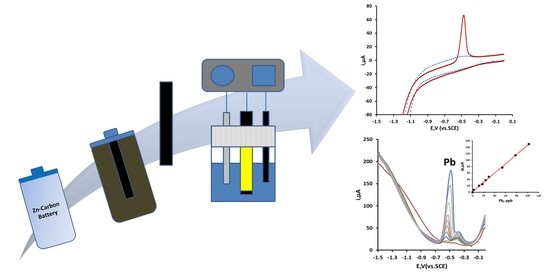
Trace Voltammetric Determination of Lead at a Recycled Battery Carbon Rod Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Apparatus
2.3. Scanning Ectron Mcroscopy (SEM) and Eergy-Dspersive X-ray Sectroscopy (EDX)
2.4. Fabrication of the Carbon Rod Electrode
2.5. Voltammetric Procedures
3. Results and Discussion
3.1. EDX Examination of the CRE
3.2. Cyclic Voltammetric Behavior of Lead at Bare CREs
3.3. Effect of Supporting Electrolyte Concentration
3.4. Differential Pulse Anodic Stripping Voltammetry
3.5. Effect of Accumulation Potential
3.6. Effect of Accumulation Time
3.7. Effect of Pb Concentration
3.8. Analytical Application
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Health and Environmental Risks (SCHER). Opinion on Lead Standard in Drinking Water; SCHER: Brussels, Belgium, 11 January 2011. [Google Scholar]
- European Commission. 1998 Council Directive (98/83/EC) of 3 November 1998 on the Quality of Water Intended for Human Consumption; Official Journal, L330/32; European Commission: Brussels, Belgium, 5 December 1998. [Google Scholar]
- World Health Organization. 2004 Guidelines for Drinking-Water Quality, 3rd ed.; Recomendations; WHO: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Laaninen, T. Members’ Research Service PE 625.179–October 2018 Revision of the Drinking Water Directive. Available online: http://www.europarl.europa.eu/RegData/etudes/BRIE/2018/625179/EPRS_BRI (accessed on 11 December 2018).
- Hayes, C.R.; Skubala, N.D. Is there still a problem with lead in drinking water in the European Union? J. Water Health 2009, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Ladapo, J.A.; Mohammed, A.K.; Nwosu, V.C. Lead Pollution in Flint, Michigan, U.S.A. and Other Cities. Int. J. Environ. Sci. Educ. 2017, 11, 1341–1351. [Google Scholar] [CrossRef]
- Harvey, P.J.; Handley, H.K.; Taylor, M.P. Wide spread copper and lead contamination of house hold drinking water, New South Wales, Australia. Environ. Res. 2016, 151, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, N.; Arain, M.A.; Badar, N.; Rasheed, M.; Haque, Z. Drinking water: A major source of lead exposure in Karachi, Pakistan. East Mediterr. Health J. 2011, 17, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Švancara, I.; Prior, C.; Hocevar, S.B.; Wang, J. A Decade with Bismuth-Based Electrodes in Electroanalysis. Electroanalysis 2010, 22, 1405–142. [Google Scholar] [CrossRef]
- Finšgar, M.; Majer, D.; Maver, U.; Maver, T. Reusability of SPE and Sb-modified SPE Sensors for Trace Pb(II) Determination. Sensors 2018, 18, 3976. [Google Scholar] [CrossRef]
- Jovanovski, V.; Hrastnik, N.I.; Hočevar, S.B. Copper film electrode for anodic stripping voltammetric determination of trace mercury and lead. Electrochem. Commun. 2015, 57, 1–4. [Google Scholar] [CrossRef]
- Czop, E.; Economou, A.; Bobrowski, A. A study of in situ plated tin-film electrodes for the determination of trace metals by means of square-wave anodic stripping voltammetry. Electrochim. Acta 2011, 56, 2206–2212. [Google Scholar] [CrossRef]
- Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Ex situ Deposited Bismuth Film on Screen-Printed Carbon Electrode: A Disposable Device for Stripping Voltammetry of Heavy Metal Ions. Electroanalysis 2010, 22, 1460–1467. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs Nanocomposite Modified Electrode for Electrochemical Determination of Copper (II), Lead (II) and Mercury (II) Ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Eisner, U.; Mark, H.B., Jr. The anodic stripping voltammetry of trace silver solutions employing graphite electrodes Application to silver analysis of rain and snow samples from silver iodide seeded clouds. Electrochemistry 1970, 24, 345–355. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P.; Cowell, D.C. Voltammetric behavior and trace determination of lead at a mercury-free screen-printed carbon electrode. Electroanalysis 2000, 12, 171–177. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hawkins, D.M.; Hart, J.P.; Cowell, D.C. Voltammetric behaviour and trace determination of copper at a mercury-free screen-printed carbon electrode. Talanta 2002, 57, 565–574. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Rymansaib, Z.; Iravani, P. Anodic stripping voltammetric determination of zinc at a 3-D printed carbon nanofiber–graphite–polystyrene electrode using a carbon pseudo-reference electrode. Sens. Actuator B Chem. 2018, 267, 476–482. [Google Scholar] [CrossRef]
- European Commission, On the Application of Commission Regulation EU 493/2012 Laying down Detailed Rules Regarding the Calculation of Recycling Efficiencies of the Recycling Processes of Waste Batteries and Accumulators. 2012. Available online: http://ec.europa.eu/environment/waste/batteries/pdf/Guidelines%20on%20RE.pdf (accessed on 27 December 2018).
- Tan, D.S.Y.; Impas, M.G.W.; Camacho, D.H.; Palisoc, S.T. Paper-based electrode using cladophora cellulose-polyaniline composite for electrochemical quantification of toxic lead (II). Cellulose Chem. Technol. 2018, 52, 853–861. [Google Scholar]
- Jackfama, T.; Moyo, M.; Nharingo, T.; Shumba, M.; Okonkwo, J. Water hyacinth modified carbon paste electrode for simultaneous electrochemical stripping analysis of Cd (II) and Pb (II). Int. J. Environ. Anal. Chem. 2019; in press. [Google Scholar] [CrossRef]
- Selvan, K.S.; Narayanan, S.S. Synthesis, structural characterization and electrochemical studies switching of MWCNT/novel tetradentate ligand forming metal complexes on PIGE modified electrode by using SWASV. Mater. Sci. Eng. C 2019, 98, 657–665. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, H.; Fang, Y.; Guan, J.; Ma, S.; Pan, Y.; Zhang, M.; Zhu, H.; Liu, X.D.; Du, M.L. Detection of trace Cd2+, Pb2+ and Cu2+ ions via porous activated carbon supported palladium nanoparticles modified electrodes using SWASV. Mater. Chem. Phys. 2019, 225, 433–442. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, F.; Gao, F.; Wang, Q. Enhanced stripping voltammetric response of Hg2+, Cu2+, Pb2+ and Cd2+ by ZIF-8 and its electrochemical analytical application. J. Electroanal. Chem. 2019, 835, 293–300. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, N.; Zhuang, H.; Liu, L.; Yang, B.; Wang, C.; Gai, Z.; Guo, F.; Li, Z.; Jiang, X. A diamond/graphite nanoplatelets electrode for anodic stripping voltammetric trace determination of Zn(II), Cd(II), Pb(II) and Cu(II). Appl. Surf. Sci. 2018, 457, 1192–1201. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Lee, W.H. A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Ping, J. Simultaneous determination of Cd(II) and Pb(II) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019, 274, 8–15. [Google Scholar] [CrossRef]
- Ramalingam Vinoth, M.; Ponnusamy Sriman, K.; Sangilimuthu, N. A nanocomposite consisting of porous graphitic carbon nitride nanosheets and oxidized multiwalled carbon nanotubes for simultaneous stripping voltammetric determination of cadmium(II), mercury(II), lead(II) and zinc(II). Microchim. Acta 2019, 186, 69. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sun, D.; Su, M.; Han, J.; Dong, C. Rapid analysis on the heavy metal content of spent zinc-manganese batteries by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2012, 44, 2469–2475. [Google Scholar] [CrossRef]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.M.; Robinson, J. Instrumental Methods in Electrochemistry, 1st ed.; Woodhead Publishing: Cambridge, UK, 2001; pp. 210–212. [Google Scholar]
- Honeychurch, K.C.; Al-Berezanchi, S.; Hart, J.P. The voltammetric behaviour of lead at a microband screen-printed carbon electrode and its determination in acetate leachates from glazed ceramic plates. Talanta 2011, 84, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry, 2nd ed.; Imperial College Press: London, UK, 2011; pp. 45–46. ISBN 978-1-78326-323-3. [Google Scholar]
- Chooto, P.; Wararatananurak, P.; Innuphat, C. Determination of trace levels of Pb(II) in tap water by anodic stripping voltammetry with boron-doped diamond electrode. Sci. Asia 2010, 36, 150–156. [Google Scholar] [CrossRef]
- Salonia, J.A.; Wuilloud, R.G.; Gásquez, J.A.; Olsina, R.A.; Martinez, L.D. Determination of lead in tap water by ICP-AES with flow injection on-line adsorption preconcentration using a knotted reactor and ultrasonic nebulization. J. Anal. At. Spectrom. 1999, 14, 1239–1243. [Google Scholar] [CrossRef]
- Zougagh, M.; García de Torres, A.; Vereda Alonso, E.; Cano Pavón, J.M. Automatic on line preconcentration and determination of lead in water by ICP-AES using a TS-microcolumn. Talanta 2004, 62, 503–510. [Google Scholar] [CrossRef]
- Pourreza, N.; Hoveizavi, R. Simultaneous preconcentration of Cu, Fe and Pb as methylthymol blue complexes on naphthalene adsorbent and flame atomic absorption determination. Anal. Chim. Acta 2005, 549, 124–128. [Google Scholar] [CrossRef]







| Working Electrode Material | Linear Range | Detection Limit | Voltammetric Technique | Sample | Ref. |
|---|---|---|---|---|---|
| Stainless steel EDTA–PANI/SWCNT nanocomposite electrode | Pb(II) ca. 400 µg/L–1500 µg/L; Cu(II) ca. 120 µg/L–6000 µg/L and Hg(II) ca. 400 µg/L–20,000 µg/L | Cu(II), 5.1 µg/L; Pb(II) 342 µg/L and Hg(II) 136 µg/L | DPASV 1 | / | [15] |
| Paper-based electrode using Cladophora rupestris cellulose coated with polyaniline (PANI) | 0.2 mg/L–1.0 mg/L | Pb(II) 72 µg/L | LSASV 2 | / | [21] |
| Carbon paste electrode modified with Eichhornia crassipes powder | Pb (II) and Cd (II) 10 µg/L 5000 μg/L for Cd(II) and Pb (II) | 4.9 μg/L Cd(II), 2.1 μg/L Pb(II) | SWASV 3 | Natural water samples | [22] |
| Multiwall carbon nanotube MWCNT/(H2bpabza) novel tetradentate carboxamide ligand modified electrode | Pb(II) 1.72 µg/L– 23.8 µg/L; Cd(II) 0.930 µg/L–12.3 µg/L | Pb(II) 0.56 µg/L, Cd(II) 0.10 µg/L | SWASV 3 | Rice and tap water samples | [23] |
| Porous activated carbon-supported, palladium nanoparticles-modified glassy carbon electrode | Cd(II) 5.6 µg/L–56.2 µg/L Pb(II) 10.3 µg/L–104 µg/L Cu(II) 3.2 µg/L −32 µg/L | Cd(II) 2.3 µg/L, Pb(II) 1.90 µg/L, Cu(II) 0.97 µg/L | SWASV 3 | / | [24] |
| Zeolitic imidazolate chitosan-modified glassy carbon electrode | Hg(II) 1.0 μM–80.0 μM, Cu(II), 64 µg/L–6.4 mg/L Pb(II) 207 µg/L−20.7 mg/L Cd(II) 112 μg/L−11.2 mg/L | Hg(II) 5.86 µg/L, Cu(II) 6.96 µg/L, Pb(II) 12.8 µg/L, Cd(II) 15.2 µg/L | DPASV 1 | Lake water | [25] |
| Fully 3-D printed carbon nanofiber–graphite–polystyrene electrode | Zn(II) 12.7 μg/L−450 μg/L | Zn(II) 8.6 μg/L | DPASV 1 | Tap water | [19] |
| Diamond/graphite nanoplatelet electrode | 10 μg/L−250 μg/L | Zn(II) 1.72 μg/L, Cd(II) 0.47 μg/, Pb(II) 4.86 μg/L Cu(II), 0.45 μg/L | DPASV 1 | / | [26] |
| Nanoporous bismuth-modified electrode | 5.0 µg/L−40 µg/L | 1.3 µg/L Cd(II), 1.5 µg/L Pb(II) | SWASV 3 | Tap water | [27] |
| Single-walled carbon, nanohorns-modified, bismuth film screen-printed electrode | 1.0 μg/L−60 μg/L | 0.2 μg/L Cd(II), 0.4 μg/L Pb(II) | SWASV 3 | Honey and milk samples | [28] |
| Porous graphitic carbon nitride nanosheets and oxidized multiwalled carbon nanotube-modified screen-printed carbon electrode | Hg(II) 4.8 µg/L− 93.0 µg/L, Pb(II) 0.35 µg/L−6.5 µg/L and 6.5 µg/L−110 µg/L, Cd(II) 4.25 µg/L−79.0 µg/L and 79.0 µg/L−251 µg/L, Zn(II) 4.2 µg/L−202 µg/L | Hg(II) 0.04 µg/L, Pb(II) 0.008 µg/L, Cd(II) 0.03 µg/L, Zn(II) 0.06 µg/L | DPASV 1 | Vegetables (cabbage and capsicum) and food products (noodles) | [29] |
| Unmodified battery carbon rod electrode | 2.8 µg/L–110 µg/L Pb(II) | 2.8 µg/L Pb(II) | DPASV 1 | Tap water | This work |
| Original Concentration, µg/L | Added, µg/L | Found, µg/L | % Recovery | |
|---|---|---|---|---|
| 1 | ND | 21.3 | 20.3 | 95.3 |
| 2 | ND | 21.3 | 19.3 | 90.6 |
| 3 | ND | 21.3 | 19.8 | 93.0 |
| 4 | ND | 21.3 | 21.5 | 101 |
| 5 | ND | 21.3 | 20.9 | 98.3 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honeychurch, K. Trace Voltammetric Determination of Lead at a Recycled Battery Carbon Rod Electrode. Sensors 2019, 19, 770. https://doi.org/10.3390/s19040770
Honeychurch K. Trace Voltammetric Determination of Lead at a Recycled Battery Carbon Rod Electrode. Sensors. 2019; 19(4):770. https://doi.org/10.3390/s19040770
Chicago/Turabian StyleHoneychurch, Kevin. 2019. "Trace Voltammetric Determination of Lead at a Recycled Battery Carbon Rod Electrode" Sensors 19, no. 4: 770. https://doi.org/10.3390/s19040770