A Potassium Ion-Exchanged Glass Optical Waveguide Sensor Locally Coated with a Crystal Violet-SiO2 Gel Film for Real-Time Detection of Organophosphorus Pesticides Simulant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. K+ Ion-Exchanged Glass OWG Preparation
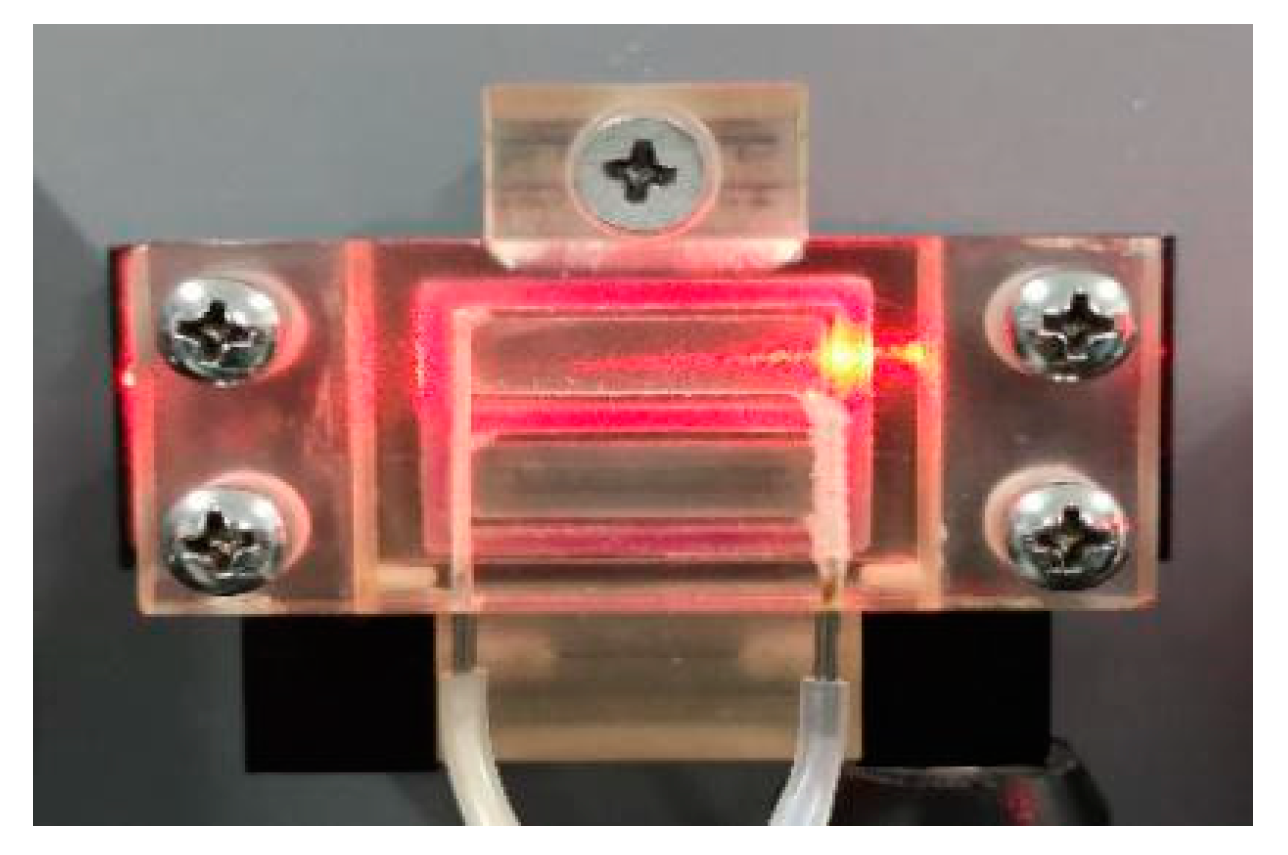
2.3. Fabrication of the CV-SiO2 Gel Film Coated PIE OWG Chip
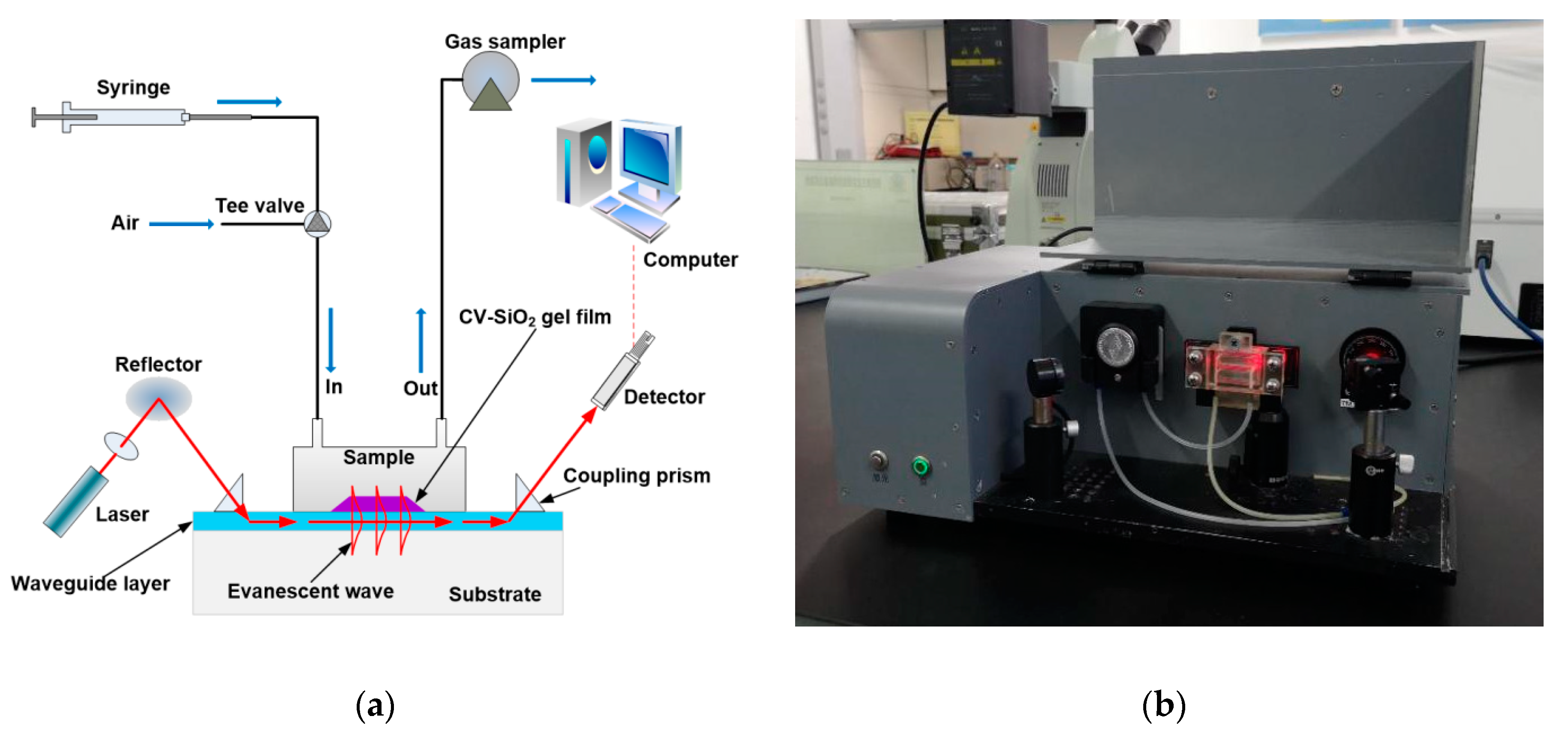
2.4. Measurement Procedures for Testing for Gas
3. Results and Discussion
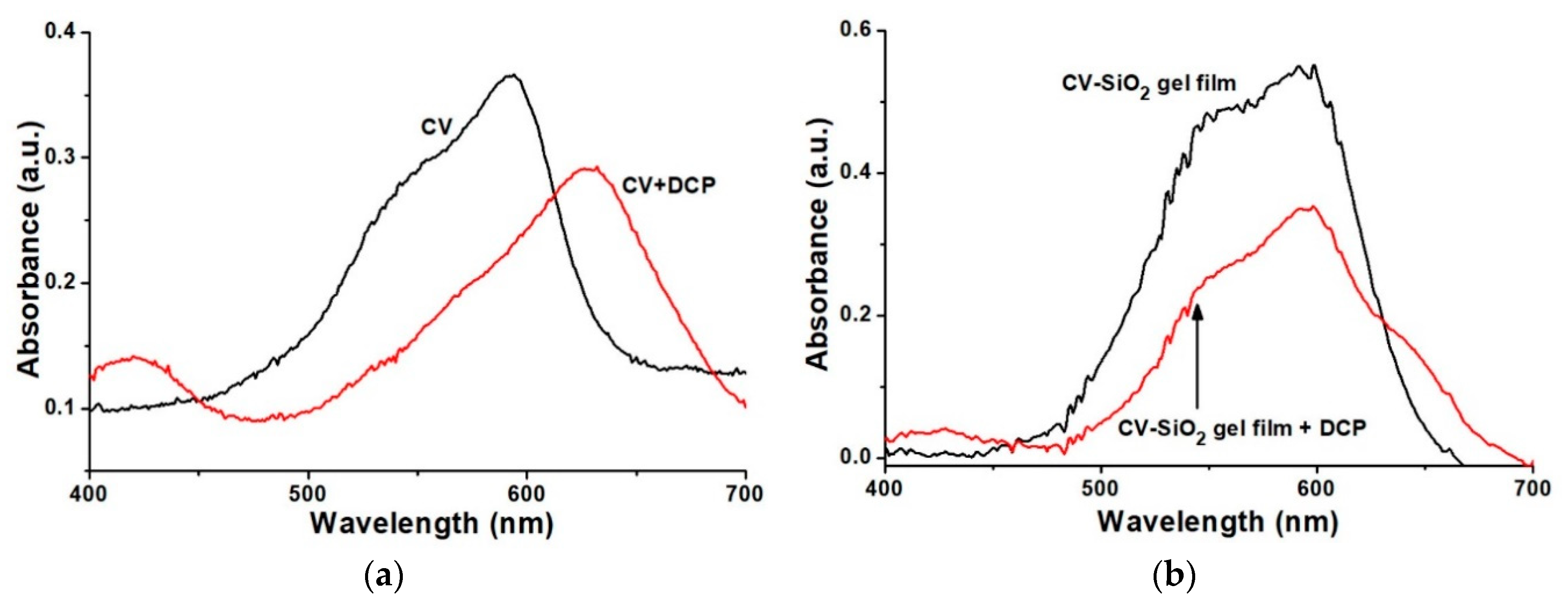
3.1. Topographical Characterization of the CV-SiO2 Gel Film
3.2. Performance of the Sensor Device
3.3. Sensing Principle
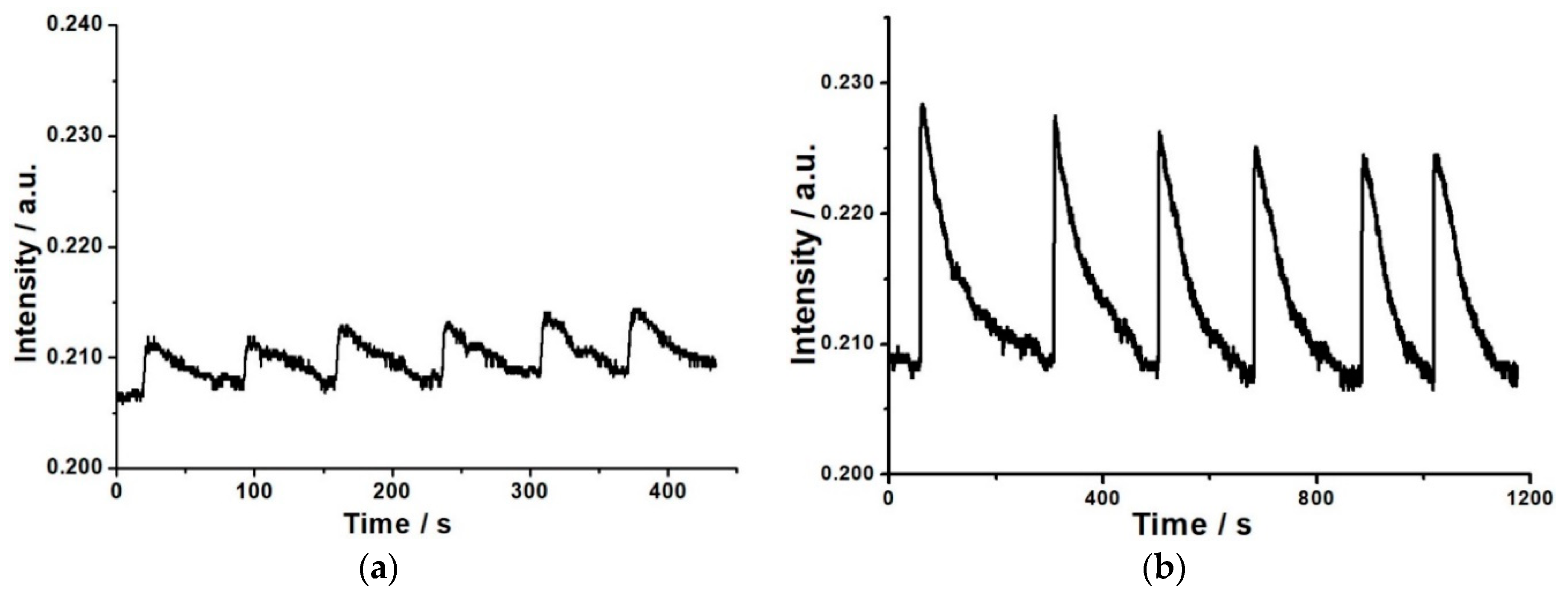
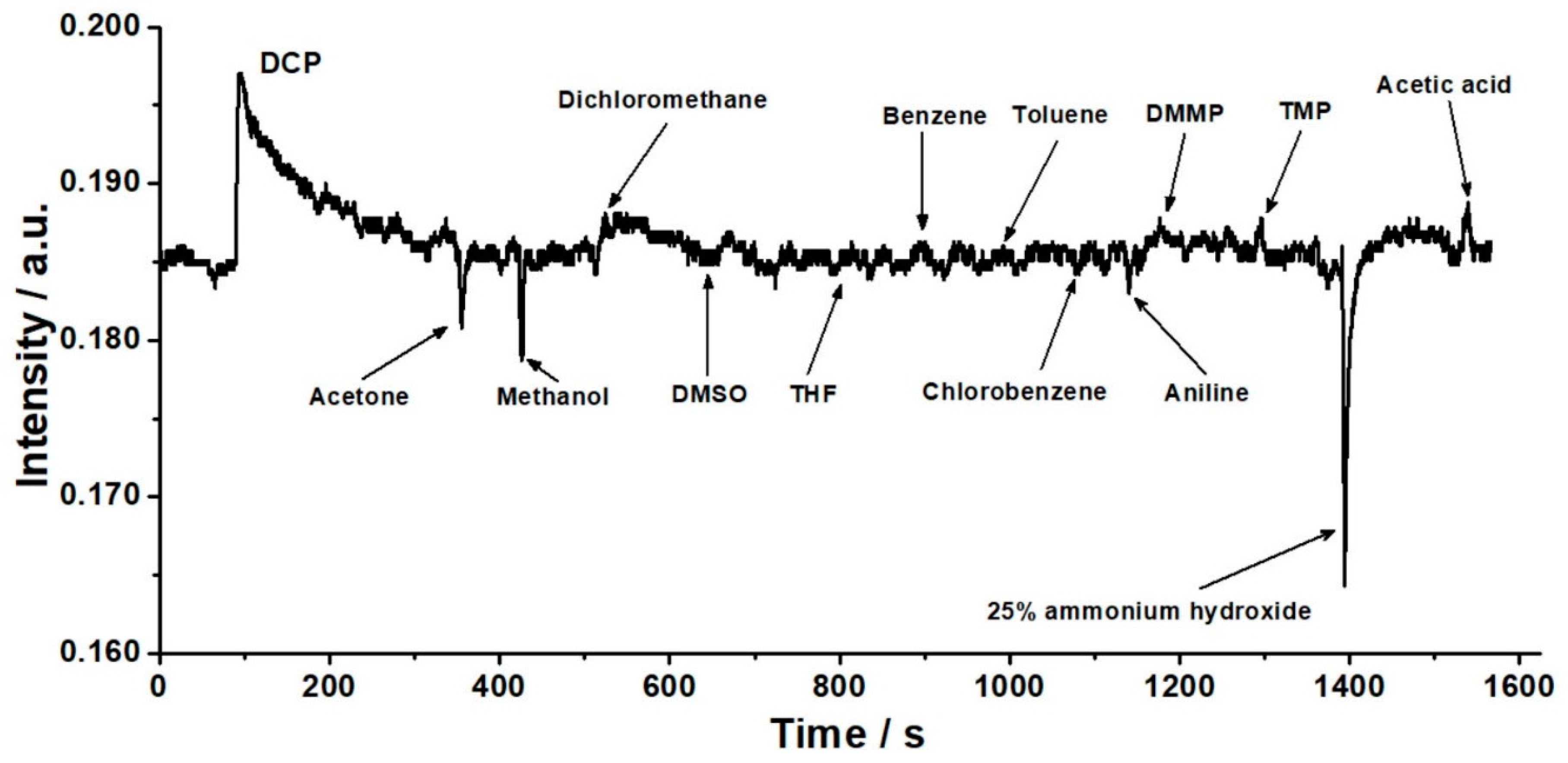
3.4. Reproducibility and Selectivity of the Response of the OWG Sensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goswami, S.; Manna, A.; Paul, S. Rapid ‘naked eye’ response of DCP, a nerve agent simulant: From molecules to low-cost devices for both liquid and vapour phase detection. RSC Adv. 2014, 4, 21984–21988. [Google Scholar] [CrossRef]
- Rusu, A.D.; Moleavin, I.A.; Hurduc, N.; Hamel, M.; Rocha, L. Fluorescent polymeric aggregates for selective response to Sarin surrogates. Chem. Commun. 2014, 50, 9965–9968. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Kaufmann, K.; Orozco, J.; Li, J.; Galarnyk, M.; Arya, G.; Wang, J. Micromotor-based on–off fluorescence detection of sarin and soman simulants. Chem. Commun. 2015, 51, 11190–11193. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, Y.; Chen, B.; Yao, S. Simultaneous determination of 102 pesticide residues in Chinese teas by gas chromatography–mass spectrometry. J. Chromatogr. B 2007, 853, 154–162. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; Knoll, F.R.N.; Apon, B.M. Supercritical fluid extraction for pesticide multiresidue analysis in honey: Determination by gas chromatography with electron-capture and mass spectrometry detection. J. Chromatogr. A 2004, 1048, 153–159. [Google Scholar] [CrossRef]
- Satoh, T.; Kishi, S.; Nagashima, H.; Tachikawa, M.; Kanamori-Kataoka, M.; Nakagawa, T.; Kitagawa, N.; Tokita, K.; Yamamoto, S.; Seto, Y. Ion mobility spectrometric analysis of vaporous chemical warfare agents by the instrument with corona discharge ionization ammonia dopant ambient temperature operation. Anal. Chim. Acta 2015, 865, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 616, 632–639. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Wang, B.; Wang, S.; Li, H.; Chen, J. Selectivity improvement of positive photoionization ion mobility spectrometry for rapid detection of organophosphorus pesticides by switching dopant concentration. Talanta 2018, 176, 247–252. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Shi, X.; Yang, F.; Meng, G.; Xiong, Q.; Ke, Y.; Wang, H.; Lu, Y.; Wu, N. Detection of dithiocarbamate pesticides with a spongelike surface-enhanced raman scattering substrate made of reduced graphene oxide-wrapped silver nanocubes. ACS Appl. Mater. Interfaces 2017, 9, 39618–39625. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, G.; Wu, M.; Chen, Z.; Lu, P.; Wu, W. Large-scale fabrication of ultrasensitive and uniform surface-enhanced Raman scattering substrates for the trace detection of pesticides. Nanomaterials 2018, 8, 520. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.J.; Li, J.J.; Li, X.; Zhao, J.W. Multi-branched gold nanostars with fractal structure for SERS detection of the pesticide thiram. Spectrochim. Acta Part A 2018, 189, 586. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhang, F.; Cao, W.; Wang, P.; Li, Z.; Ding, Z. A naphthalimide-based fluorescent turn-on sensor for the selective detection of diethyl chlorophosphate. ChemistrySelect 2018, 3, 13470–13471. [Google Scholar] [CrossRef]
- Boroduleva, A.Y.; Wu, J.; Yang, Q.; Li, H.; Zhang, Q.; Li, P.; Eremin, S.A. Development of fluorescence polarization immunoassays for parallel detection of pesticides carbaryl and triazophos in wheat grains. Anal. Methods 2017, 9, 6814–6822. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Jia, M.; Li, B.; Rong, J.; Yang, X. Deposition of CdTe quantum dots on microfluidic paper chips for rapid fluorescence detection of pesticide 2,4-D. Analyst 2019, 144, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- So, H.S.; Angupillai, S.; Son, Y.A. Prompt liquid-phase visual detection and low-cost vapor-phase detection of DCP, a chemical warfare agent mimic. Sens. Actuators B 2016, 235, 447–456. [Google Scholar] [CrossRef]
- Costero, A.M.; Gil, S.; Parra, M.; Mancini, P.M.E.; Martínez-Máñez, R.; Sancenóna, F.; Royo, S. Chromogenic detection of nerve agent mimics. Chem. Commun. 2008, 6002–6004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yimit, A.; Itoh, K.; Murabayashi, M. Detection of ammonia in the ppt range based on a composite optical waveguide pH sensor. Sens. Actuators B 2003, 88, 239–245. [Google Scholar] [CrossRef]
- Nizamidin, P.; Yimit, A.; Abdurrahman, A.; Itoh, K. Formaldehyde gas sensor based on silver-and-yttrium-co doped-lithium iron phosphate thin film optical waveguide. Sens. Actuators B 2013, 176, 460–466. [Google Scholar] [CrossRef]
- Horváth, R.; Pedersen, H.C.; Skivesen, N.; Selmeczi, D.; Larsen, N.B. Optical waveguide sensor for on-line monitoring of bacteria. Opt. Lett. 2003, 28, 1233–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekacs, I.; Farkas, E.; Gemes, B.L.; Takacs, E.; Szekacs, A.; Horvath, R. Integrin targeting of glyphosate and its cell adhesion modulation efects on osteoblastic MC3T3-E1 cells revealed by label-free optical biosensing. Sci. Rep. 2018, 8, 17401. [Google Scholar] [CrossRef]
- Farkas, E.; Szekacs, A.; Kovacs, B.; Olah, M.; Horvath, R.; Szekacs, I. Label-free optical biosensor for real-time monitoring the cytotoxicity of xenobiotics: A proof of principle study on glyphosate. J. Hazard. Mater. 2018, 351, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Qi, Z. Determination of surface protein coverage by composite waveguide based polarimetric interferometry. Analyst 2011, 136, 5277–5282. [Google Scholar] [CrossRef] [PubMed]
- Yimit, A.; Huang, X.; Xu, Y.; Amemiya, T.; Itoh, K. Development of a Composite Optical Waveguide Sensor for Immunoglobulin G. Chem. Lett. 2003, 32, 86–87. [Google Scholar] [CrossRef]
- Lu, D.; Li, J.; Qi, Z. Nonspecific detection of lead ions in water using a simple integrated optical polarimetric interferometer. J. Appl. Phys. 2013, 113, 213109. [Google Scholar] [CrossRef]
- Mohemaiti, M.; Keram, A.; Nezamidin, P.; Yimit, A. Preparation of zinc oxide thin film/tin-diffused optical waveguide sensor and gas-sensing detection. Acta Chim. Sin. 2011, 69, 1840–1844. [Google Scholar]
- Nizamidin, P.; Yimit, A.; Wang, J.D.; Itoh, K. Optical properties and sensing applications of lithium iron phosphate thin films. Thin. Solid Films 2012, 520, 6250–6255. [Google Scholar] [CrossRef]
- Abdurahman, R.; Yimit, A.; Ablat, H.; Mahmut, M.; Wang, J.D.; Itoh, K. Optical waveguide sensor of volatile organic compounds based on PTA thin film. Anal. Chim. Acta 2010, 658, 63–67. [Google Scholar] [CrossRef]
- Qi, Z.; Honma, I.; Zhou, H. Chemical gas sensor application of open-pore mesoporous thin films based on integrated optical polarimetric interferometry. Anal. Chem. 2006, 78, 1034–1041. [Google Scholar] [CrossRef]
- Zhu, M.; Kari, N.; Yan, Y.; Yimit, A. The fabrication and gas sensing application of a fast-responding m-CP-PVP composite film/potassium ion-exchanged glass optical waveguide. Anal. Methods 2017, 9, 5494–5501. [Google Scholar] [CrossRef]
- Mahmut, M.; Yimit, A.; Abliz, S. Detection of hydrogen chloride gas by congo red cross-linked poly(vinylalcohol) thin film/K+ ion-exchanged glass optical waveguide. Chin. J. Anal. Chem. 2008, 36, 1435–1439. [Google Scholar]
- Lenza, R.F.S.; Vasconcelos, W.L. Synthesis and properties of microporous sol-gel silica membranes. J. Non-Cryst. Solids 2000, 273, 164–169. [Google Scholar] [CrossRef]
- Qi, Z.; Matsuda, N.; Santos, J.; Itoh, K.; Takatsu, A.; Kato, K. A Study of Molecular adsorption of bromothymol blue by optical waveguide spectroscopy. Langmuir 2003, 19, 214–217. [Google Scholar] [CrossRef]
- Belger, C.; Weis, J.G.; Egap, E.; Swager, T.M. Colorimetric Stimuli-Responsive Hydrogel Polymers for the Detection of Nerve Agent Surrogates. Macromolecules 2015, 48, 7990–7994. [Google Scholar] [CrossRef] [Green Version]
- Gotor, R.; Costero, A.M.; Gil, S.; Parra, M.; Martínez-Máñez, R.; Sancenón, F. A Molecular Probe for the Highly Selective Chromogenic Detection of DFP, a Mimic of Sarin and Soman Nerve Agents. Chem. Eur. J. 2011, 17, 11994–11997. [Google Scholar] [CrossRef] [PubMed]
- Costero, A.M.; Parra, M.; Gil, S.; Gotor, R.; Martínez-Máñez, R.; Sancenón, F.; Royo, S. Selective Detection of Nerve Agent Simulants by Using Triarylmethanol-Based Chromogenic Chemodosimeters. Eur. J. Org. Chem. 2012, 2012, 4937–4946. [Google Scholar] [CrossRef]
- Cui, W.; Jia, R.; Nie, L.; Zhang, S. Molecular Structure and Function of Organic Reagents Frequently Used in Analytical Chemistry. Univ. Chem. 2018, 33, 100–112. [Google Scholar]
- Rocha, W.R.M.; Pilling, S. Determination of optical constants n and k of thin films from absorbance data using Kramers-Kronig relationship. Spectrochim. Acta Part A 2014, 123, 436–446. [Google Scholar] [CrossRef]
- Yao, J.; Fu, Y.; Xu, W.; Fan, T.; Gao, Y.; He, Q.; Zhu, D.; Cao, H.; Cheng, J. Concise and efficient fluorescent probe via an intromolecular charge transfer for the chemical warfare agent mimic diethylchlorophosphate vapor detection. Anal. Chem. 2016, 88, 2497–2501. [Google Scholar] [CrossRef]
- Du, B.; Tong, Z.; Mu, X.; Liu, S.; Xu, J.; Liu, Z.; Qi, Z.M.; Ding, Z. Detection of diethyl chlorophosphate using a composite optical waveguide sensor. Anal. Methods 2019, 11, 1208–1213. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Tong, Z.; Mu, X.; Xu, J.; Liu, S.; Liu, Z.; Cao, W.; Qi, Z.-M. A Potassium Ion-Exchanged Glass Optical Waveguide Sensor Locally Coated with a Crystal Violet-SiO2 Gel Film for Real-Time Detection of Organophosphorus Pesticides Simulant. Sensors 2019, 19, 4219. https://doi.org/10.3390/s19194219
Du B, Tong Z, Mu X, Xu J, Liu S, Liu Z, Cao W, Qi Z-M. A Potassium Ion-Exchanged Glass Optical Waveguide Sensor Locally Coated with a Crystal Violet-SiO2 Gel Film for Real-Time Detection of Organophosphorus Pesticides Simulant. Sensors. 2019; 19(19):4219. https://doi.org/10.3390/s19194219
Chicago/Turabian StyleDu, Bin, Zhaoyang Tong, Xihui Mu, Jianjie Xu, Shuai Liu, Zhiwei Liu, Wei Cao, and Zhi-Mei Qi. 2019. "A Potassium Ion-Exchanged Glass Optical Waveguide Sensor Locally Coated with a Crystal Violet-SiO2 Gel Film for Real-Time Detection of Organophosphorus Pesticides Simulant" Sensors 19, no. 19: 4219. https://doi.org/10.3390/s19194219



