Monitoring of Adult Zebrafish Heart Regeneration Using High-Frequency Ultrasound Spectral Doppler and Nakagami Imaging
Abstract
:1. Introduction
2. Method
2.1. Pulsed Wave Spectral Doppler
2.2. Nakagami Imaging
2.3. Experimental Setup and Evaluation Metric
3. Results and Discussion
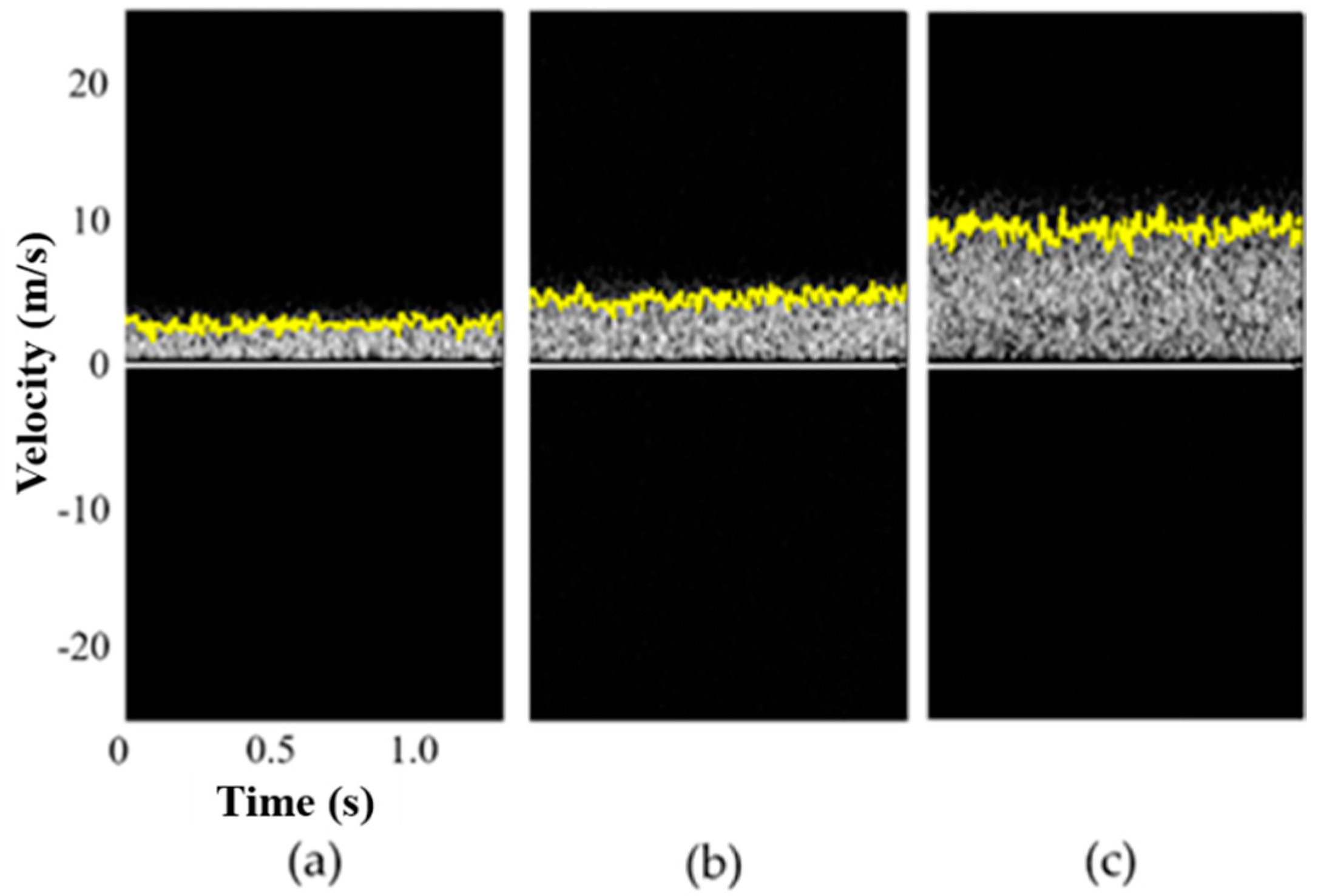
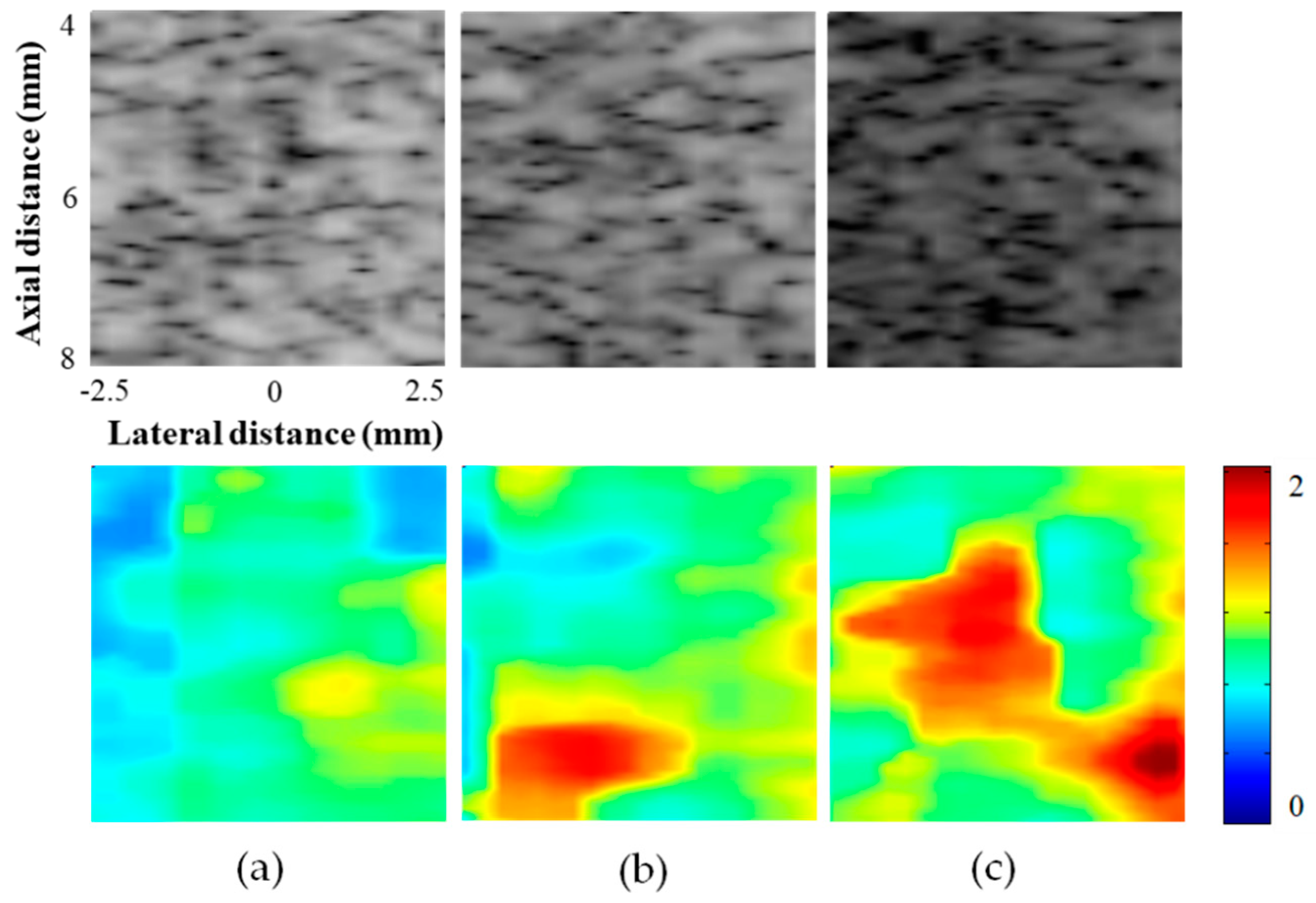
3.1. Phantom Experiments
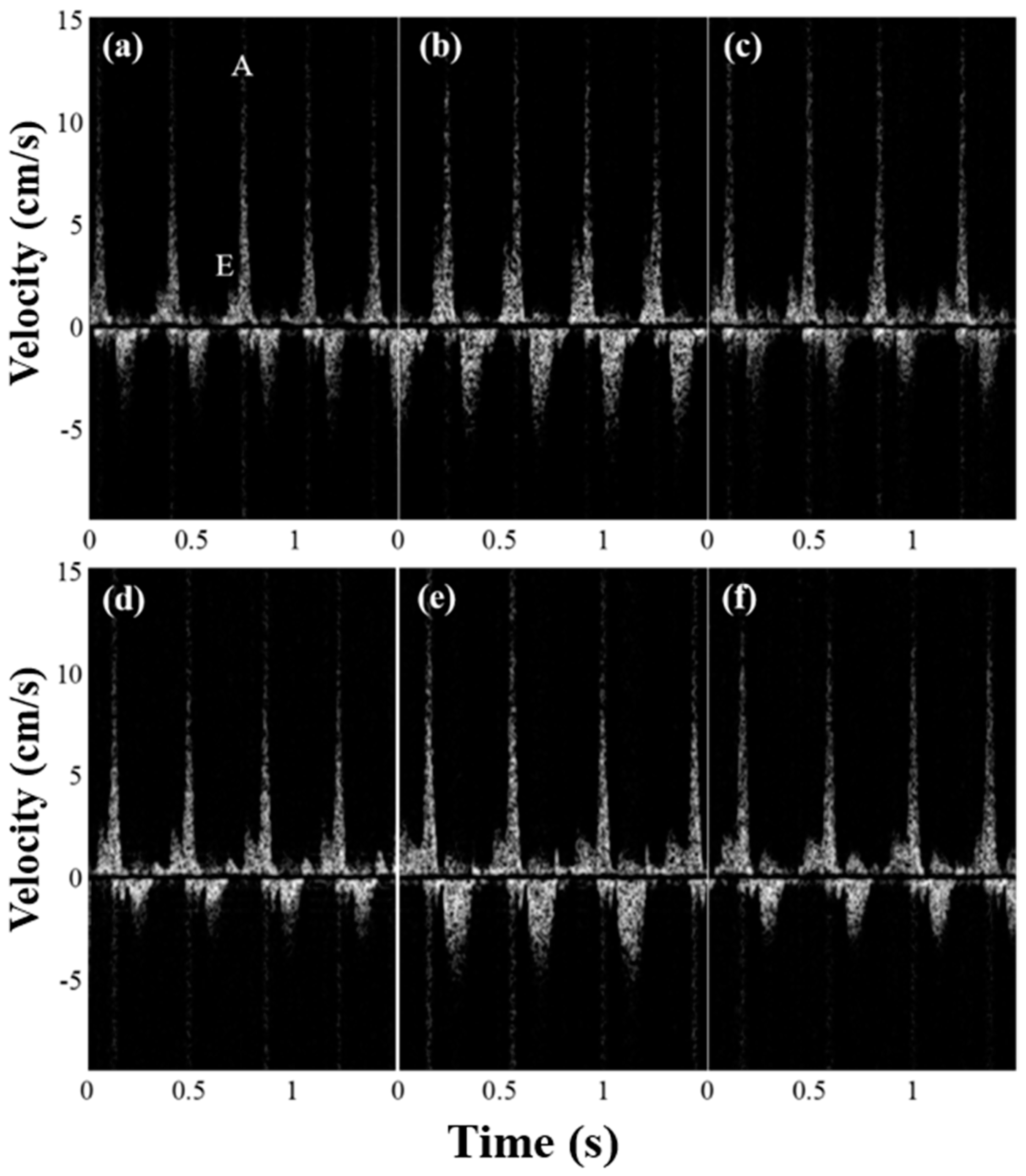
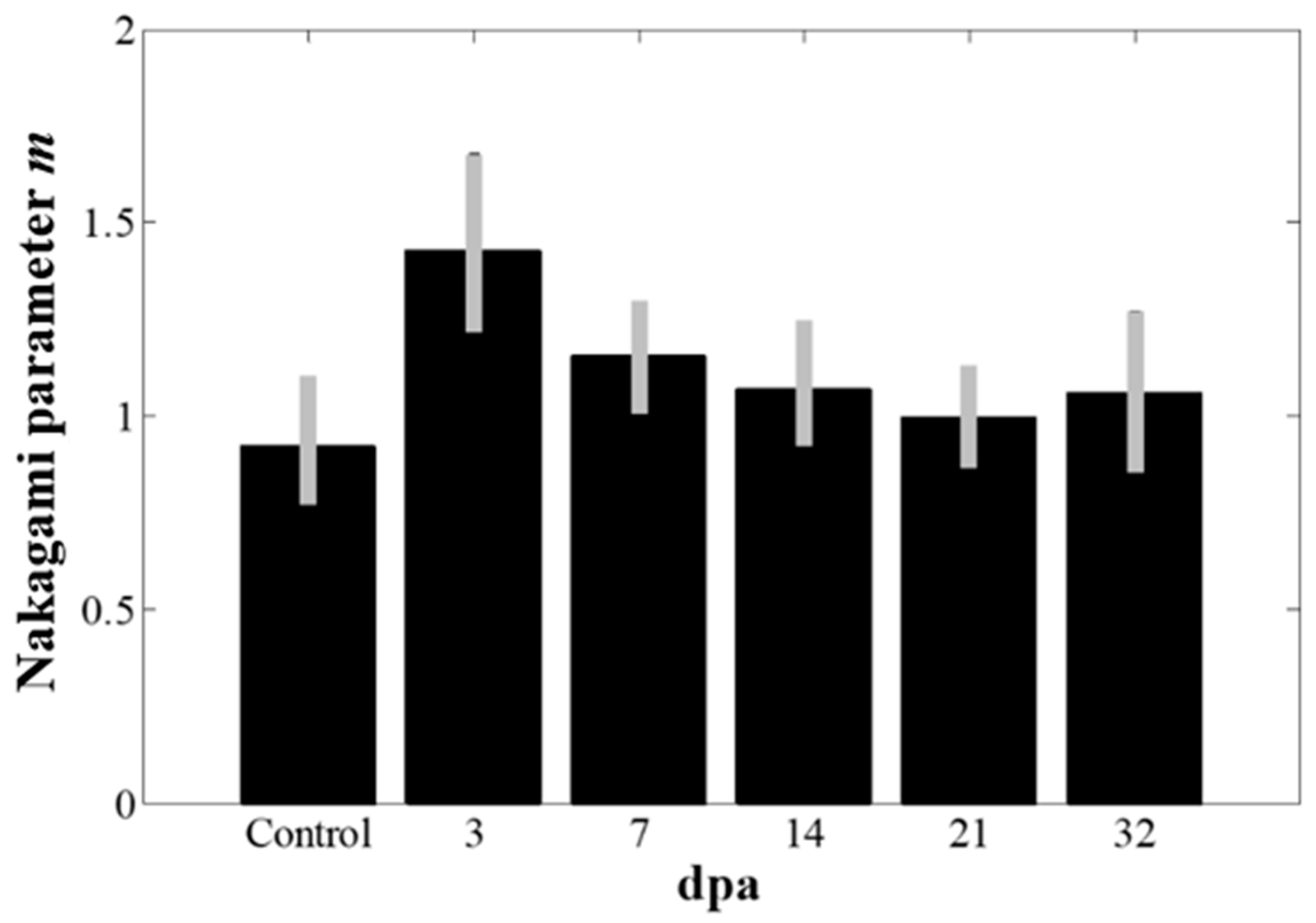
3.2. In Vivo Experiments
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Thisse, C.; Zon, L. Organogenesis: Heart and blood formation from the zebrafish point of view. Science 2002, 295, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Bailey, T.J.; Hyde, D.R.; Poss, K.D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013, 29, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.T.; Pomerantsev, E.V.; Mably, J.D.; Macrae, C.A. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol. Genomics 2010, 42, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; Leblanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Belmonte, J.C.I. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Sedmera, D.; Guldberg, R.E.; Markwald, R.R. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev. Dyn. 2007, 236, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kabli, S.; Alia, A.; Spaink, H.P.; Verbeek, F.J.; de Groot, H.J. Magnetic resonance microscopy of the adult zebrafish. Zebrafish 2006, 3, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; North, T.E.; Zon, L.I. Ultrasound biomicroscopy permits in vivo characterization of zebrafish liver tumors. Nat. Methods 2007, 4, 551–553. [Google Scholar] [CrossRef]
- Sun, L.; Xu, X.; Richard, W.D.; Feng, C.; Johnson, J.A.; Shung, K.K. A high-frame rate duplex ultrasound biomicroscopy for small animal imaging in vivo. IEEE Trans. Biomed. Eng. 2008, 55, 2039–2049. [Google Scholar] [CrossRef]
- Park, J.; Huang, Y.; Chen, R.; Lee, J.; Cummins, T.M.; Zhou, Q.; Lien, C.L.; Shung, K.K. Pulse inversion chirp coded tissue harmonic imaging (pI-cTHI) of zebrafish heart using high frame rate ultrasound biomicroscopy. Ann. Biomed. Eng. 2013, 41, 41–52. [Google Scholar] [CrossRef]
- Liu, T.; Lee, P.; Huang, C.; Sun, L.; Shung, K.K. A Study of the Adult Zebrafish Ventricular Function by Retrospective Doppler-Gated Ultrahigh-Frame-Rate Echocardiography. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2013, 60, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Park, J.; Kim, J.; Kim, H.H.; Lee, C.; Hwang, J.Y.; Lien, C.; Shung, K.K. High-frequency dual mode pulsed wave Doppler imaging for monitoring the functional regeneration of adult zebrafish hearts. J. R. Soc. Interface 2015, 12, 20141154. [Google Scholar] [CrossRef] [PubMed]
- Insana, M.F.; Wagner, R.F.; Brown, D.G.; Hall, T.J. Describing small-scale structure in random media using pulse-echo ultrasound. J. Acoust. Soc. Am. 1990, 87, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.M. A general statistical model for ultrasonic backscattering from tissues. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2000, 47, 727–736. [Google Scholar] [CrossRef]
- Shankar, P.M. Statistical modeling of scattering from biological media. J. Acoust. Soc. Am. 2002, 111, 2463. [Google Scholar] [CrossRef]
- Yoon, C. Spectrum analysis for assessing red blood cell aggregation using high-frequency ultrasound array transducer. Biomed. Eng. Lett. 2017, 4, 273–279. [Google Scholar] [CrossRef]
- Shankar, P.M.; Dumane, V.A.; Reid, J.M.; Genis, V.; Forsberg, F.; Piccoli, C.W.; Goldgerg, B.B. Classification of ultrasonic B-mode images of breast masses using Nakagami distribution. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2001, 48, 569–580. [Google Scholar] [CrossRef]
- Shankar, P.M.; Dumane, V.A.; George, T.; Piccoli, C.W.; Reid, J.M.; Forsberg, F.; Goldgerg, B.B. Classification of breast masses in ultrasonic B scans using Nakagami and K distributions. Phys. Med. Biol. 2003, 48, 2229–2240. [Google Scholar] [CrossRef]
- Tsui, P.H.; Chang, C.C. Imaging local scatter concentrations by the Nakagami statistical model. Ultrasound Med. Biol. 2007, 33, 608–619. [Google Scholar] [CrossRef]
- Tsui, P.H.; Yeh, C.K.; Chang, C.C.; Liao, Y.Y. Classification of breast masses by ultrasonic Nakagami imaging: A feasibility study. Phys. Med. Biol. 2008, 53, 6027–6044. [Google Scholar] [CrossRef]
- Tsui, P.H.; Huang, C.C.; Chang, C.C.; Wang, S.H.; Shung, K.K. Feasibility study of using high-frequency ultrasonic Nakagami imaging for characterizing the cataract lens in vitro. Phys. Med. Biol. 2007, 52, 6413–6425. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Li, C.H.; Tsui, P.H.; Chang, C.C.; Kuo, W.H.; Chang, K.J.; Yeh, C.K. Strain-compounding technique with ultrasound Nakagami imaging for distinguishing between benign and malignant breast tumors. Med. Phys. 2012, 39, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Geng, X.; Yeh, T.S.; Liu, H.L.; Tsui, P.H. Monitoring radiofrequency ablation with ultrasound Nakagami imaging. Med. Phys. 2013, 40, 072901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, P.H.; Ho, M.C.; Tai, D.I.; Lin, Y.H.; Wang, C.Y.; Ma, H.Y. Acoustic structure quantification by using ultrasound Nakagami imaging for assessing liver fibrosis. Sci. Rep. 2016, 6, 33075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrbach, D.; Wodlinger, B.; Wen, J.; Mamou, J.; Feleppa, E. High-Frequency Quantitative Ultrasound for Imaging Prostate Cancer Using a Novel Micro-Ultrasound Scanner. Ultrasound Med. Biol. 2018, 44, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Liu, H.L.; Li, D.W.; Li, M.L. Ultrasonic Nakagami Imaging of High-intensity Focused Ultrasound-induced Thermal Lesions in Porcine Livers: Ex Vivo Study. Ultrasonic Imaging 2018, 40, 310–324. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Q.; Wu, W.; Wu, S.; Tsui, P.H. Hepatic Steatosis Assessment Using Quantitative Ultrasound Parametric Imaging Based on Backscatter Envelope Statistics. Appl. Sci. 2019, 9, 661. [Google Scholar] [CrossRef]
- Tsui, P.H.; Wan, Y.L.; Tai, D.I.; Shu, Y.C. Effects of estimators on ultrasound Nakagami imaging in visualizing the change in the backscattered statistics from a Rayleigh distribution to a pre-Rayleigh distribution. Ultrasound Med. Biol. 2015, 41, 2240–2251. [Google Scholar] [CrossRef]
- Yoon, C.; Kim, H.H.; Shung, K.K. Development of a low-complexity, cost-effective digital beamformer architecture for high-frequency ultrasound imaging. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2017, 64, 1002–1008. [Google Scholar] [CrossRef]
- Cannon, L.M.; Fagan, A.J.; Browne, J.E. Novel tissue mimicking materials for high frequency breast ultrasound phantoms. Ultrasound Med. Biol. 2011, 37, 122–135. [Google Scholar] [CrossRef]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A Test in Context: E/A and E/e′ to Assess Diastolic Dysfunction and LV Filling Pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Kim, G.D.; Yoo, Y.; Song, T.K.; Chang, J.H. Frequency equalized compounding for effective speckle reduction in medical ultrasound imaging. Biomed Signal Process Control 2013, 8, 876–887. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, S.; Yoon, C.; Lien, C.-L.; Song, T.-K.; Shung, K.K. Monitoring of Adult Zebrafish Heart Regeneration Using High-Frequency Ultrasound Spectral Doppler and Nakagami Imaging. Sensors 2019, 19, 4094. https://doi.org/10.3390/s19194094
Yeo S, Yoon C, Lien C-L, Song T-K, Shung KK. Monitoring of Adult Zebrafish Heart Regeneration Using High-Frequency Ultrasound Spectral Doppler and Nakagami Imaging. Sensors. 2019; 19(19):4094. https://doi.org/10.3390/s19194094
Chicago/Turabian StyleYeo, Sunmi, Changhan Yoon, Ching-Ling Lien, Tai-Kyong Song, and K. Kirk Shung. 2019. "Monitoring of Adult Zebrafish Heart Regeneration Using High-Frequency Ultrasound Spectral Doppler and Nakagami Imaging" Sensors 19, no. 19: 4094. https://doi.org/10.3390/s19194094




