed with polyethylene oxide-CuCl
2 complex for making surface cells by applying a thin paste of polyaniline dispersed in PEO-CuCl
2 complex on inter-digited PCB [
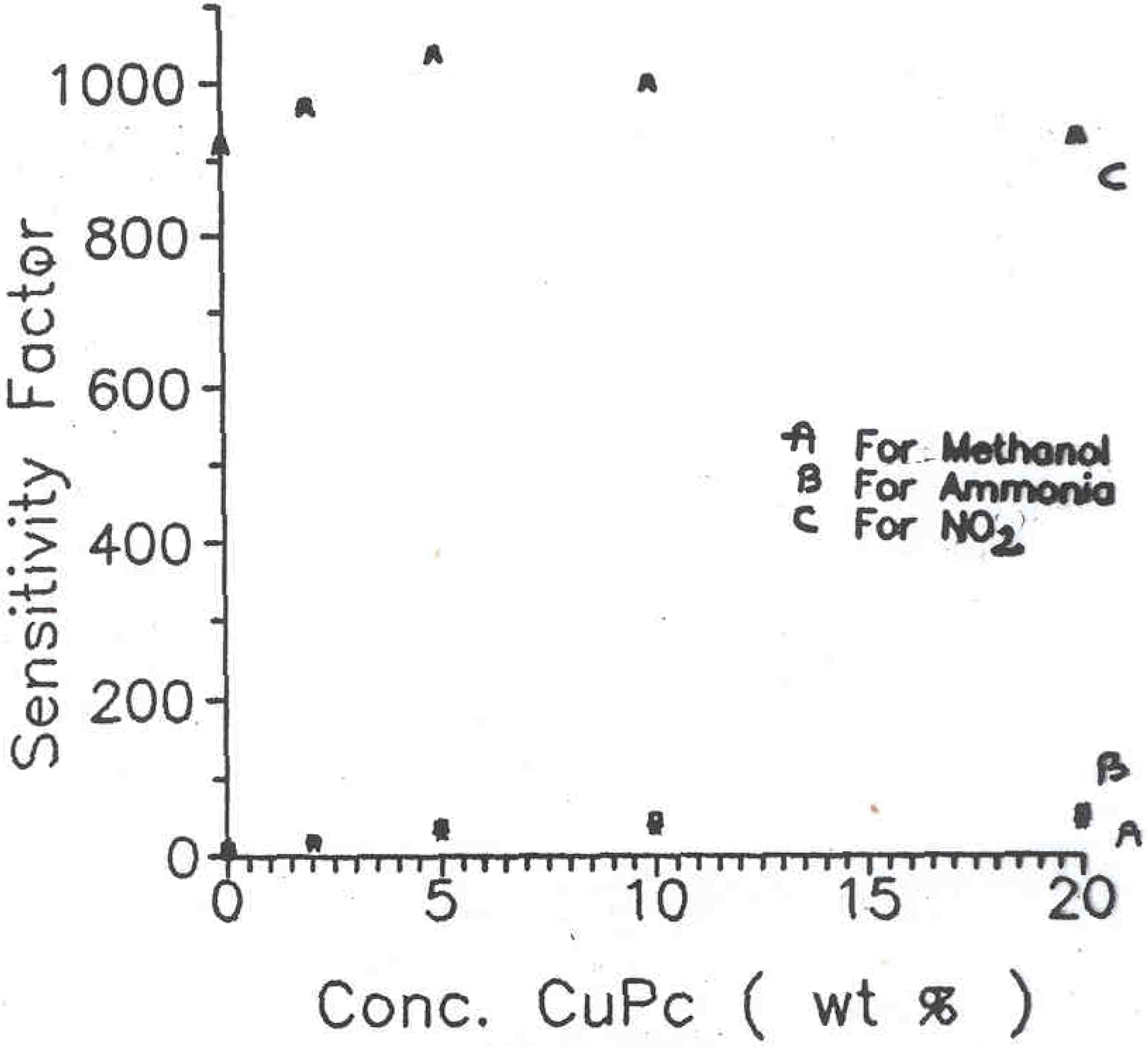
14]. Such surface cell type sensors were prepared for different compositions of PANI-CuPc. These samples were then exposed to various chemical vapours such as methanol, nitrogen dioxide and ammonia gas in a specially designed pre-evacuated glass chamber. The sensitivity factor (S) obtained for different phthalocyanine concentration in polyaniline samples are shown in
Figure 4. The curves A, B and C correspond to sensitivity on exposure to the vapours of methanol, NH
3 and NO
2 respectively. The sensitivity factor S can be calculated using the expression,
where
Rv is the resistance after the exposure to chemical vapour and
Ro is the initial resistance. The pure polyaniline was not found to be much sensitive to chemical vapours, whereas PANI-CuPc polymers show enhanced sensitivity.
Figure 4.
Plots of sensitivity factor versus CuPc concentration for (A) Methanol (B) Ammonia (C) Nitrogen dioxide vapors with PANI-CuPc/ PEO/ CuCl2 Inter-digited electrodes
Figure 4.
Plots of sensitivity factor versus CuPc concentration for (A) Methanol (B) Ammonia (C) Nitrogen dioxide vapors with PANI-CuPc/ PEO/ CuCl2 Inter-digited electrodes
The sensitivity to methanol vapours was comparatively low (S in the range of 10 to 20) for pure polyaniline sample but almost twice (S = 55 to 65) for the PANI-CuPc samples. The exposure of these samples to ammonia vapour showed slightly higher sensitivity as compared to methanol. However, it is quite interesting to note the tremendous sensitivity of these samples to the vapours of nitrogen dioxide. In this case, the sensitivity factor of about 900 to 1100 was obtained depending upon the phthalocyanine concentration in the polymeric sample. The results obtained from these investigations are compiled in
Table 1.
The action of chemical vapours on such sensor system would proceed as follows. Firstly the PEO-CuCl2 matrix, which is the major component, absorbs the vapour which then has to diffuse through the inter-domain spaces and reach the conducting polymer (polyaniline) moieties. It then interacts with the impurity states in PANI and transfer electronic charge. The transfer of electrons leads to a lowering of the potential barrier at the interface, giving rise to an increase in conductivity (or decrease in resistivity). The enhancement of sensitivity of PANI – CuPc to NO2 is mainly due to the CuPc groups and there seems to be an optimum concentration required for maximum sensitivity.
Table 1.
Sensitivity of PANI-CuPc polymers with chemical vapours.
Table 1.
Sensitivity of PANI-CuPc polymers with chemical vapours.
| Sr. No. | CuPc content by Wt. % | Sensitivity Factor (S) |
|---|
| Methanol | Ammonia | Nitrogen dioxide |
|---|
| 1 | 0.0 | 11.0 | 6.0 | 100.0 |
| 2 | 2.0 | 20.0 | 18.0 | 970.0 |
| 3 | 5.0 | 40.0 | 30.0 | 1040.0 |
| 4 | 10.0 | 49.0 | 37.0 | 1000.0 |
| 5 | 20.0 | 57.0 | 44.0 | 930.0 |
The PPy and PPy-CuPc polymers obtained in its highly conducting form were exposed to the various chemical vapours such as methanol, ammonia and nitrogen dioxide15. The results obtained from this study are shown in
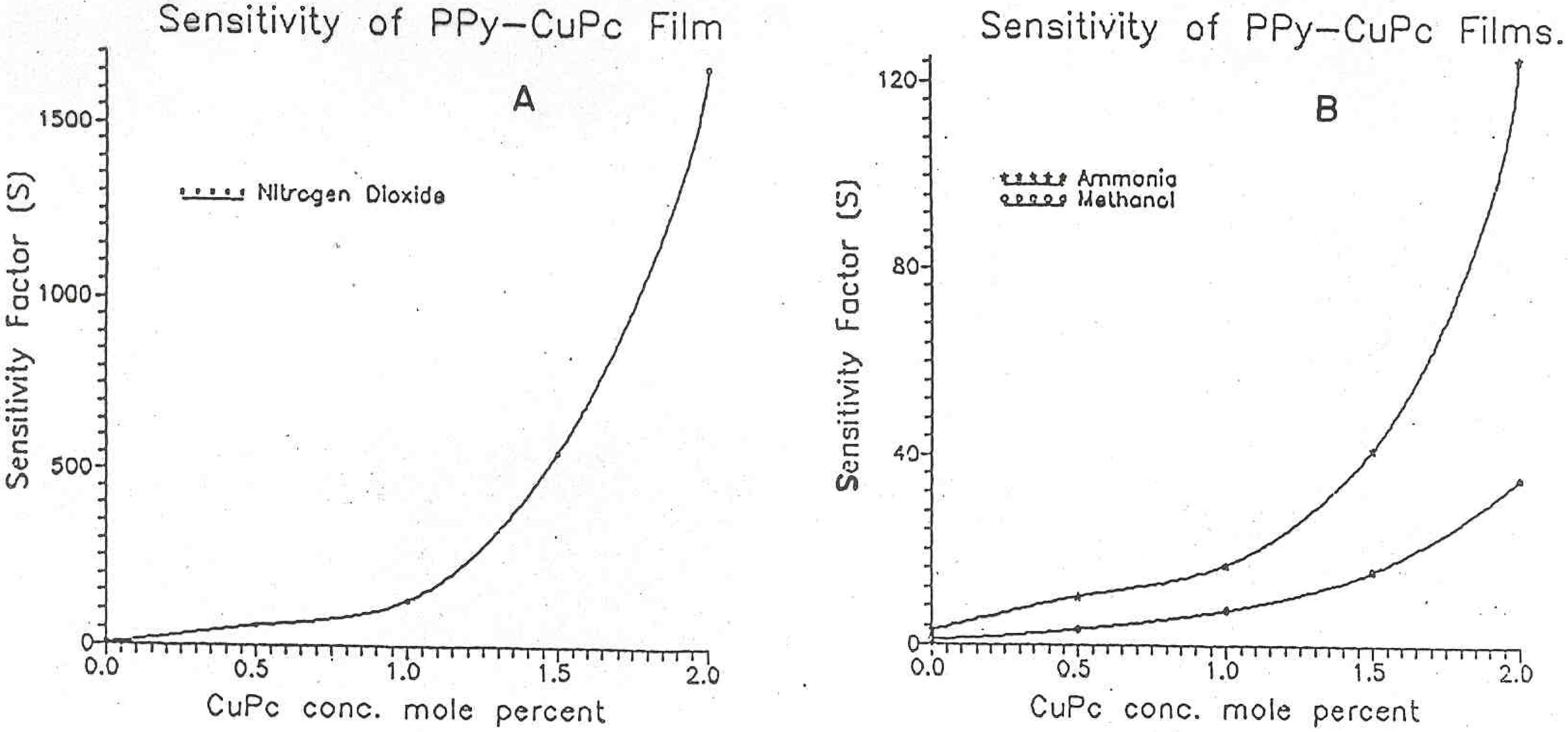
Figure 5 and the data is presented in
Table 2. The curve A represents the sensitivity towards nitrogen dioxide gas, whereas curve B shows the sensitivity towards the ammonia and methanol vapours. From these curves it can be seen that PPy-CuPc polymers show moderate sensitivity towards the methanol and ammonia vapours. To mention here, the ammonia vapour sensitivity is higher than that of methanol vapour. It is quite interesting to note that PPy-CuPc polymers show tremendous sensitivity towards nitrogen dioxide vapours. This very high response to nitrogen dioxide vapours can be explained as follows.
Figure 5.
Plots of sensitivity factor versus CuPc concentration for PPy-CuPc polymers Nitrogen dioxide (B) Ammonia and Methanol
Figure 5.
Plots of sensitivity factor versus CuPc concentration for PPy-CuPc polymers Nitrogen dioxide (B) Ammonia and Methanol
Table 2.
Sensitivity of PPy-CuPc polymers with chemical vapours.
Table 2.
Sensitivity of PPy-CuPc polymers with chemical vapours.
| Sr. No. | CuPc content by Wt. % | Sensitivity Factor (S) |
|---|
| Methanol | Ammonia | Nitrogen dioxide |
|---|
| 1 | 0.0 | 1.00 | 3.00 | 5.00 |
| 2 | 0.5 | 3.25 | 10.00 | 55.00 |
| 3 | 1.0 | 7.25 | 16.75 | 125.00 |
| 4 | 1.5 | 15.50 | 41.50 | 555.00 |
| 5 | 2.0 | 35.00 | 125.00 | 1665.00 |
Nitrogen dioxide was reported [
16,
17], to have large effects (6 to 8 orders of magnitude) on the conductivity of phthalocyanine films. Chadwick et.al. [
18], interpreted that a charge transfer complex is formed between a phthalocyanine donor and nitrogen dioxide acceptor, and the charge carriers are the holes produced in the phthalocyanine matrix. Nitrogen dioxide is π-electron acceptor, and accepted electron would delocalize over the nitrogen dioxide planar structure. Since the hole is also delocalized over the phthalocyanine structure the coulombic force between the opposite charges is weakened and charge carrier movement is facilitated. Ammonia is a competing electron donor and can displace phthalocyanine in the interaction. Thus, we can observe the less response towards the ammonia and on the other hand, tremendous response for nitrogen dioxide vapours in case of PPy polymers containing CuPc.
The various PT-CuPc polymers obtained were fabricated as a chemical sensor device using inter-digited gold films with PEO-CuCl
2-Polymer mixture. This sensor [
19], was then subjected to exposure of various chemical vapours such as methanol, ammonia and nitrogen dioxide etc. The results obtained in each case are plotted in
Figure 6 and the data is presented in
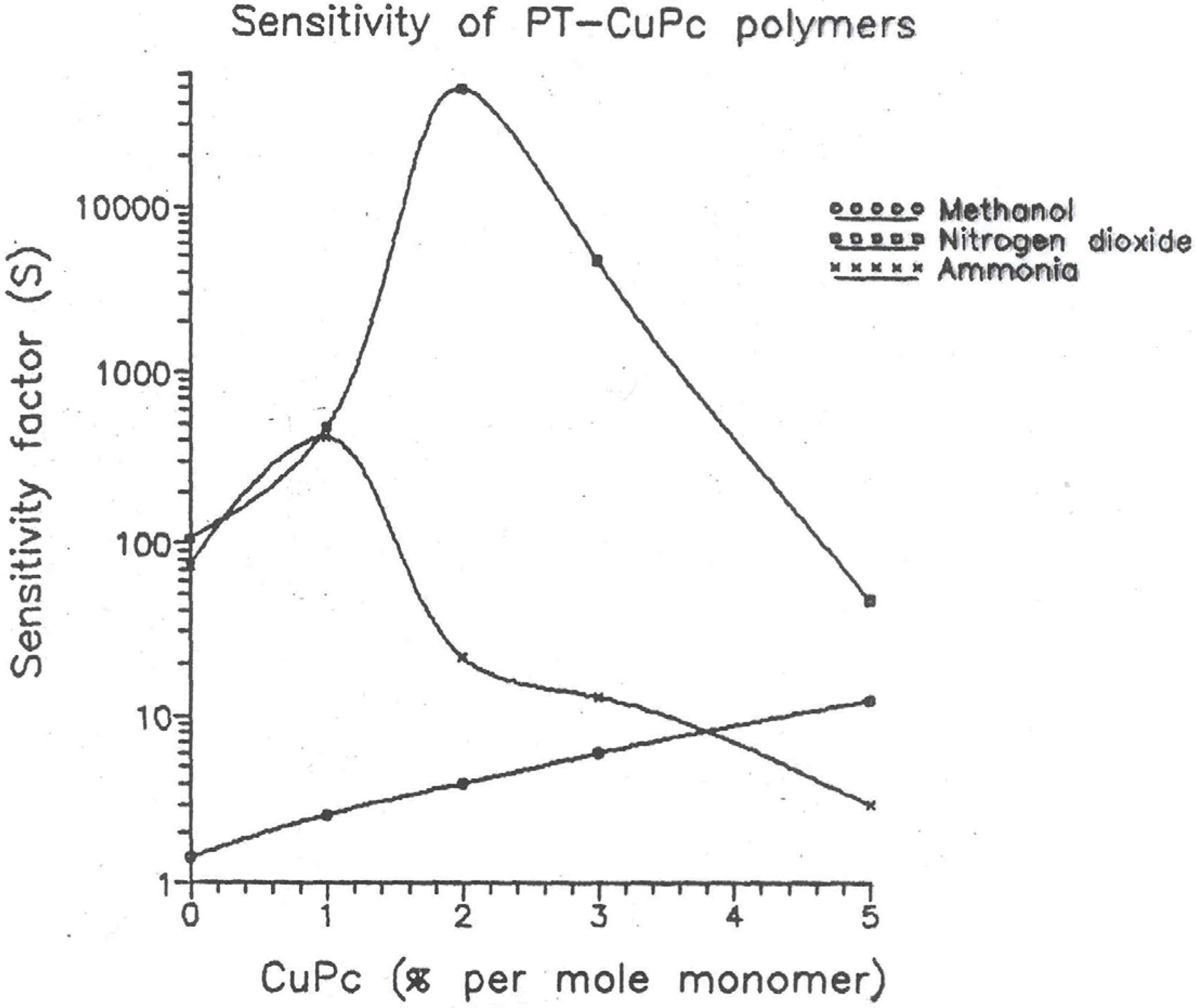
Table 3. The figure shows the sensitivity factor versus CuPc concentration curves.
Figure 6.
Plots of sensitivity factor versus CuPc concentration for Methanol, ammonia and nitrogen dioxide vapors measured with PT-CuPc/ PEO/ CuCl2 inter-digited electrodes.
Figure 6.
Plots of sensitivity factor versus CuPc concentration for Methanol, ammonia and nitrogen dioxide vapors measured with PT-CuPc/ PEO/ CuCl2 inter-digited electrodes.
Table 3.
Sensitivity of PT-CuPc polymers with chemical vapours.
Table 3.
Sensitivity of PT-CuPc polymers with chemical vapours.
| Sr. No. | CuPc content by Wt. % | Sensitivity Factor (S) |
|---|
| Methanol | Ammonia | Nitrogen dioxide |
|---|
| 1 | 0.0 | 1.43 | 77.33 | 105.26 |
| 2 | 1.0 | 2.60 | 416.60 | 476.20 |
| 3 | 2.0 | 4.00 | 22.00 | 50000.00 |
| 4 | 3.0 | 6.92 | 13.33 | 4761.90 |
| 5 | 5.0 | 12.50 | 3.00 | 47.62 |
The action of chemical vapours, on this composite system would proceed as follows. Firstly, PEO-CuCl2 which is a major component would absorb the vapour, which then diffuses through inter-domain spaces and reach the conducting polymer moieties, where it would interact with the impurity states and transfer electronic charge. Transfer of electron leads to a lowering of the potential barrier at the interface, giving rise to an increase in conductivity.
It is interesting to note that sensitivity increases initially with increasing phthalocyanine content to about 3% concentration for ammonia and nitrogen dioxide vapours but then decreases for higher concentration of phthalocyanine. This peculiar behaviour can be explained as follows: The pure PT is an amorphous polymer. Through this amorphous structure diffusion of these gases would to very easy. Hence they show greater sensitivity. As the phthalocyanine content in the polymer increases, the polymer becomes more crystalline. The diffusion of the gas molecules through this well ordered crystal lattice is very difficult. Hence after certain level of CuPc concentration there is a sudden drop in sensitivity.