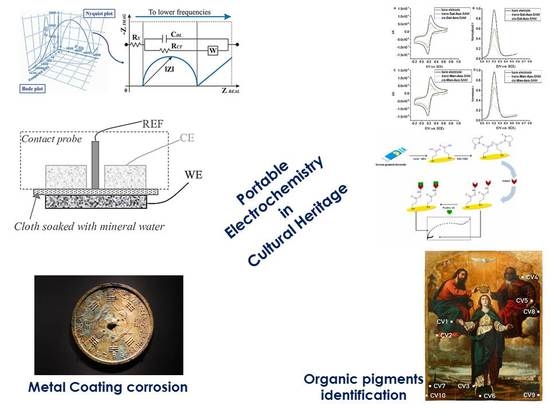
Smart Electrochemical Portable Tools for Cultural Heritage Analysis: A Review
Abstract
:1. Introduction
2. Electrochemical Impedance Spectroscopy in CH
EIS for Evaluating Corrosion Events and Analyzing Metallic Protective Layers of CH Objects and Surfaces
3. Electrochemical Devices to Apply in CH
3.1. Electrochemical Small Tools to Apply in Diagnosis and Preservation of CH
Portable Electrochemical Sensor Prototypes in CH Field Applications
3.2. Portable Electrochemical Immunosensors to Apply in CH Fields
3.3. Portable Electrochemical Biosensors to Apply in CH
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Price, C.; Hallam, D.; Heath, G.; Creagh, D.; Ashton, J. An electrochemical study of waxes for bronze sculpture. In Proceedings of the International Conference on Metal Conservation, Semur en Auxois, France, 25–28 September 1995; pp. 233–241. [Google Scholar]
- Otieno-Alego, V.; Hallam, D.; Viduka, A.; Heath, G.; Creagh, D. Electrochemical evaluation of the anti-corrosion performance of waxy coatings for outdoor bronze conservation. In Proceedings of the International Conference on Metal Conservation, Draguigan, France, 27–29 May 1998; pp. 315–319. [Google Scholar]
- Letardi, P.; Beccaria, A.; Marabelli, M.; D’Ercoli, G. Application of electrochemical impedance measurements as a tool for the characterization of the conservation and protection state of bronze works of art. In Proceedings of the International Conference on metals conservation, Draguignan-Figanières, France, 27–29 May 1998; pp. 303–308. [Google Scholar]
- Bendezú, R.D.P.H.; Gonçalves, R.P.; Neiva, A.C.; De Melo, H.G. EIS and Microstructural Characterization of Artificial Nitrate Patina Layers Produced at Room Temperature on Copper and Bronze. J. Braz. Chem. Soc. 2007, 18, 54–64. [Google Scholar] [CrossRef]
- Rocca, E.; Mirambet, F. The electrochemical techniques for the diagnosis and restoration treatments of technical and industrial heritage: Three examples of metallic artefacts. J. Solid State Electrochem. 2010, 14, 415–423. [Google Scholar] [CrossRef]
- Sabǎu Chelaru, J.D.; Mureşan, L.M.; Soporan, V.F.; Nemeş, O.; Kolozsi, T. A study on the corrosion resistance of bronzes covered with artificial patina. Int. J. Conserv. Sci. 2011, 2, 109–116. [Google Scholar]
- Degrigny, C.; Crawford, J.; Debattista, R. The “Drop” Ecorr vs time monitoring technique: A possible spot test for metal artefacts? In Proceedings of the Interim Meeting of the ICOM-CC Metal WG, Amsterdam, The Netherlands, 17–21 September 2007; Volume 3, p. 71. [Google Scholar]
- Fabjan, E.Š.; Kosec, T.; Kuhar, V.; Legat, A. Corrosion stability of different bronzes in simulated Urban rain. Materiali in Tehnologije 2011, 45, 585–591. [Google Scholar]
- Balbo, A.; Chiavari, C. Effectiveness of corrosion inhibitor films for the conservation of bronzes and gilded bronzes. Corros. Sci. 2012, 59, 204–212. [Google Scholar] [CrossRef]
- Martini, B.C.; Passarini, F.; Motori, A.; Bignozzi, M.C. Atmospheric corrosion of Cor-Ten steel with different surface finish: Accelerated ageing and metal release. Mater. Chem. Phys. 2012, 136, 477–486. [Google Scholar]
- De Cristofaro, N.; Gallese, F.; Laguzzi, G.; Luvidi, L. Selection of bronze alloys with reduced lead content suitable for outdoor sculptures. Mater. Chem. Phys. 2012, 132, 458–465. [Google Scholar] [CrossRef]
- Elsener, B.; Alter, M.; Lombardo, T.M.; Wörle, L.M.; Cocco, F.; Fantauzzi, M.; Palomba, S.; Rossi, A. A non-destructive in-situ approach to monitor corrosion inside historical brass wind instruments. Microchem. J. 2016, 124, 757–764. [Google Scholar] [CrossRef]
- Elsener, B.; Cocco, F.; Fantauzzi, M.; Palomba, S.; Rossi, A. Determination of the corrosion rate inside historical brass wind instruments—Proof of concept. Mater. Corros. 2016, 67, 1336–1343. [Google Scholar] [CrossRef]
- Robinson, A.M.; Harroun, S.G.; Bergman, J.C.; Brosseaur, L. Portable Electrochemical Surface-Enhanced Raman Spectroscopy System for Routine Spectroelectrochemical Analysis. Anal. Chem. 2012, 843, 1760–1764. [Google Scholar] [CrossRef]
- Dowsett, M.G.; Adriaens, A. Cell for Simultaneous Synchrotron Radiation X-ray and Electrochemical Corrosion Measurements on Cultural Heritage Metals and Other Materials. Anal. Chem. 2006, 78, 3360–3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentini, F.; Bicchieri, M.; Calcaterra, A.; Talamo, M. Raman, X-Ray Fluorescence Spectroscopies and Graphene Oxide Modified Screen Printed Electrodes to Identify the Pigments and Earth Present in Ancient Leather Samples. Electroanalysis 2017, 29, 2873–2881. [Google Scholar] [CrossRef]
- Domenèch-Carbò, A.; Domenèch-Carbò, M.T.; Moya-Moreno, M.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Identification of inorganic pigments from paintings and polychromed sculptures immobilized into polymer film electrodes by stripping differential pulse voltammetry. Anal. Chim. Acta 2000, 407, 275–289. [Google Scholar] [CrossRef]
- Gaetani, C.; Gheno, G.; Borroni, M.; De Wael, K.; Moretto, L.M.; Ugo, P. Nanoelectrode ensemble immunosensing for the electrochemical identification of ovalbumin in works of art. Electrochim. Acta 2019, 312, 72–79. [Google Scholar] [CrossRef]
- Sciutto, G.; Prati, S.; Mazzeo, R.; Zangheri, M.; Roda, A.; Bardini, L.; Valenti, G.; Rapino, S.; Marcaccio, M. Localization of proteins in paint cross-sections by scanning electrochemical microscopy as an alternative immunochemical detection technique. Anal. Chim. Acta 2014, 831, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sciutto, G.; Zangheri, M.; Prati, S.; Guardigli, M.; Mirasoli, M.; Mazzeo, R.; Roda, A. Immunochemical Micro Imaging Analyses for the Detection of Proteins in Artworks. Anal. Chem. Cult. Herit. 2016, 374, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bashir, R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 26, 135–150. [Google Scholar] [CrossRef]
- Scarano, S.; Carretti, E.; Dei, L.; Baglioni, P.; Minunni, M. Coupling non invasive and fast sampling of proteins from work of art surfaces to surface plasmon resonance biosensing: Differential and simultaneous detection of egg components for cultural heritage diagnosis and conservation. Biosens. Bioelectron. 2016, 85, 83–89. [Google Scholar] [CrossRef]
- Kassal, P.; Horak, E.; Sigurnjak, M.; Steinberg, M.D.; Steinberg, I.M. Wireless and mobile optical chemical sensors and biosensors. Rev. Anal. Chem. 2018, 37, 17–24. [Google Scholar] [CrossRef]
- Chechirlian, S.; Eichner, P.; Keddam, M.; Takenouti, H.; Mazille, H. A specific aspect of impedance measurements in low conductivity media. Artefacts and their interpretations. Electrochim. Acta 1990, 35, 1125–1131. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS or the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2010, 14, 381–391. [Google Scholar] [CrossRef]
- Letardi, P. Radiation in Art and Archeometry; Creagh, D.C., Bradley, O.A., Eds.; Elsevier Science B.V: Amsterdam, The Netherlands, 2000; pp. 15–39. [Google Scholar]
- Letardi, P.; Beccaria, A.; Marabelli, M.; D’Ercoli, G. Developpments of Electrochemical Impedance Spectroscopy as a Tool for Outdoors Bronze Corrosion Characterization. In Proceedings of the 2nd International Congress on Science and Tecnology for the Safeguard of Cultural Heritage in the Mediterranean Basin, Paris, France, 5–9 July 2000; pp. 407–411. [Google Scholar]
- Letardi, P.; Albini, M.; Joseph, E. EIS measurements for treatment testing: the case of a bio-based method applied on outdoor bronze statues in Switzerland. Available online: https://www.scienceopen.com/hosted-document?doi=10.14293/S2199-1006.1.SOR-.PPANDZU.v1 (accessed on 27 September 2019).
- Letardi, P. Laboratory and field test on patinas and protective coating systems for outdoor bronze monuments. In Proceedings of the International Conference on Metals Conservation, Published by the National Museum of Australia, ABN 70 592 297 967, Canberra, Australia, 4–8 October 2004; pp. 379–387. [Google Scholar]
- Ramírez Barat, B.; Cano, E. Advances for in-situ EIS measurements and their interpretation for the diagnostic of metallic cultural heritage: A review. ChemElectroChem 2018. [Google Scholar] [CrossRef]
- Cano, E.; Crespo, A.; Lafuente, D.; Ramirez Barat, B. A novel gel polymer electrolyte cell for in-situ application of corrosion electrochemical techniques. Electrochem. Commun. 2014, 41, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Ramírez Barat, B.; Cano, E. The use of agar gelled electrolyte for in situ electrochemical measurements on metallic cultural heritage. Electrochim. Acta 2015, 182, 751–762. [Google Scholar] [CrossRef]
- Newton, C.J.; Sykes, J.M. A galvanostatic pulse technique for investigation of steel corrosion in concrete. Corros. Sci. 1988, 28, 1051–1074. [Google Scholar] [CrossRef]
- Di Turo, F.; Matricardi, P.; Di Meo, C.; Mazzei, F.; Favero, G.; Zane, D. PVA hydrogel as polymer electrolyte for electrochemical impedance analysis on archaeological metals. J. Cult. Herit. 2019, 37, 113–120. [Google Scholar] [CrossRef]
- Carullo, A.; Ferraris, F.; Parvis, M.; Vallan, A.; Angelini, E.; Spinelli, P. Low-cost electrochemical impedance spectroscopy system for corrosion monitoring of metallic antiquities and works of art. IEEE Trans. Instrum. Meas. 2000, 49, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Angelini, E.; Carullo, A.; Corbellini, S.; Ferraris, F.; Gallone, V.; Grassini, S.; Parvis, M.; Vallan, A. Handheld-impedance-measurement system with seven-decade capability and potentiostatic function. IEEE Trans. Instrum. Meas. 2006, 55, 436–441. [Google Scholar] [CrossRef]
- Letardi, P.; Spiniello, R. Characterisation of bronze corrosion and protection by contact-probe electrochemical impedance measurements. In Proceedings of the International Conference on Metals Conservation, Santiago, Chile, 2–6 April 2001; pp. 316–319. [Google Scholar]
- Cano, E.; Bastidas, D.M.; Argyropoulos, V.; Siatou, A. Electrochemical techniques as a tool for testing the efficiency of protection Systems for historical 22 steel objects. In Proceedings of the International Conference on Conservation Strategies for Saving Indoor Metallic Collections, Cairo, Egypt, 25 February–1 March 2007. [Google Scholar]
- Joseph, E.; Letardi, P.; Mazzeo, R.; Prati, S.; Vandini, M. Innovative Treatments for the Protection of Outdoor Bronze Monuments. In Proceedings of the Interim Meeting of ICOM-CC Metal WG, Amsterdam, The Netherlands, 17–21 September 2007; pp. 71–77. [Google Scholar]
- Mazzeo, R.; Bittner, S.; Farron, G.; Fontinha, R.; Job, D.; Joseph, E.; Letardi, P.; Mach, M.; Prati, S.; Salta, M.; et al. Development and Evaluation of New Treatments for Outdoor Bronze Monuments. In Conservation Science 2007; Townsend, J.H., Toniolo, L., Cappitelli, F., Eds.; Archetype: London, UK, 2008. [Google Scholar]
- Denissen, P.J.; Garcia, J.S. Reducing subjectivity in EIS interpretation of corrosion and corrosion inhibition processes by in-situ optical analysis. Electrochim. Acta 2019, 293, 514–524. [Google Scholar] [CrossRef]
- Sansonetti, A.; Colella, M.; Letardi, P.; Salvadori, B.; Striova, J. Laser cleaning of a nineteenth-century bronze sculpture: In situ multi-analytical evaluation. Stud. Conserv. 2015, 60, S28–S33. [Google Scholar] [CrossRef]
- Letardi, P.; Salvadori, B.; Galeotti, M.; Cagnini, A.; Porcinai, S.; Santagostino Barbone, A.; Sansonetti, A. An in situ multi-analytical approach in the restoration of bronze artefacts. Microchem. J. 2016, 125, 151–158. [Google Scholar] [CrossRef]
- Clare, T.L.; Lins, P.A. Evaluation of fluorinated protective coatings for outdoor metals. In Proceedings of the Interim Meeting of the ICOM-CC Metal WG, Amsterdam, The Neatherlands, 17–21 September 2007; p. 8387. [Google Scholar]
- Grassini, S.; Angelini, E.; D’Agostino, R.; Palumbo, F.; Ingo, G.M. Advanced plasma treatment for cleaning and protecting precious metal artefacts. In Strategies for Saving our Cultural Heritage, Proceedings of the International Conference on Conservation Strategies for Saving Indoor Metallic Collections, Cairo, Egypt, 25 February–1 March 2007; Argyropoulos, V., Hein, A., Abdel Harith, M., Eds.; TEI of Athens: Athens, Greece, 2007; pp. 127–131. [Google Scholar]
- Angelini, E.; Grassini, S.; Corbellini, S.; Ingo, G.M.; De Caro, T. Potentialities of XRF and EIS portable instruments for the characterisation of ancient artefacts. Appl. Phys. A Mater. Sci. Process 2006, 83, 643–649. [Google Scholar] [CrossRef]
- McNamara, C.J.; Breuker, M.; Helms, M.; Perry, T.D.; Mitchell, R. Biodeterioration of Incralac used for the protection of bronze monuments. J. Cult. Herit. 2004, 5, 361–364. [Google Scholar] [CrossRef]
- Hallam, D.; Thurrowgood, D.; Otieno-Alego, V.; Creagh, D.; Viduka, A.; Heath, G. Studies of commercial protective petrochemical coatings on ferrous surfaces of historical and museum objects. In Metal 2001: proceedings of the international conference on metals conservation = Actes de la conférence internationale sur la conservation des métaux = Actas del congreso internacional sobre la conservacion de metales: Santiago, Chile, 2–6 April 2001; Western Australian Museum: Perth, Australia, 2004; pp. 297–303. ISBN 1-920843-17-5. [Google Scholar]
- Clare, T.L.; England, A.; Swartz, N.; Hosbein, K. Onsite electrochemical monitoring method for early detection of coating failure. In Proceedings of the Interim Meeting of the ICOM-CC Metal Working Group Conference Proceedings, Edinburgh, UK, 16–20 September 2013; pp. 89–94, ISBN 9781849171427. [Google Scholar]
- Ramírez Barat, B.; Cano, E.; Letardi, P. Advances in the design of a gel-cell electrochemical sensor for corrosion measurements on metallic cultural heritage. Sens. Actuators B 2018, 261, 572–580. [Google Scholar] [CrossRef]
- Ramírez Barat, B.; Crespo, A.; García, E.; Díaz, S.; Cano, E. An EIS study of the conservation treatment of the bronze sphinxes at the Museo Arqueológico Nacional (Madrid). J. Cult. Herit. 2017, 24, 93–99. [Google Scholar] [CrossRef]
- Ellingson, L.A.; Shedlosky, T.J.; Bierwagen, G.P.; De la Rie, E.R.; Brostoff, L.B. The use of electrochemical impedance spectroscopy in the evaluation of coatings for outdoor bronze. Stud. Conserv. 2004, 49, 53–62. [Google Scholar] [CrossRef]
- Crespo, A.; Ramírez Barat, B.; Diaz, I.; Cano Díaz, E. Assessment of the protective properties of patinas on contemporary sculpture made out of weathering steel. In Proceedings of the ICOM-CC 18th Triennial Conference, Brigland, Janet edt International Council of Museums (ICOM), Copenhagen, Denmark, 4–8 September 2017. [Google Scholar]
- Rodríguez-Acuña, F.; Genescá, J.; Uruchurtu, J. Electrochemical evaluation of patinas formed on nineteenth century bronze bells. J. Appl. Electrochem. 2010, 40, 311–320. [Google Scholar] [CrossRef]
- Hosbein, K.N.; Swartz, N.A.; Clare, T.L. Electrochemical Identification and Categorization of the Protective Quality of Intact and Damaged Coatings. Electroanalysis 2014, 26, 1935–1944. [Google Scholar] [CrossRef]
- Zhang, X.; He, W.; Odnevall Wallinder, I.; Pan, J.; Leygraf, C. Determination of instantaneous corrosion rates and runoff rates of copper from naturally patinated copper during continuous rain events. Corros. Sci. 2002, 44, 2131–2151. [Google Scholar] [CrossRef]
- Alexander, C.L.; Tribollet, B.M.; Orazem, E. Contribution of Surface Distributions to Constant-Phase-Element (CPE) Behavior: 1. Influence of Roughness. Electrochim. Acta 2015, 173, 416–424. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Brug, G.J.; Van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Feng, Y.; Teo, W.-K.; Siow, K.-S.; Tan, K.-l.; Hsieh, A.-K. The corrosion behavior of copper in neutral tap water. Part I: Corrosion mechanisms. Corros. Sci. 1996, 38, 369–385. [Google Scholar] [CrossRef]
- Feng, Y.; Teo, W.-K.; Siow, K.-S.; Hsieh, A.-K. The corrosion behavior of copper in neutral tap water. Part II: Determination of corrosion rates. Corros. Sci. 1996, 38, 387–395. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, J.G. Copper corrosion in potable water distribution systems: Influence of copper products on the corrosion behavior. Mater. Lett. 2004, 58, 2002–2006. [Google Scholar] [CrossRef]
- Valcarce, M.B.; De Sanchez, S.R.; Vazquez, M. A comparative analysis of copper and brass surface films in contact with tap water. J. Mater. Sci. 2006, 41, 1999–2007. [Google Scholar] [CrossRef]
- Yohai, L.; Vázquez, M.; Valcarce, M.B. Brass corrosion in tap water distribution systems inhibited by phosphate ions. Corros. Sci. 2011, 53, 1130–1136. [Google Scholar] [CrossRef] [Green Version]
- Mansfeld, F. An Introduction to Electrochemical Impedance Measurement; Technical Report No. 26 Part No.: BTR026 Issue: AB: May 1999, Solartron Limited; University of Southern California: Los Angeles, CA, USA, 1999. [Google Scholar]
- Sandberg, J.; Wallinder, I.O.; Leygraf, C.; Le Bozec, N. Corrosion-induced copper runoff from naturally and pre-patinated copper in a marine environment. Corros. Sci. 2006, 48, 4316–4338. [Google Scholar] [CrossRef]
- Otmačić Ćurković, H.; Kosec, T.; Marušić, K.; Legat, A. An electrochemical impedance study of the corrosion protection of artificially formed patinas on recent bronze. Electrochim. Acta 2012, 83, 28–39. [Google Scholar] [CrossRef]
- Marušića, K.; Otmačić-Ćurkovića, H.; Horvat-Kurbegović, Š.; Takenoutic, H.; Stupnišek-Lisaca, E. Comparative studies of chemical and electrochemical preparation of artificial bronze patinas and their protection by corrosion inhibitor. Electrochim. Acta 2009, 54, 7106–7113. [Google Scholar] [CrossRef]
- Marusic, H.; Otmacic-Curkovic, K.; Takenouti, H.; Mance, A.D.; Stupinsek-Lisac, E. Corrosion Protection of Synthetic Bronze Patina. Chem. Biochem. Eng. Q. 2007, 21, 71–76. [Google Scholar]
- Rahmounia, K.; Takenoutia, H.; Hajjaji, N.; Srhiri, A.; Robbiola, L. Protection of ancient and historic bronzes by triazole derivatives. Electrochim. Acta 2009, 54, 5206–5215. [Google Scholar] [CrossRef]
- Papadopoulou, O.; Delagrammatikas, M.; Vassiliou, P.; Angelini, E.; Grassini, S.; Ingo, G.M.; Gouda, V. Degradation phenomena of bronze artefacts in coastal archaeological environments of the Mediterranean basin. In Proceedings of the Conference: EUROCORR 2015—The European Corrosion Congress, Graz, Austria, 6–10 September 2015. [Google Scholar]
- Grassini, S.; Corbellini, S.; Parvis, M.; Angelini, E.; Zucchi, F. A simple Arduino-based EIS system for in situ corrosion monitoring of metallic works of art. J. Int. Meas. Confed. 2018, 114, 508–514. [Google Scholar] [CrossRef]
- Cano, E.; Bastidas, D.M.; Argyropoulos, V.; Fajardo, S.; Siatou, A.; Bastidas, J.M.; Degrigny, C. Electrochemical characterization of organic coatings for protection of historic steel artefacts. J. Solid State Electrochem. 2010, 14, 453–463. [Google Scholar] [CrossRef]
- Brunoro, G.; Frignani, A.; Colledan, A.; Chiavari, C. Organic films for protection of copper and bronze against acid rain corrosion. Corros. Sci. 2003, 45, 2219–2231. [Google Scholar] [CrossRef]
- England, A.H.; Clare, T.L. Synthesis and Characterization of Flexible Hydrogel Electrodes for Electrochemical Impedance Measurements of Protective Coatings on Metal Sculptures. Electroanalysis 2014, 26, 1059–1067. [Google Scholar] [CrossRef]
- Muresana, L.; Varvara, S.; Stupnišek-Lisac, E.; Otmačić, H.; Marušićc, K.; Horvat-Kurbegović, S.; Robbiola, L.; Rahmounif, K.; Takenouti, H. Protection of bronze covered with patina by innoxious organic substances. Electrochim. Acta 2007, 52, 7770–7779. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Brosseau, C.L.; Casadio, F.; Van Duyne, R.P. Revealing the invisible: Using surface-enhanced Raman spectroscopy to identify minute remnants of color in Winslow Homer’s colorless skies. J. Raman Spectrosc. 2011, 42, 1305–1310. [Google Scholar] [CrossRef]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 2002. [Google Scholar]
- MacLeod, I.D. Stabilization of Corroded Aluminum. In Proceedings of the 8th Triennial ICOM-CC Meeting, Sydney, Australia, 6–11 September 1987; Grimstad, K., Ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 1987; pp. 1079–1085. [Google Scholar]
- Ghosh, P.K.; Mau, A.W.-H.; Bard, A.J. Clay-modified electrodes: Part II. Electrocatalysis at bis(2,2’-bipyridyl) (4,4’-dicarboxy-2,2’-bipyridyl)Ru(II)-dispersed ruthenium dioxide—hectorite layers. J. Electroanal. Chem. 1984, 169, 315–317. [Google Scholar] [CrossRef]
- VL.6-16/97: Sample from a Red Brown Zone in the Canvas Painting ‘Virgen de Gracia’ Painted by Vicente López Y Portaña (Spanish, 1772-1850), Virgin of the Immaculate Conception, oil on canvas, around 1795-1800. Available online: http://ceres.mcu.es/pages/ResultSearch?txtSimpleSearch=La%20Coronaci%F3n%20de%20la%20Virgen&simpleSearch=0&hipertextSearch=1&search=simpleSelection&MuseumsSearch=MNR%7C&MuseumsRolSearch=17&(accessed on 27 September 2019).
- RO.16-2/98: Sample from the Red Robes of a Main Character in the Frescoes of the Virgen del Rosario (1940) Church in Valencia (Spain), Painted by José Ferrandis Ros (1901-1981). Available online: http://www.jdiezarnal.com/valenciaiglesiadenuestrasenoradelrosario.html(accessed on 27 September 2019).
- De Leo, M.; Pereira, F.C.; Moretto, L.M.; Scopece, P.; Polizzi, S.; Ugo, P. Towards a better understanding of gold electroless deposition in track-etched templates. Chem. Mater. 2007, 19, 5955–5964. [Google Scholar] [CrossRef]
- Menon, V.P.; Martin, C.R. Fabrication and evaluation of nanoelectrode ensembles. Anal. Chem. 1995, 67, 1920–1928. [Google Scholar] [CrossRef]
- Bottari, F.; Oliveri, P.; Ugo, P. Electrochemical immunosensor based on ensemble of nanoelectrodes for immunoglobulin IgY detection: Application to identify hen’s egg yolk in tempera paintings. Biosens. Bioelectron. 2014, 52, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Cennini, C. Il libro dell’arte; Frezzato, F., Ed.; Neri Pozza: Vicenza, Italy, 2004. [Google Scholar]
- Dolci, L.S.; Sciutto, G.; Guardigli, M.; Rizzoli, M.; Prati, S.; Mazzeo, R.; Roda, A. Ultrasensitive chemiluminescent immunochemical identification and localization of protein components in painting cross-sections by microscope low-light imaging. Anal. Bioanal. Chem. 2008, 392, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sciutto, G.; Dolci, L.S.; Guardigli, M.; Zangheri, M.; Prati, S.; Mazzeo, R.; Roda, A. Single and multiplexed immunoassays for the chemiluminescent imaging detection of animal glues in historical paint cross-sections. Anal. Bioanal. Chem. 2013, 45, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Sciutto, G.; Litti, L.; Lofrumento, C.; Prati, S.; Ricci, M.; Gobbo, M.; Roda, A.; Castellucci, E.; Meneghetti, M.; Mazzeo, R. Alternative SERRS probes for the immunochemical localization of ovalbumin in paintings: An advanced mapping detection approach. Analyst 2013, 138, 4532–4541. [Google Scholar] [CrossRef] [PubMed]
- Sciutto, G.; Dolci, L.S.; Buragina, A.; Prati, S.; Guardigli, M.; Mazzeo, R.; Roda, A. Development of a multiplexed chemiluminescent immunochemical imaging technique for the simultaneous localization of different proteins in painting micro cross-sections. Anal. Bioanal. Chem. 2011, 399, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamental and Applications; John Wiley & Sons: New York, NY, USA, 1980; pp. 316–367. [Google Scholar]
- Ruan, C.; Yang, L.; Li, Y. Immunobiosensor chips doe detection of Escherichia coli O157:H7 using electrochemical impedance spectroscopy. Anal Chem. 2002, 74, 4814–4820. [Google Scholar] [CrossRef]
- Laureyn, W.; Frederix, F.; Van Gerwen, P.; Maes, G. Nanoscaled interdigitated gold electrodes for impedimetric immunosensing. In Transducers’99, Digest of Technical Papers, Proceedings of the 10th International Conference on Solid-State Sensors and ActuatorsSendai, Japan, 7–10 June 1999; pp. 1884–1885.
- Radke, S.M.; Alocilja, E.C. A high-density microelectrode array biosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y.; Srinivasan, B.; Tung, S. A label-free, microfluidic and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticle immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sens. Actuators B Chem. 2007, 128, 99–107. [Google Scholar] [CrossRef]
- Carretti, E.; Natali, I.; Matarrese, C.; Bracco, P.; Weiss, R.G.; Baglioni, P.; Salvini, A.; Dei, L. A new family of high viscosity polymeric dispersions for cleaning easel paintings. J. Cult. Herit. 2010, 11, 373–380. [Google Scholar] [CrossRef]

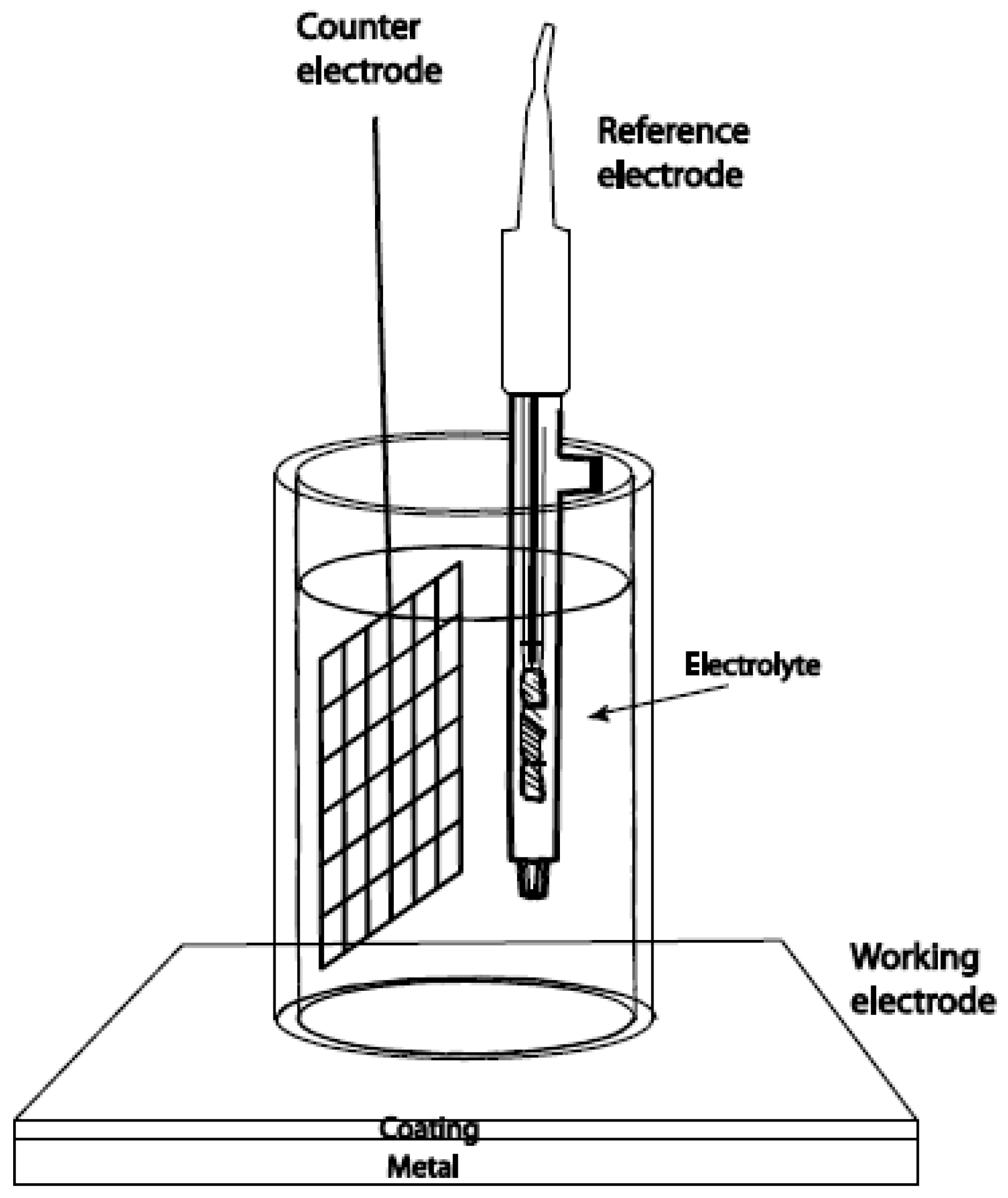










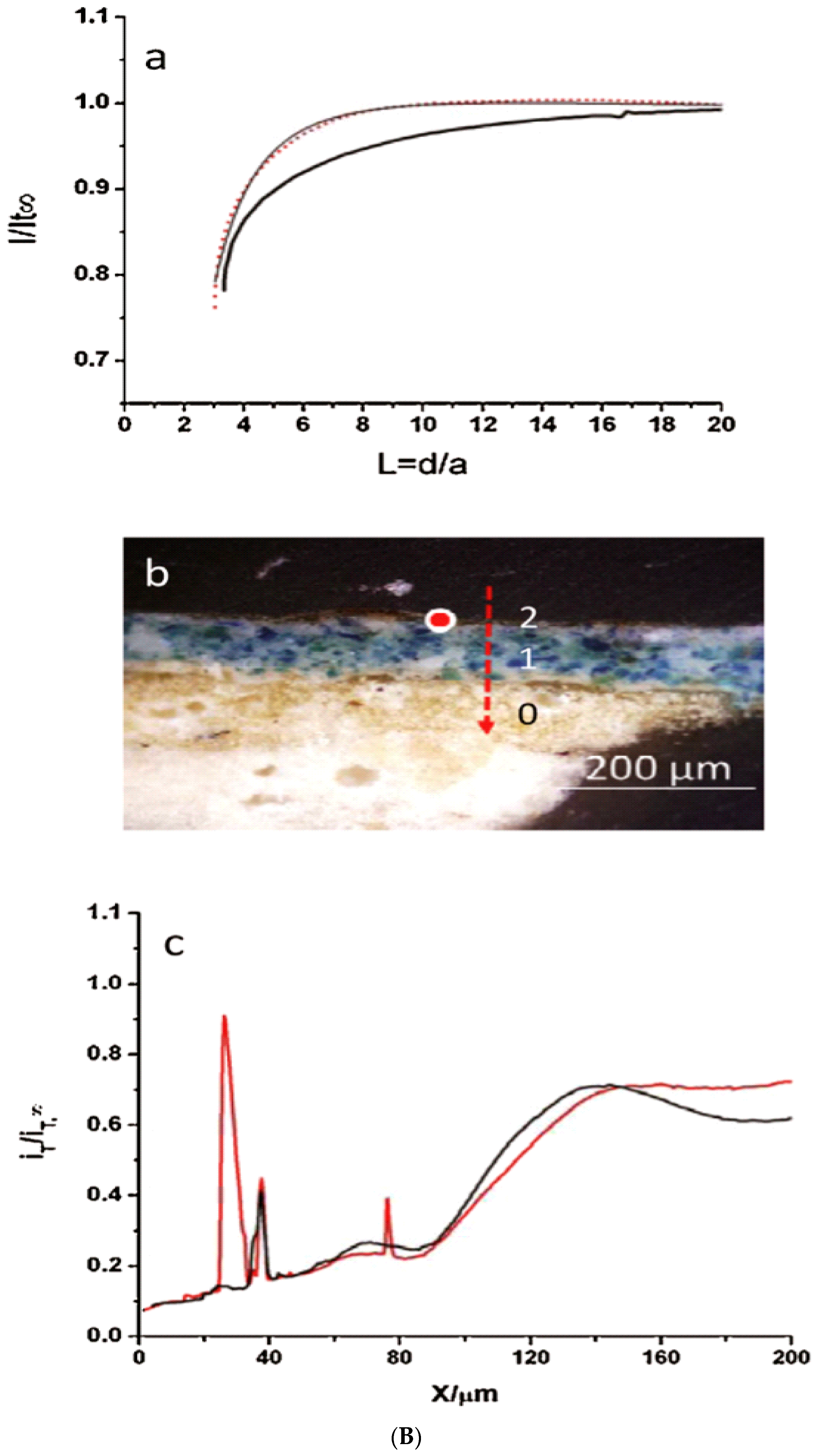


| Original Metal Samples | Equivalent Circuit (ECs) and Electrical Elements | Real Case of Study: Original Metal Coatings/Layers | References |
|---|---|---|---|
| Clean metals | Randles circuit with Re that is the electrolyte resistance; CPEdl is the double-layer capacitance; Rct is the charge transfer resistance, and finally W is the Warburg impedance for copper ions, during their diffusion through the oxide film | The Randles circuit was analytically standardize and validated, by using the standard corrosion events of copper in slightly mineralized neutral aqueous solutions (such as tap water, as conventional working medium) | [60,61] |
| Clean metals | A variation of the previous Randles circuit consists of the charge transfer resistances of cathodic and anodic processes, are in parallel in different branches of the circuit | The EIS spectra, acquired with the modified Randles circuit, was applied to recorded EIS profiles for artistic bronze coupons in artificial rain | [60] |
| Clean metals | Other authors have applied a simple two nested (R-CPE) couple circuit, but fitting results showing the exponent of the second CPE close to 0.5, suggesting a diffusion impedance, both in copper and brass | Copper and brass surfaces for application of the two nested (R-CPE) couple of electrochemical circuits | [62,63,64] |
| Metals with patinaCopper and alloys | The first EC (where the pair CPEdl-Rct is not considered to be electrical equivalent element) represents the double-layer patina morphology, quite similar to the anodized layers in aluminum model, which consist of a thin barrier layer covered by a porous outer layer | This equivalent circuit describes the outdoor copper and bronze patinas, exhibiting a double-layer structure. In particular, the inner layer contains cuprous oxide materials and an outer layer, appears more porous for the presence of different cupric compounds, depending on the environment to which the object is exposed and located | [65] |
| Metals with patina Copper and alloys | The second EC with two nested (R-CPE) couple circuit, represents the impedance of the inner and outer patina layer. Considering an exponent value of 0.5 for the CPE in the inner layer, a Warburg/W impedance, could replace the CPE final circuit. | The same EC was applied to study the response of bronze roman coins and natural copper patinas, formed during 1–3 years in Chile in different environments, with different thickness and porosity depending on their location | [66] |
| Metals with patina Copper and alloys | Three nested (R-CPE) circuits were reported in the literature, to explain the electrochemical output signals regarding artificial patinas, putting in Na2SO4-NaHCO3, as working electrolytes. The first (R-CPE) pair represents the resistance and capacitance of the patina, the second (R-CPE), at intermediate frequencies, represents the corrosion process on the metal surface, while third (R-CPE) couple that corresponds to the low-frequency loop is explained as a result of oxidation–reduction processes of the corrosion products taking place at the electrode surface | A first example of this three nested (R-CPE) circuits is the EIS spectrum profiles of samples, collected by a brass object, excavated from the archaeological area of Tharros, in 0.1 M NaCl (as working electrolyte). Another example concerns archaeological bronze coins, working with NaCl 0.3 M-5% agar electrolyte and mineral water. This EC is depicted in a different order (Re[(Rct-CPEdl)(Rpl-CPEpl)]) that is mathematically. Equivalent as EC and which best represents the original sample | [67,68,69,70,71] |
| Metals with patina Iron and steel | Two-cell EC have been applied to describe the impedance of the two interfaces: metal/rust layer and rust layer/electrolyte, respectively. A third time constant seems to be present at low frequencies, applying the R(RC(C[RW])) model and performing measurements in the G-PE cell | Regarding the Two-cell EC circuits electrochemical studies were carried out on weathering steel sculptures from Adriana Veyrat; Politecnico di Torino performed EIS measurements on historic iron surface/coating belonging to the Notre-Dame Cathedral of Amiens and the Metz Cathedral, in France | [72] |
| Evaluation of coatings | A metal-coating system is a capacitor and a resistance in parallel, according to the capacitance (Ccoat) and resistance (Rcoat) of the metal coating in series with the resistance of the working electrolyte, (Re). In highly protective coatings, Rcoat is very high and the system becomes Ccoat in series with Re (no current crosses the resistance). When the coating deteriorates the circuit, changes and the main electric components/elements are Cdl (the double-layer capacitance and Rct (the charge transfer resistance) of the corrosion process that occurs at the metal-electrolyte interface. This circuit was applied to the characterization of organic coatings, including varnishes and waxes for bronze and historic steel artwork objects | Not reported cases of studies on original samples | [73,74,75] |
| Evaluation of inhibitors | The EC circuits are the same of the clean surfaces, only differing in the values of different parameters | Not reported cases of studies on original samples | [76,77] |
| Electrochemical Configurations | Quantitative Analysis | Qualitative Analysis | Main Advantages | Main Disadvantages |
|---|---|---|---|---|
| Setup1: | ---------------------------- | ✓ | easy sensor geometry for in situ manipulation | Stiff (not flexible) contact probe and the liquid conductive electrolytes provoke electrochemical contact problems toward the surface of cultural heritage, compromising the final electrochemical measurements |
| Setup2: | ---------------------------- | ✓ | Flexible cell geometries suitable for CH surfaces (with significant roughness) Solid conductive electrolytes to guarantee the electrochemical/electrical contact toward CH surfaces | The difficulty of processing the acquired experimental data by ECs, working with Nyquist plot |
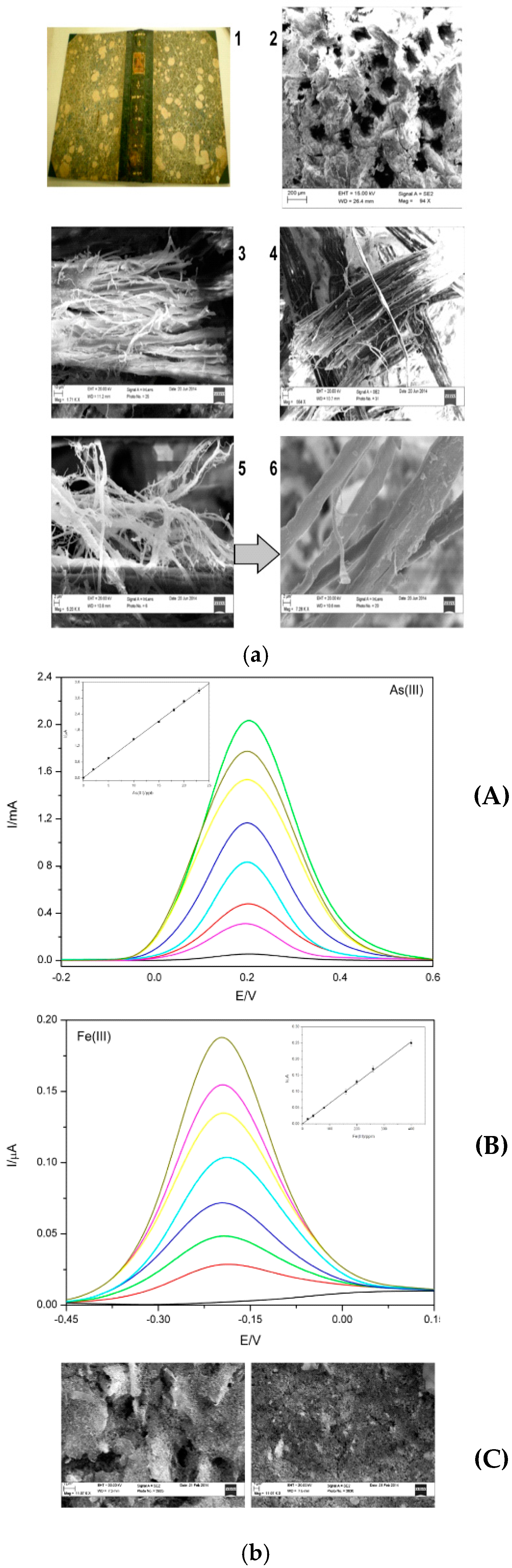
| Sample As(III) | GO/SPEs by SWCSV (μg/L) | Total Inorganic As: [As(III) + As(V)] Total Inorganic Fe: [FeO + Fe3O4 + Fe2O3] (μg/L) | ICP-MS (μg/L) | P Value (t-Test)a |
|---|---|---|---|---|
| 1 | 992 ± 2 | 101 ± 2 | 100.80 ± 0.5 | 0.01 |
| 2 | 100 ± 1 | 100 ± 1 | 100.00 ± 0.02 | 0.03 |
| 3 | 102 ± 2 | 103 ± 1 | 103.05 ± 0.5 | 0.02 |
| Sample Fe(III) | ||||
| 1 | 399 ± 1 | 400 ± 1 | 400 ± 1 | 0.04 |
| 2 | 400 ± 2 | 400 ± 2 | 400 ± 0,2 | 0.01 |
| 3 | 402 ± 1 | 403 ± 0.3 | 403 ± 1 | 0.03 |
| Electrochemical Devices | Quantitative Analysis | Qualitative Analysis | Main Advantages | Main Disadvantages |
|---|---|---|---|---|
| Raman spectrometer, equipped with a potentiostatic apparatus | LOD = 5 ppm (mg/L) for melamine | ✓ | low cost measurement, no-time consuming analysis, high sensitivity, low detection of limit | Non-electroactive pigments and organic binders cannot quantify by electrochemistry |
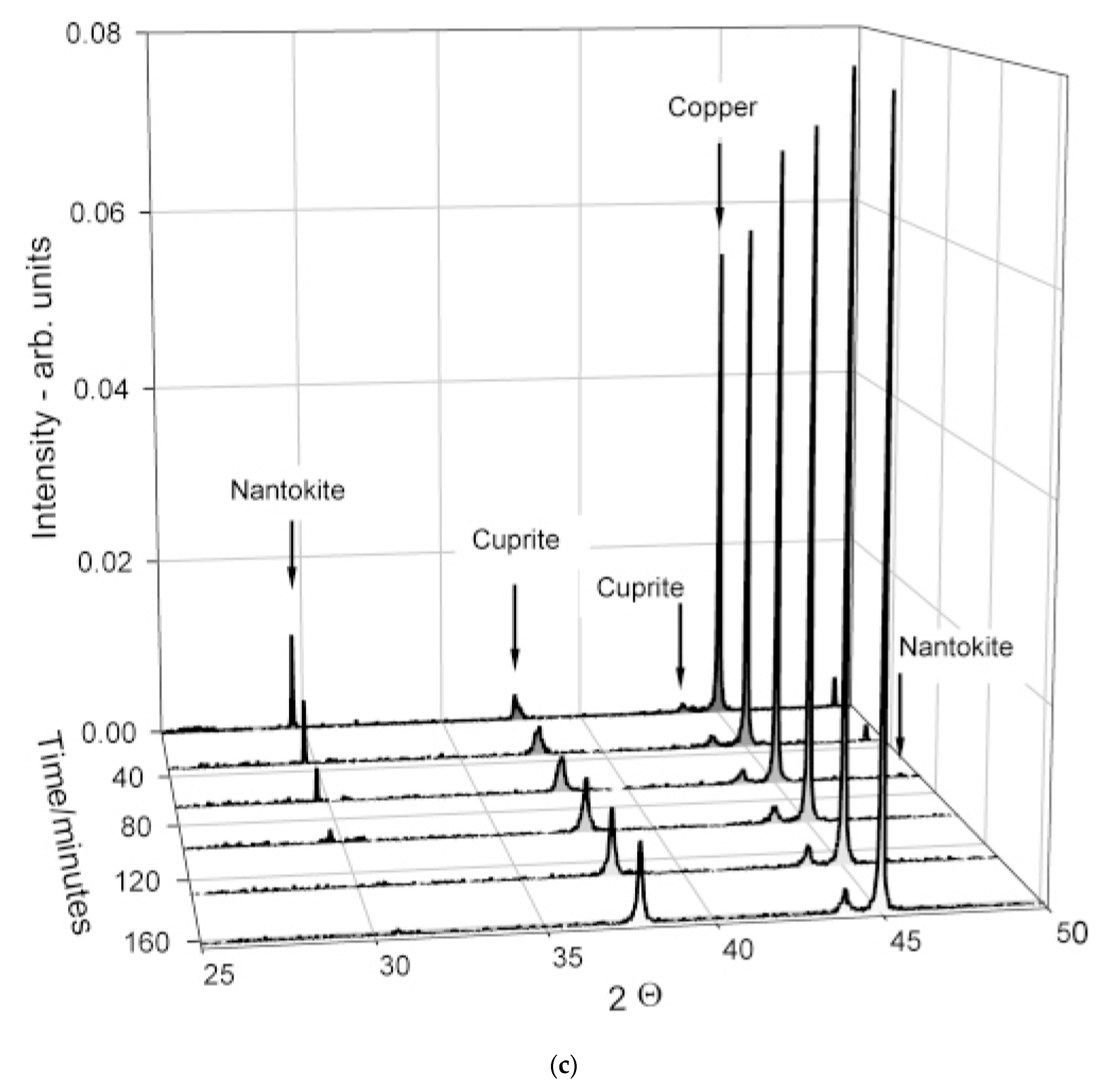
| Electrochemical cell, which also works like a Bragg tool | ---------------------------- | ✓ | to detect different crystallographic phases during serious corrosion events, to detect the corrosion potential Ecorr, both parameters, unequivocally provide important information on the nature of metallic corrosion phenomena | not all the corrosion products, to which different crystallographic phases belong, have electrochemical activity, therefore the disadvantage is that there is only qualitative crystallographic information |
| Screen-Printed Electrodes (SPEs) chemically modified with Graphene Oxide (GO); GO/SPE | See Table 3 | ✓ | Voltammetric techniques are useful to understand two important aspects, as -the chemical-physical composition of the colors; -the oxidation status and conservation conditions of metallic elements contained in organic pigments, useful to select the best restoration strategies | Particularly useful only in the case of metals contained in inks, colored pigments and organic compounds The absence of metals makes the application of this device more difficult, as the organic component (very often) does not have a marked electrocatalytic activity |
| micro-sample coatings in Paraloid B72 film-modified electrodes, combined with | ---------------------------- | ✓ | micro-sample coatings in Paraloid B72-film provide the possibility of pre-concentrating the pigments to increase the Signal/Noise ratio, especially in the presence of traces of organic materials | micro-sample coatings in Paraloid B72-film are not so selective and for this purpose several interferences and/or passivation/fouling effects can occur during analysis/measurements |
| Immunosensor Prototype | Quantitative Analysis | Qualitative Analysis | Main Advantages | Main Disadvantages |
|---|---|---|---|---|
| Nano-Electrode Ensembles (NEEs) immunosensors for the detection of ovalbumin in paintings | ✓ | ----------------------------------- | High selectivity and specificity for the proteins and organic binder recognition, more than FTIR traditional method | No quantification of the organic component in paintings and this could represent a lack of useful information for restorers |
| Nano-Electrode Ensembles (NEEs) immunosensors for the detection of egg yolk in tempera and paintings | ✓ | ----------------------------------- | High selectivity and specificity for the proteins and egg yolk recognition, more than FTIR traditional method | No quantification of the organic component in paintings and this could represent a lack of useful information for restorers |
| Scanning Electro-Chemical Microscopy (SECM) immunosensors | ✓ | ------------------------------------ | Immunochemical stratigraphic SECM is excellent to selectively identify the organic components in the painting layers | Low reproducibility due to the passivation/fouling of the scanning/electrode-based tip Low Signal/to Noise Ratio |
| Biosensor Prototype | Quantitative Analysis | Qualitative Analysis | Main Advantages | Main Disadvantages |
|---|---|---|---|---|
| Impedance biosensor devices, recording Faradaic signal, in the presence of the electroactive probes | ✓ | Linear range of concentration: 6.0 × 104–6.0 × 107 cells/mL; Limit Of Detection (L. O. D.): 6.0 × 102 cells/mL. | High selectivity and sensitivity toward the organic binder recognition, more than FTIR traditional method. The biosensor regeneration opportunity | The inadequacy, in some case studies, of the Randles model equivalent electrical circuit to describe the electrochemical impedance biosensor prototypes |
| Impedance biosensor devices, recording non-Faradaic signal, without the electroactive probes | ✓ | Linear range of concentration: 104–107 cfu/mL; Limit Of Detection (L. O. D.): 1.0 × 102 cells/mL. | High Signal/Noise ratio Lower LOD | Possible Fouling and passivation phenomenon with interference on the output signals due to the stable chemical bonds between bacteria suspension and the immobilized antibodies |
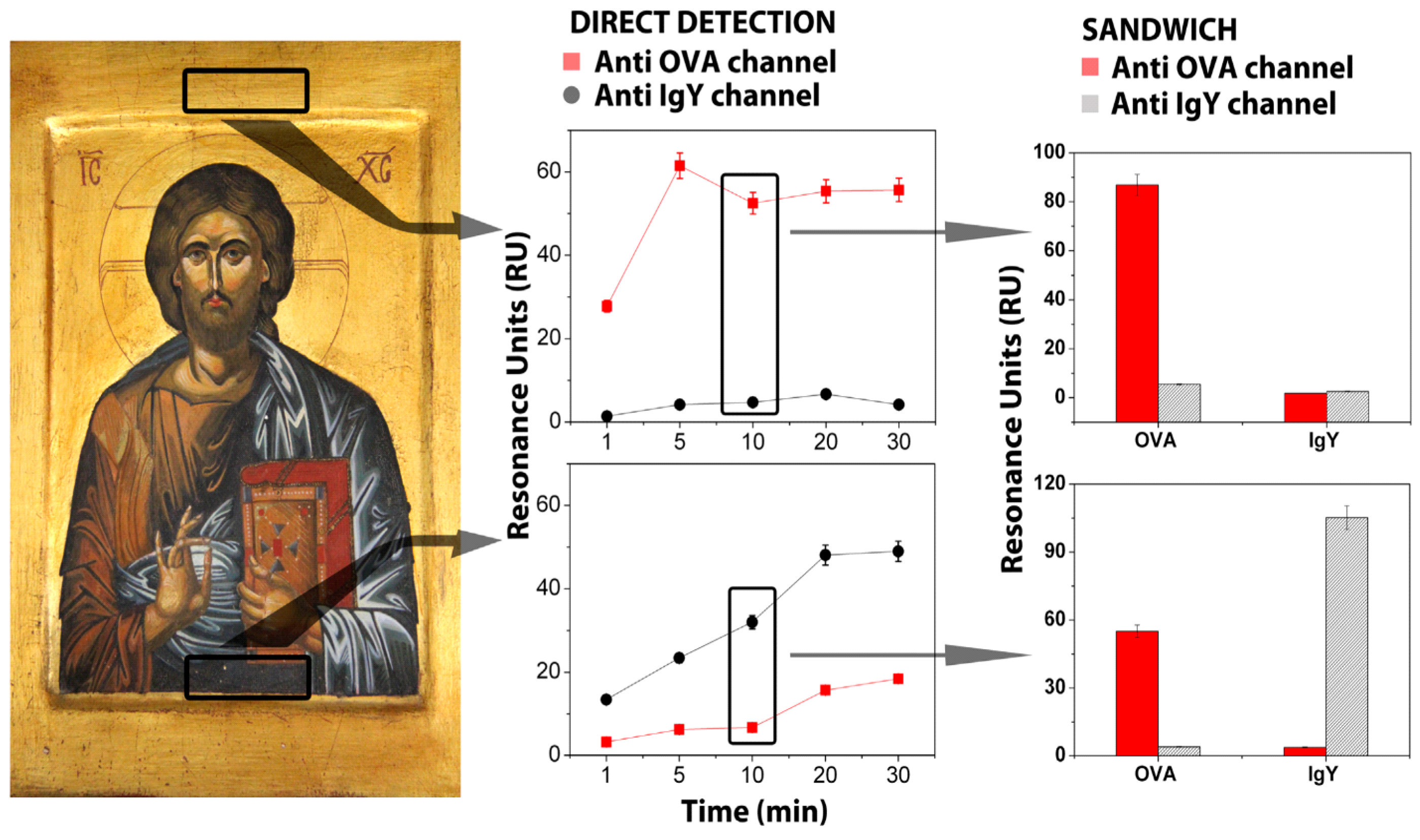
| Surface Plasmon Resonance-based biosensors | ✓ | The direct assay of albumen/yolk mixture recognized the presence of both chemical analytes (140.0 ± 5.6 RU) for (anti-OVA) channel, (123.3 ± 4.9 RU) for (anti-IgY) channel. | The experimental results confirmed that very short application times were enough to extract useful information and, at the same time, they were ideal for avoiding invasive treatments on original artwork surfaces | The main analytical problem could be related to the interference when in absence of Highly Viscous Polymeric Dispersions (HVPDs) extracts from original samples |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, F. Smart Electrochemical Portable Tools for Cultural Heritage Analysis: A Review. Sensors 2019, 19, 4303. https://doi.org/10.3390/s19194303
Valentini F. Smart Electrochemical Portable Tools for Cultural Heritage Analysis: A Review. Sensors. 2019; 19(19):4303. https://doi.org/10.3390/s19194303
Chicago/Turabian StyleValentini, Federica. 2019. "Smart Electrochemical Portable Tools for Cultural Heritage Analysis: A Review" Sensors 19, no. 19: 4303. https://doi.org/10.3390/s19194303