Introduction
Mn(II) can be determined in several ways: iodometrically
1, by differential stripping voltammetry
2, polarographically
3, fluorometrically
4, amperometrically
5, by indirect titration with EDTA
6, sprectrophotometrically
7,8, by the potentiometric titration with Mn(VII) in pyrophosphate media
9 as well as by potentiometric titration with polyamine ligands in acetonitrile
10.
Fe(II) can be determined by volumetric titration with cerium(IV) using ferroin as indicator
11, by liquid chromatography
12, photometrically
13,14, polarographically
15, ferrocenometrically
16, by using XeF
2 as a standard titrating solution
17 as well as potentiometrically
10,18,19,20,21,22.
Hexacyanoferrate(II) can be determined by infrared spectroscopy
23, or amperometrically
24,25, coulometrically
26,27 as well as potentiometrically
28,29,30,31,32.
Arsenic(III) can be determined potentiometrically and by visual titration with bromoamine
28, by potentiometric stripping analysis
33, AFS method
34, cerimetrically
11,35, by potentiometric titration with XeF
2 and also bipotentiometrically with Mn(III) and Mn(IV).
Oxalate can be determined chromatorgraphically
36, spectrophotometrically
37, gasometrically
38 as well as by potentiometric precipitation titrations.
From this short survey of methods for the determination of reducing substances it is seen that the potentiometric methods are of great importance for these determinations.Therefore, it is desirable to reveal and develop new sensors for the TEP detection. In the hitherto published papers the following indicator electrodes were used: Cu-wire, graphite electrode, Pt-electrode, Ag-electrode coated with Ag2S and Ag3Fe(CN)6 and picrate ion-selective electrode. All theafore-mentioned determinations were carried out either in aqueous or nonaqueous media, and are based on redox reactions, complex formation reactions or formation of precipitates.
In addition, Au, stainless steel, various inert electrodes can be also used as indicator electrodes in redox titrations, and one among the latter ones is the pyrite electrode which was found to be very effective in acid-base titrations in aqueous
40 and non-aqueous
41 solutions, as well as for complexometric titrations
42,43 and redox titrations
44,32.
In this paper results obtained in investigating pyrite as sensor of the indicator electrode in redox titrations with standard Mn(VII) solution, are presented.
Results and Discussion
The electronic structure of crystalline pyrite indicates that this mineral is a low-spin complex with d2sp3 hybridization. All hybrid orbitals are occupied with electrons which makes pyrite a diamagnetic and non-reactive compound. Since pyrite is a semiconductor of n or p type, it is possible to use it, on account of its chemical inactivity, for themeasurements of the redox potentials.
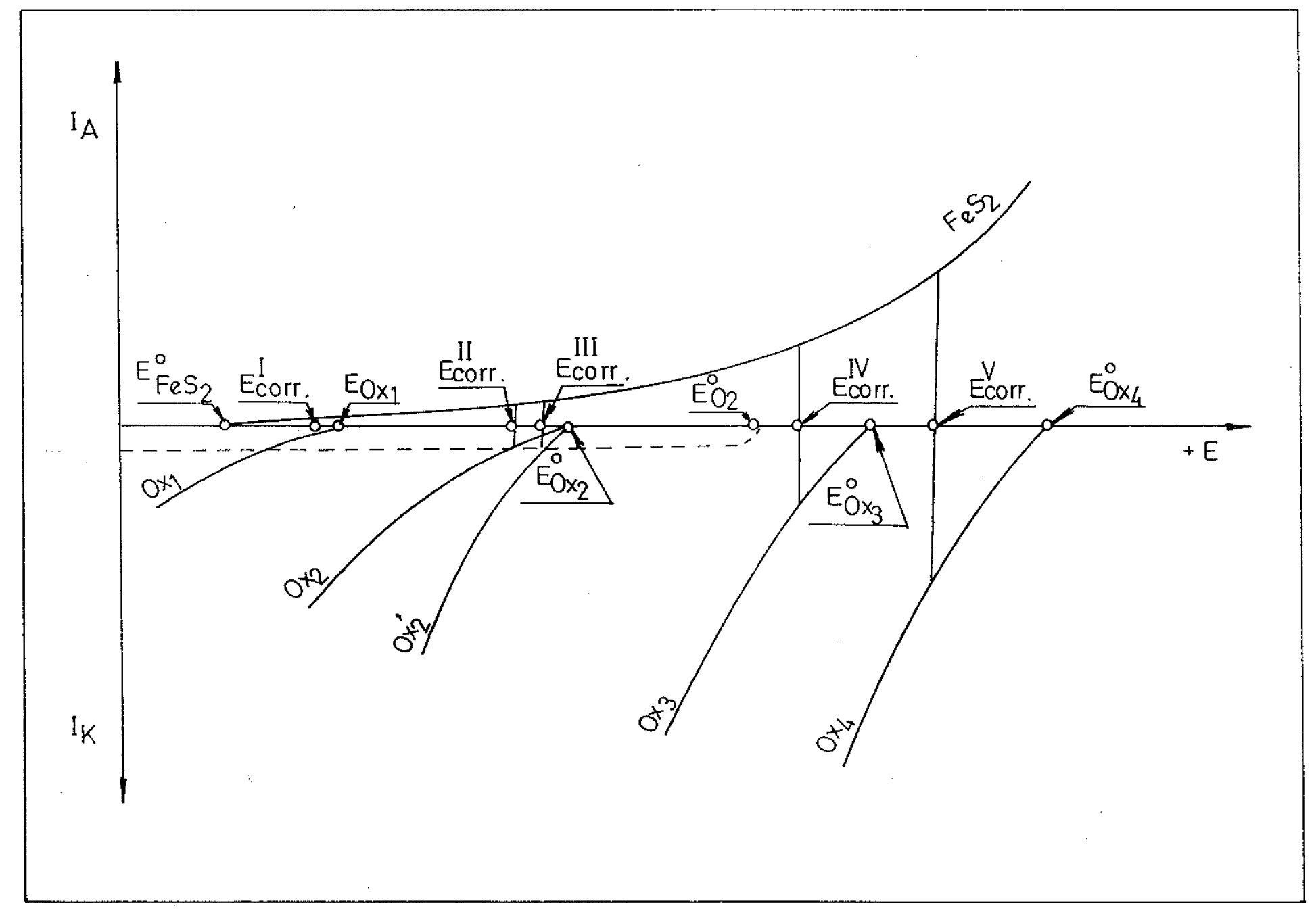
The potential changes in potentiometric titrations in this case can be explained by polarization curves for pyrite and the components taking part in the redox reaction (
Fig.1.).
Figure 1.
Anodic and cathodic polarization curves by means of which potential changes at the titration end-point are explained [Ox1, Ox2 (Ox’2), Ox3 and Ox4 are different oxidants].
Figure 1.
Anodic and cathodic polarization curves by means of which potential changes at the titration end-point are explained [Ox1, Ox2 (Ox’2), Ox3 and Ox4 are different oxidants].
It is known that in acid media pyrite dissolves slightly (becomes corroded), giving rise to soluble products
45:
The rest potential of pyrite in open-circuit is 0.62 V vs. SCE, and at more positive potentials the dissolutions of pyrite occurs, which may be shown by the anodic polarization curve. The degree of pyrite oxidation is equivalent to the corrosion current and depends on several parameters (temperature, state of pyrite surface, oxidant concentration, oxidant nature, acidity of medium etc.). In potentiometric titrations, however, a polished mineral (small surface area), room temperature and low oxidant concentrations are used, so that all these conditions ensure an insignificant dissolution of pyrite. These data indicate that in the abscence of oxidizing agents, the value of the corrosion potential will be more negative (
Fig.1.). Under these conditions the value of the corrosion current is also small. Such a state of the pyrite electrode occurs at the beginning of the potentiometric titrations. The efect of oxygen in the solution may be eliminated by bubbling nitrogen through the solution. Besides, the initial solutions may contain some ions which might cause the corrosion of pyrite at low potentials (E
Icorr.). It may be assumed that the solutions a contains a reducing species (Red
2) which on oxidationgives Ox
2, when being titrated with the oxidant Ox
3. Under these conditions the following reaction takes place in the solution:
where Ox
2/Red
2 and Ox
3/Red
3 denote corresponding redox pairs. After the first addition of the oxidizing agent Ox
3 a corrosion potential E
IIcorr. is established at the pyrite electrode, whereby pyrite is oxidized by the oxidizing form of the reducing species ( Ox
2). On further addition of the oxidizing agent Ox
3, the concentration of the Ox
2 species increases (Ox’
2) and that results in the shift of the corrosion potential to E
IIIcorr. At the end-point, when in the solution the oxidant Ox
3 is in excess, the corrosion potential is abruptly shifted towards the more positive region, since under these conditions the dissolution of pyrite is defined by the Ox
3/Red
3 system. This change of the corrosion potential from E
IIIcorr. to E
IVcorr. makes possible the applications of the pyrite electrode as the indicator electrode in potentiometric redox titrations. From
Fig.1. it is also seen that higher potential changes of the pyrite electrode at the titration end-point are observed in case oxidizing agents with more positive equilibrium potentials (stronger oxidants ) are used. The reagents which display a great overvoltage in cathodic reduction at pyrite are not suitable as titrating agents, since the potential changes at the titration end-point in that case are small. Similar consideration hold for titrations of oxidizing agents with reducing agents, but then, the corrosion potential of pyrite is shifted towards the more negative region.
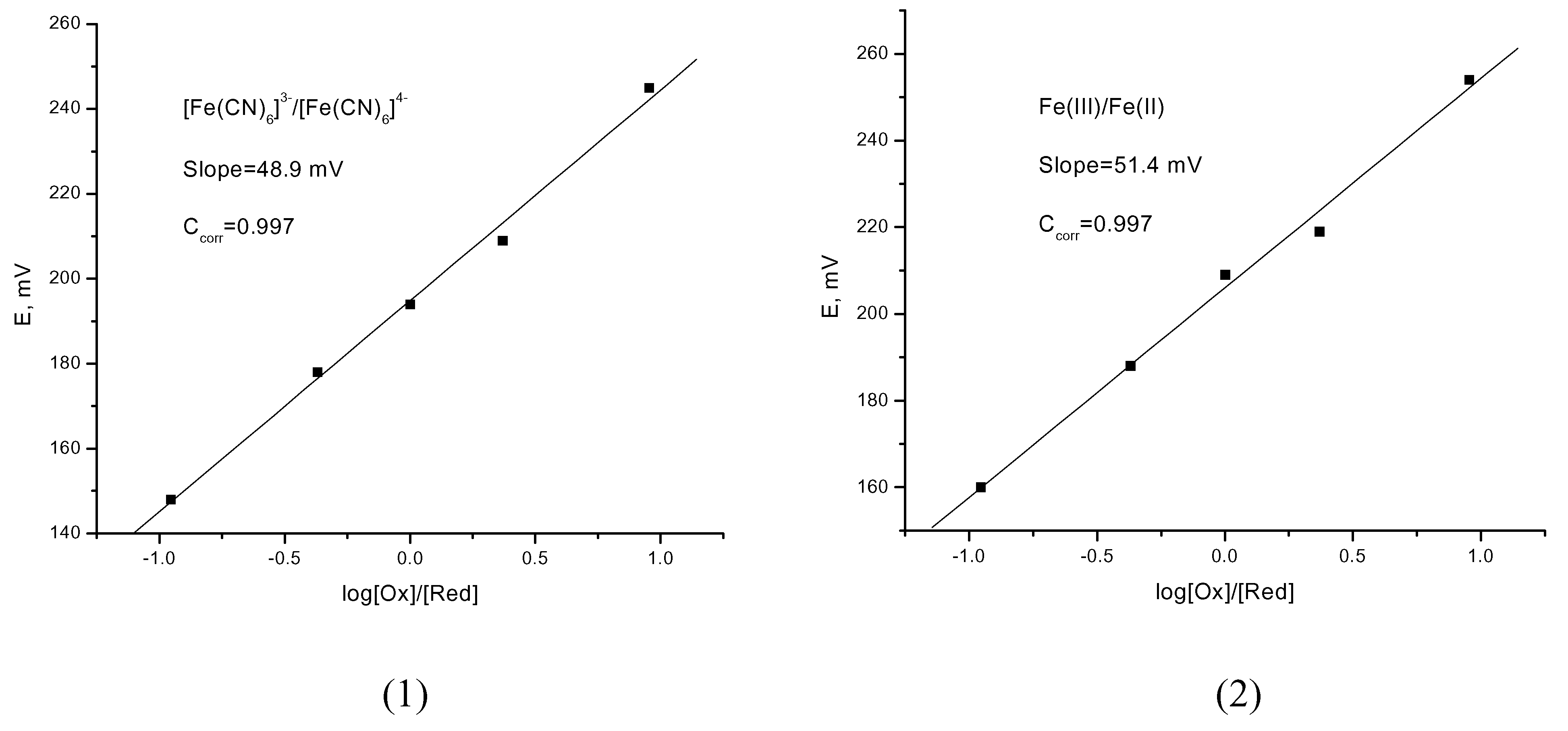
The measurements of the standard potential were carried out with solutions having different c
Ox/c
Red rations of the Fe(III)/Fe(II) and Fe(CN)
63−/Fe(CN)
64− systems, and the results obtained are shown in
Fig. 2.
The calculated correlation coefficients (ccorr) shown the linearity of the results obtained, whereas the hitherto performed investigations point out the effectiveness of the pyrite electrode as the indicator electrode for potentiometric titrations.
Figure 2.
Plots of E against log (cOx/cRed): (1) Fe(CN)63−/Fe(CN)64−; (2) Fe(III)/Fe(II).
Figure 2.
Plots of E against log (cOx/cRed): (1) Fe(CN)63−/Fe(CN)64−; (2) Fe(III)/Fe(II).
The chemical process taking place in the potentiometric titration of the Mn(II) with permanganate in pyrophosphate media is given by the following equation:
The main factor of this process is the concentration of H
2P
2O
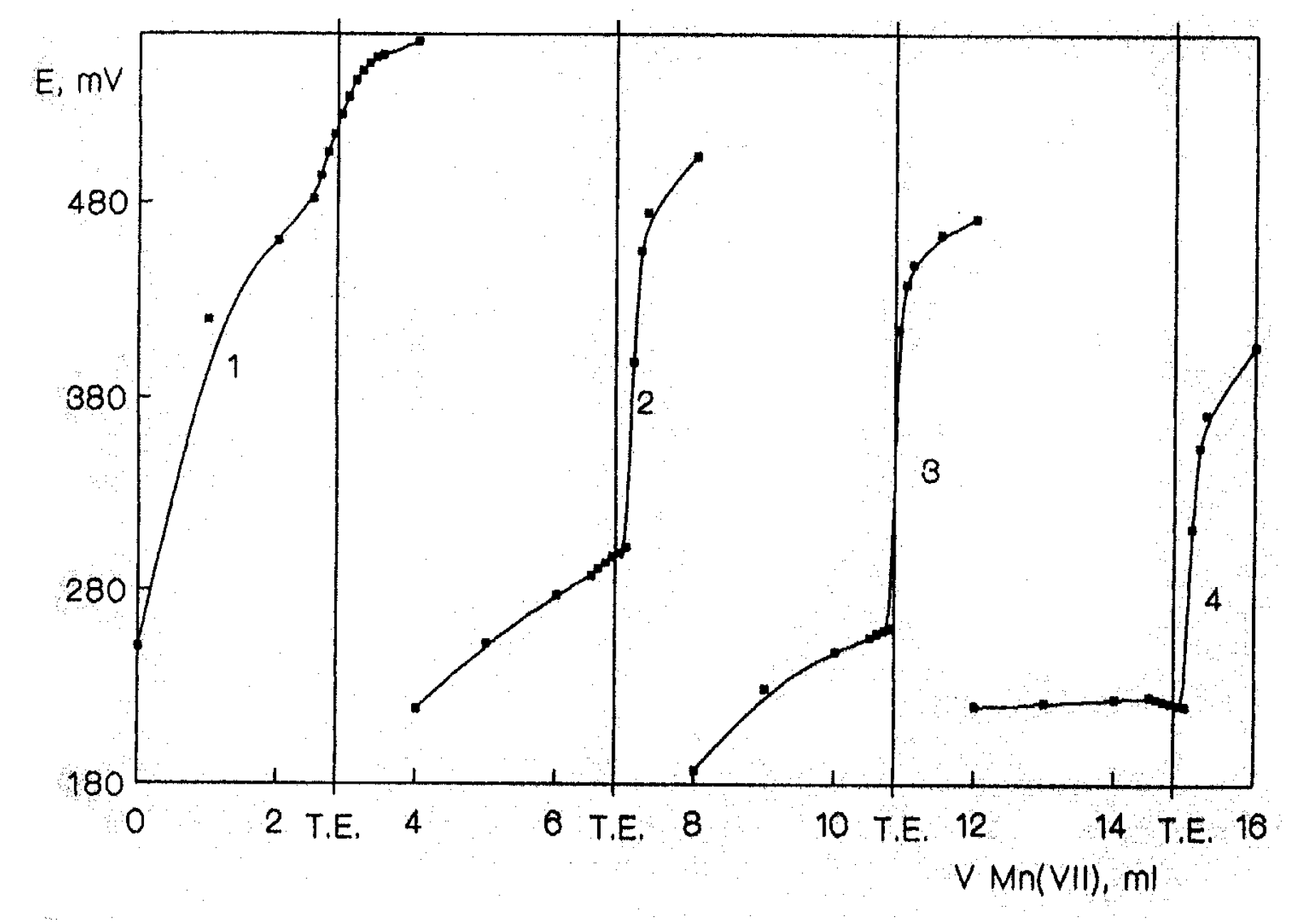
72− on account of the stabilization of Mn(III) in the form of the pyrophosphate complex. The titrations were performed at pH values 4.0, 5.0, 6.0 and 7.0 (
Fig. 3).
Figure 3.
Potentiometric titrations of Mn(II) in pyrophosphate at various pH values (1. pH 4.00; 2. pH 5.00; 3. pH 6.00; 4. pH 7.00).
Figure 3.
Potentiometric titrations of Mn(II) in pyrophosphate at various pH values (1. pH 4.00; 2. pH 5.00; 3. pH 6.00; 4. pH 7.00).
From the graph in
Fig. 3. it is seen that pH 6.0 is the optimum pH, and that the tirations at pH-values 4.0, 5.0 and 7.0 do not afford good results since the TEP is shifted. At pH 6.0 the concentration of H
2P
2O
72− ion in the solution is maximal which enables the stabilization of Mn(III) form, a decrease of the standard Mn(III)/Mn(II) potential and shifts the reaction to the right.
Pastor et al
9. carried out the determination of Mn(II) under the same conditions but they applied Pt and graphite electrodes as indicator electrodes. Comparison of results obtained by the application of the pyrite, Pt and graphite electrodes, is given in
Table 1.
Table 1.
Comparison of the results obtained with the pyrite, Pt and carbon indicator electrode in pyrophosphate media.
Table 1.
Comparison of the results obtained with the pyrite, Pt and carbon indicator electrode in pyrophosphate media.
| Titrated ion | Titrant | pH | Electrode | Potential change at the TEP | Reference |
|---|
| Mn(II) | Mn(VII) | 6.2 | Pt | 110 - 130 | 9 |
| Mn(II) | Mn(VII) | 6.2 | Carbon | 165 - 190 | 9 |
| Mn(II) | Mn(VII) | 6.0 | FeS2 | 100 - 190 | Results presented in this paper |
From
Table 1 it is seen that the pyrite electrode is applicable to the titrations of Mn(II) in pyrophosphate media at pH 6.0 with the same effectiveness as the Pt and graphite electrodes. The total potential changes range from 200 to 260 mV, whereas the jumps at the TEP from 100 to 190 mV/0.1 mL, depending on Mn(II) concentration in the titrated solution.
An increase in sulphuric acid concentration has a small effect on the increase of the total potential changes in the course of iron(II) titration, but not on the potential changes at the TEP.
However, an increase in H
3PO
4 concentration effects considerably both the potential changes in the course of the titration and the potential changes at the TEP. These observations are expected since Fe(III) which is formed in the course of the titration, in phosphoric acid solution forms the complex Fe(HPO
4)
+ whose formation constant is 2.3*10
9(46). The formation of such a stable complex causes a decrease of the potential of the Fe(III)/Fe(II) system and in this manner the potential differences with respect to the Mn(VII)/Mn(II) is increased. All that results in an increase of total potential changes in the course of the titration andalso of potential changes at the TEP (
Table 2).
Table 2.
Results obtained in potentiometric titrations of reducing substances with standard potassium permanganat solution (electrode couple FeS2-SCE).
Table 2.
Results obtained in potentiometric titrations of reducing substances with standard potassium permanganat solution (electrode couple FeS2-SCE).
| Taken (mol) | cacid (mol.dm3) | Titrant | Total change of E (mV) | Change of E at the TEP (mV/0.1ml) | Found |
|---|
| Fe(II)1 | | Mn(VII) | | | |
| 0.0027 | 0.1 | | 280 - 360 | 90 - 120 | 99.4±0.1 |
| 0.0027 | 4.5 | | 340 - 350 | 90 - 120 | 99.7±0.2 |
| 0.0010 | 0.5 | | 290 - 300 | 140 - 150 | 100.6±0.4 |
| 0.0019 | 0.5 | | 305 - 310 | 100 - 130 | 100.1±0.3 |
| 0.0029 | 0.5 | | 745 - 750 | 450 - 500 | 100.0±0.0* |
| Mn(VII) | | Fe(II) | | | |
| 0.0007 | 1.5 | | 260 - 270 | 50 - 60 | 99.9±0.2 |
| 0.0007 | 4.5 | | 260 - 300 | 40 - 70 | 99.2±0.2 |
| 0.0004 | 1.5 | | 280 - 290 | 75 - 80 | 99.2±0.1 |
| 0.0006 | 1.5 | | 270 - 290 | 50 - 60 | 100.0±0.6 |
| Fe(II)2 | | Mn(VIII) | | | |
| 0.0029 | 0.1 | | 390 - 400 | 180 - 240 | 100.5±0.1 |
| 0.0029 | 1.5 | | 440 - 470 | 260 - 330 | 100.5±0.1 |
| 0.0021 | 0.5 | | 430 - 440 | 260 - 270 | 100.0±0.0 |
| 0.0037 | 0.5 | | 440 - 445 | 240 - 250 | 99.9±0.1 |
| | Fe(II) | | | |
| 0.0007 | 0.1 | | 340 - 350 | 130 - 160 | 99.5±0.2 |
| 0.0007 | 1.5 | | 430 - 440 | 130 - 190 | 99.8±0.3 |
| 0.0004 | 0.5 | | 410 - 420 | 190 - 200 | 99.2±0.1 |
| 0.0007 | 0.5 | | 400 - 405 | 170 - 190 | 99.8±0.1 |
| Fe(CN)64− | | Mn(VII)1 | | | |
| 0.0031 | 0.1 | | 470 - 480 | 180 - 190 | 99.9±0.0 |
| 0.0031 | 1.5 | | 370 - 380 | 60 - 65 | 99.9±0.1 |
| 0.0018 | 0.5 | | 380 - 390 | 100 - 120 | 100.4±0.1 |
| 0.0038 | 0.5 | | 400 - 410 | 90 - 100 | 100.3±0.1 |
| 0.0031 | 0.5 | | 730 - 770 | 430 - 460 | 100.0±0.0* |
| Mn(VII) | | Fe(CN)64− | | | |
| 0.0004 | 0.1 | | 320 - 330 | 140 - 150 | 100.3±0.1 |
| 0.0004 | 1.5 | | 205 - 210 | 30 - 35 | 99.9±0.3 |
| 0.0004 | 0.5 | | 260 - 270 | 75 - 80 | 99.9±0.3 |
| 0.0005 | 0.5 | | 280 - 285 | 60 - 80 | 99.6±0.2 |
| Fe(CN)64− | | Mn(VII)2 | | | |
| 0.0031 | 0.5 | | 440 - 450 | 150 - 180 | 99.7±0.1 |
| 0.0031 | 4.5 | | 330 - 350 | 20 - 40 | 99.7±0.2 |
| 0.0012 | 0.5 | | 420 - 430 | 170 - 180 | 101.1±0.1 |
| 0.0038 | 0.5 | | 440 - 470 | 130 - 150 | 100.1±0.1 |
| 0.0031 | 0.5 | | 710 - 780 | 440 - 510 | 100.0±0.0* |
| As(III)3 | | Mn(VII) | | | |
| 0.0013 | 1.2 | | 230 - 290 | 150 - 200 | 100.7±0.1 |
| 0.0020 | 1.2 | | 240 - 270 | 150 - 200 | 99.9±0.1 |
| As(III)1 | | Mn(VII) | | | |
| 0.0020 | 0.1 | | 280 - 330 | 160 - 230 | 100.3±0.2 |
| 0.0020 | 1.5 | | 260 - 380 | 200 - 310 | 100.2±0.1 |
| 0.0009 | 0.5 | | 230 - 250 | 150 - 160 | 98.9±0.1 |
| 0.0020 | 0.5 | | 300 - 340 | 180 - 270 | 100.1±0.1 |
| 0.0013 | 0.5 | | 690 - 740 | 350 - 620 | 100.0±0.0* |
| C2O42− | | Mn(VII)1 | | | |
| 0.0014 | 0.1 | | 180 - 250 | 90 - 140 | 101.8±0.2 |
| 0.0014 | 4.5 | | 230 - 280 | 40 - 50 | 101.9±0.8 |
| 0.0014 | 0.5 | | 220 - 280 | 110 - 160 | 101.0±0.2 |
| 0.0020 | 0.5 | | 230 - 320 | 120 - 200 | 100.4±0.1 |
| 0.0014 | 0.5 | | 550 - 560 | 360 - 420 | 100.0±0.0* |
The increase in H2SO4 and H3PO4 concentrations has a more significant influence of potential changes in titrations of hexacyanoferrate(II). At lower H2SO4 concentrations the process of hexacyanoferrate(II) oxidation takes place more readily since a small amount of H+ is available for the formation of H4Fe(CN)6.
With increasing H
+ concentration the reaction of H
4Fe(CN)
6 formation is favoured, and in conditions of satisfactory high H
+ concentration (4.5 M H
2SO
4) the process of hexacyanoferrate(II) oxidation is very slow. At lowe H
2SO
4 concentrations (0.1, 0.5 and 1.5 M) the process of hexacyanoferrate(II) oxidation proceeds with a more considerable decrease in potential shanges with the increasing acidity. However, since H
3PO
4 is a weaker acid than H
2SO
4, a H
3PO
4 solution of the same concentration as that of H
2SO
4 will contain less H
+ ions, and therefore, with increasing H
3PO
4 concentration the formation of H
4Fe(CN)
6 will have less priority. On account of these reasons the titration of hexacyanoferrate(II) in 4.5 M H
3PO
4 is possible (
Table 2).
With standard solutions of Mn(III) in 1.0 M H
2SO
4 and Mn(IV) in 2.0 M H
2SO
4 the bipotentiometric titrations (Pt-electrodes) of hexacyanoferrate(II) were performed with a total potential change of 400-750 mV. The results obtained with Mn(III) were 99.2+0.2% and those with Mn(IV) 100.2+0.3%. Hexacyanoferrate(II) can be determined also by potentiometric precipitation reactions with AgNO
3, the indicator electrode being based on a mixture of Ag
2S and Ag
4Fe(CN)
6, whereby the obtained jumps amounted to about 150 mV/0.1 mL
30, or a picrate ion-selective electrode can be used.
The titrations of As(III) were carried out in HCl (1.2 M) and H
2SO
4 (0.1 - 4.5 M). Because the reaction of As(III) with Mn(VII) is slow, it had to be catalyzed. In titrations of As(III) in HCl, only the concentration of As(III) was changed. The changes in As(III) concentration had no significant effect either on the potential changes or on the accuracy of the results. An increasein H
2SO
4 concentration in titrations of As(III) had a more significant effect on potential changes. With increasing acidity the oxidizing ability of Mn(VII) is increased, and be cause of that the difference in potential is increased which causes an increase in potential changes (
Table 2).
The titrations of oxalate were carried out in H
2SO
4 (0.1-4.5 M). It is known that the presence of higher amounts of H
2SO
4 inhibits the reaction of oxalate with Mn(VII) making it slow, which has a considerable effect on the decrease of potential change at the TEP (with increasing acidity the potential change is decreased for about 90 mV/0.1 mL) (
Table 2).
In reversed titrations the behaviour of the pyrite electrode was similar to that in direct titrations. The total potential changes and the potential changes at the TEP were smaller with respect to those in direct titrations. The highest changes at the TEP were observed in titrations of Mn(VII) with iron(II) in 1.5 M H3PO4 (130 - 190 mV/0.1 mL), whereas the smallest ones in titrations of Mn(VII) with iron in H2SO4 (50 - 60 mV/0.1 mL).
The accuracy and the reproducibility in direct and reverse titrations were satisfactory (
Table 2).
In all direct and reverse titrations, in the presence of MnO4− ion in excess, potential oscillations were observed (±5mV). These oscillations are most probably due to unequal rates of MnO4− reduction on the pyrite surface. A change in the reduction rate (cathodic current density) causes a change in the position of the corrosion potential, which gives rise to its oscillations.
In the course of the titration and also at the TEP the potential was rapidly established (<1 ) except in titrations of oxalate where on account of a slow reaction a somewhat longer time-interval was required, as well as in the determination of As(III) where the reaction had to be catalyzed.
Data in Tables show that the behaviour of pyrite in all the investigated systems is in accordance with the expected one: higher jumps were obtained with those systems where the difference between the standard potential values was greater. Also higher jumps at the TEP were registered in cases where the medium can influence the reaction equilibrium (titration of Fe(II) in H3PO4 or Mn(II) in pyrophosphate medium at the pH 6.0). In addition, taking into account the accuracy and the reproducibility as well as therate of potential establishment at the FeS2 electrode in the course of the titration and at the TEP, it may be concluded that pyrite as sensor of the indicator electrode is suitable for the TEP detection in redox potentiometric titrations where standard Mn(VII) solution is used as the titrant.