An Affordable Method for Evaluation of Ataxic Disorders Based on Electrooculography
Abstract
:1. Introduction
2. Related Work
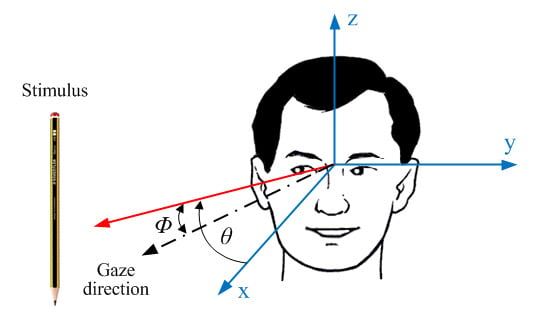
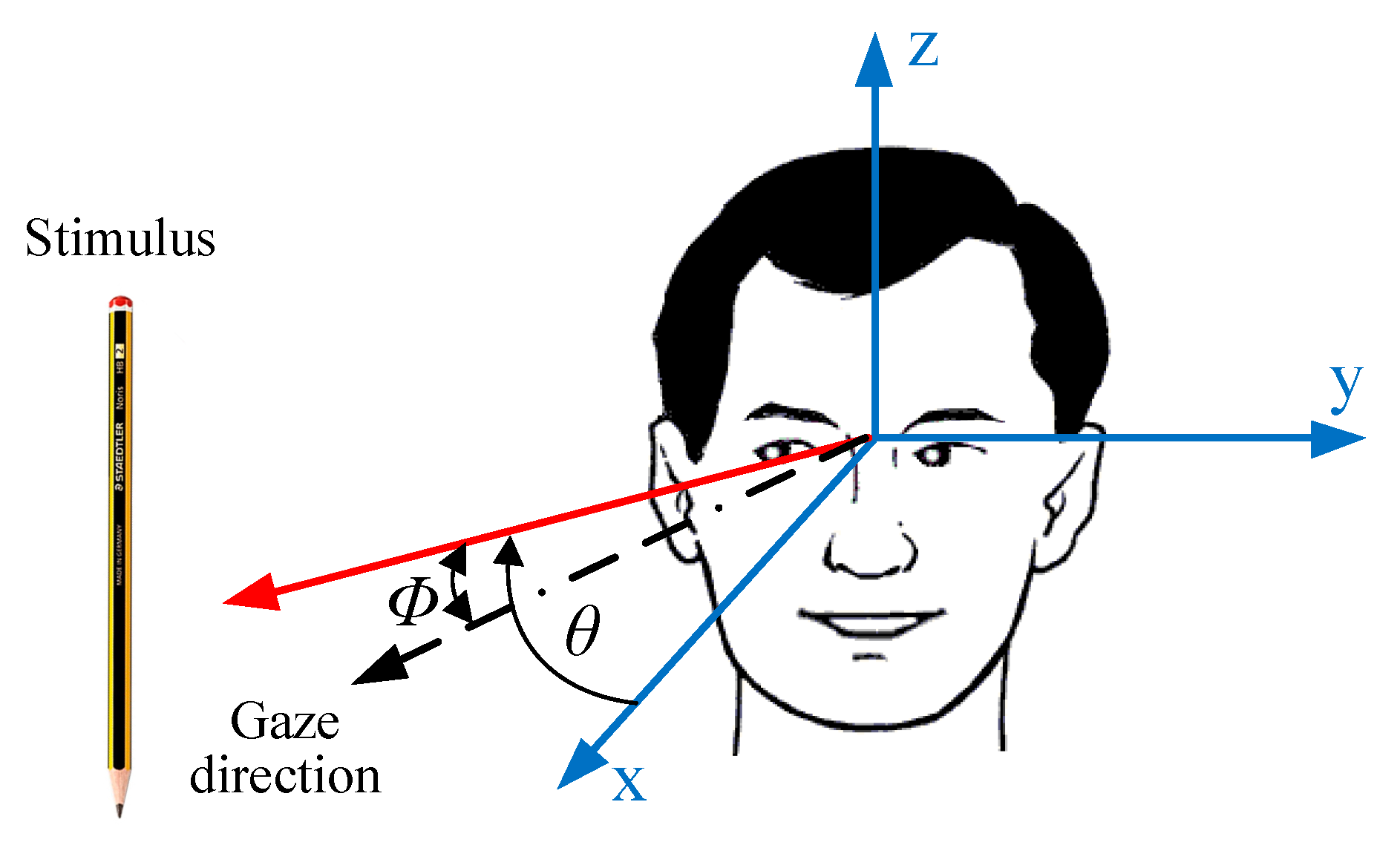
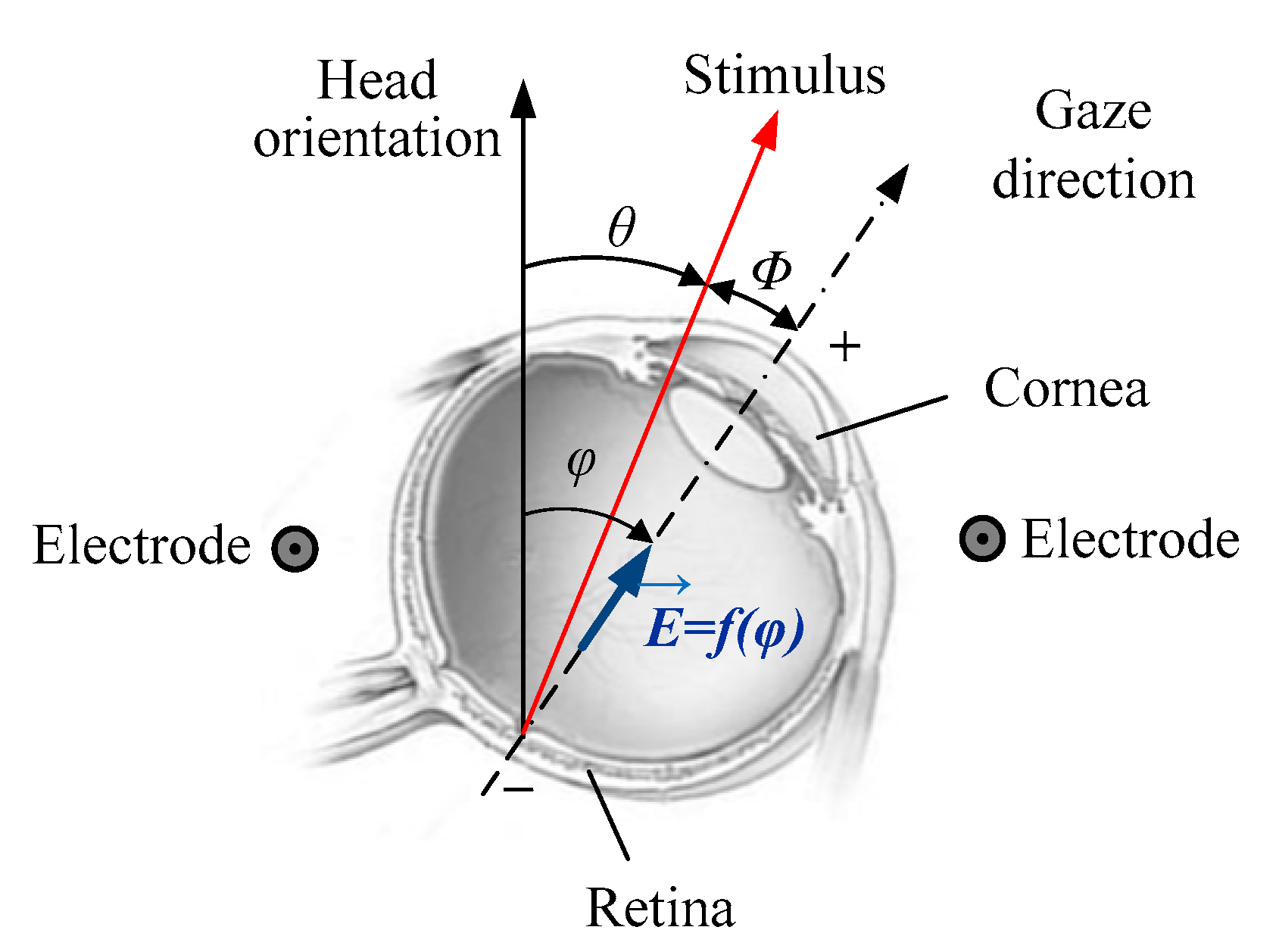
3. Electrooculography
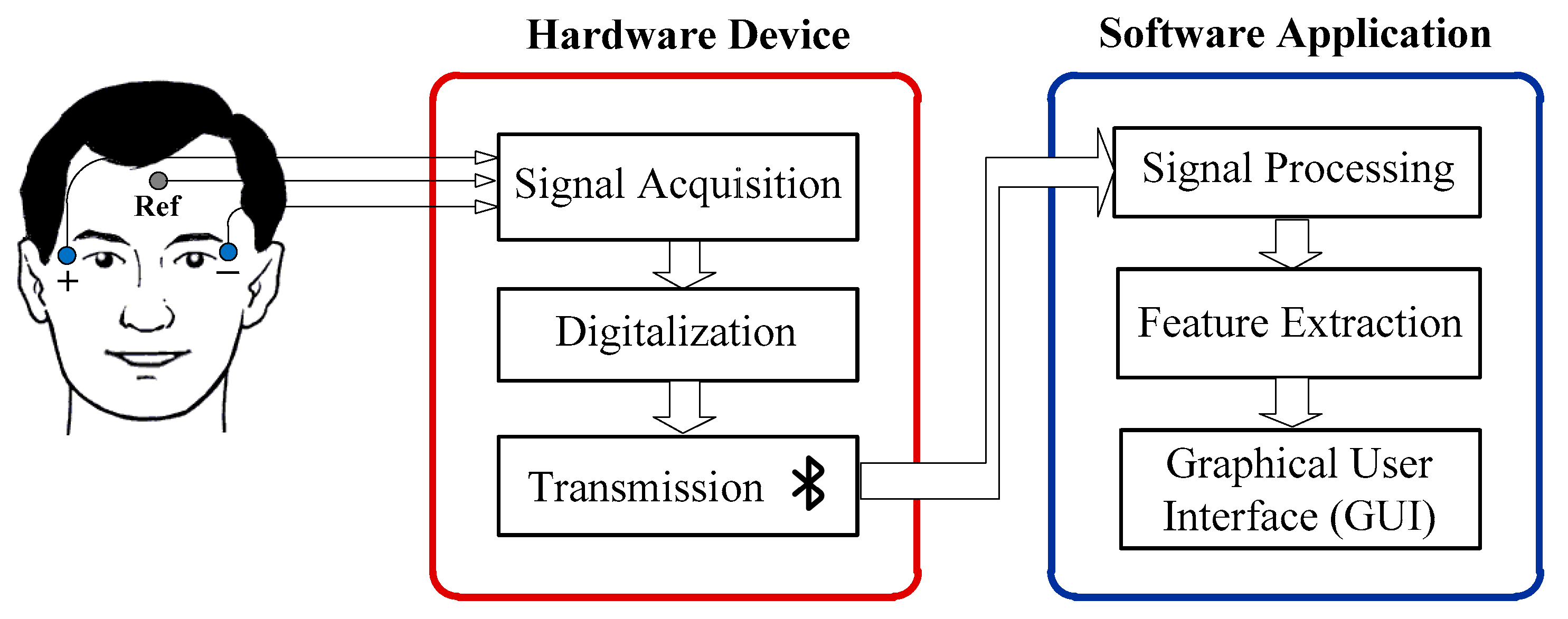
4. Materials and Methods
4.1. Hardware Device
4.2. Software Application
- BlueGainRequestData requests a packet of data from BlueGain.
- BlueGainRetrieveData retrieves data from BlueGain.
- BlueGainResetSampling resets the sampling of BlueGain and clears the buffer.
- crsFindSerialPort finds the serial port of the computer where the Bluetooth device is connected.
- crsOpenSerialPort opens the port that was previously found by the former function.
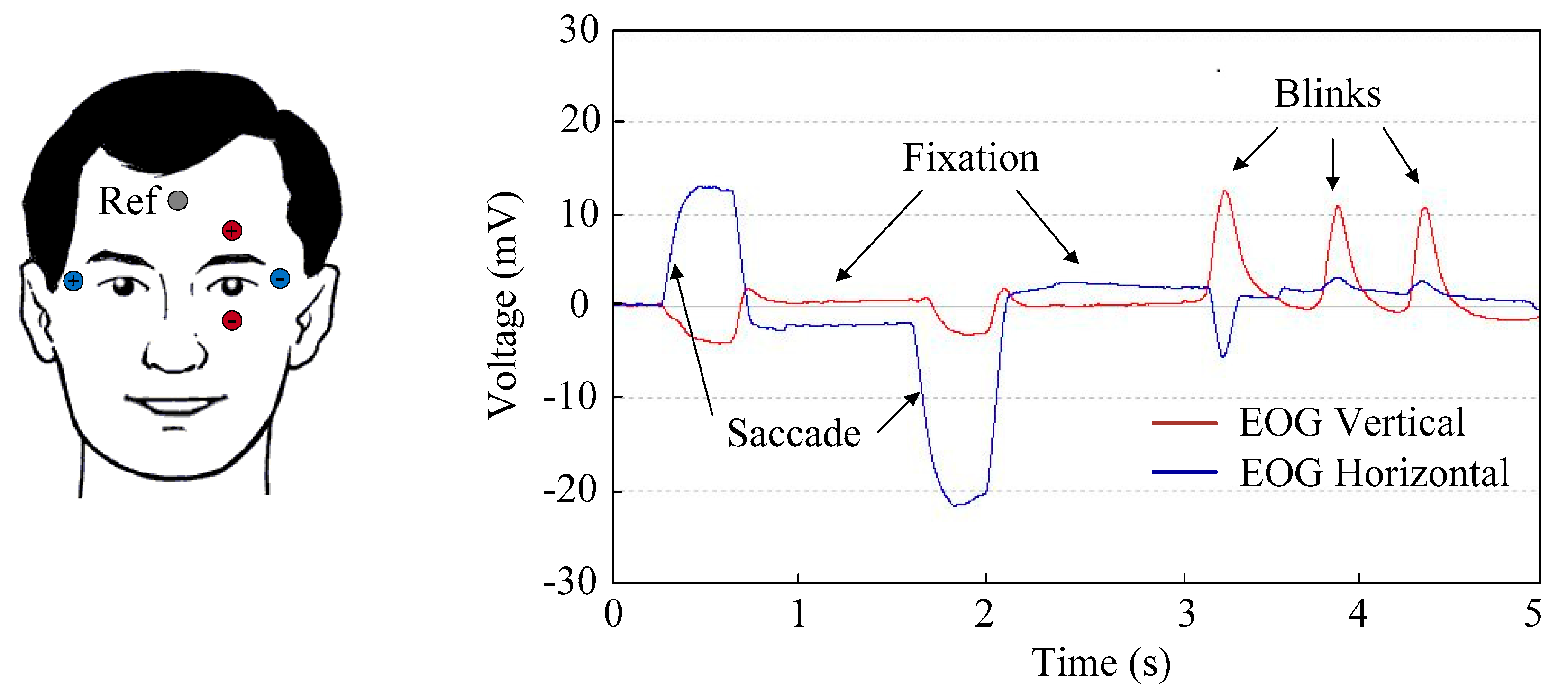
4.2.1. EOG Signal Processing
4.2.2. Feature Extraction
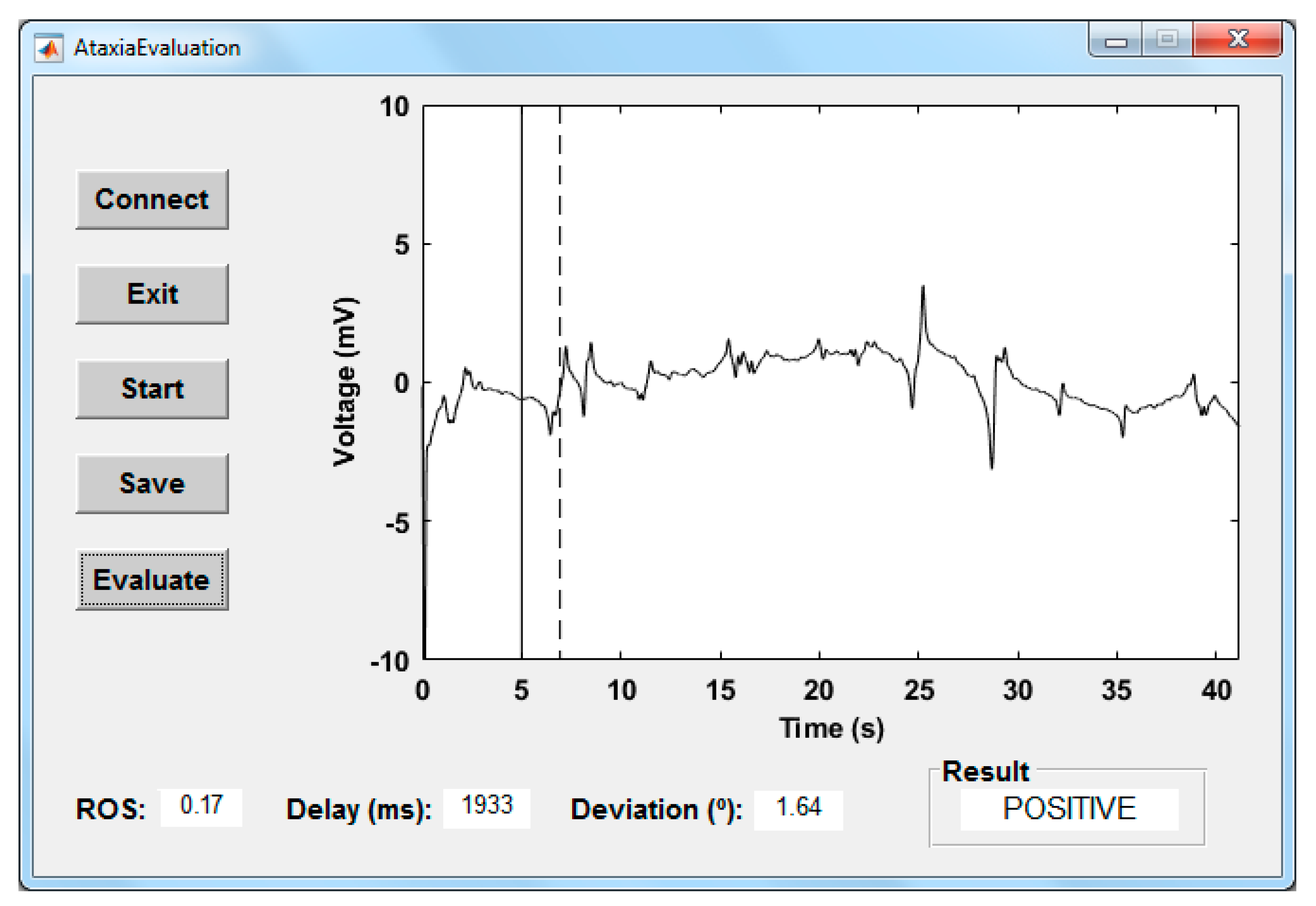
4.2.3. Graphical User Interface
5. Experimental Results
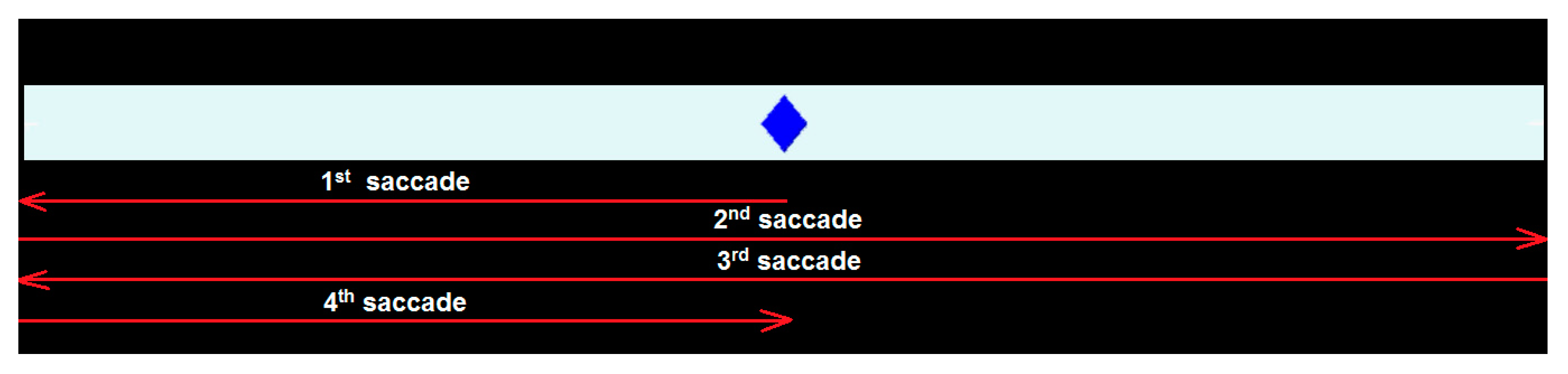
5.1. Experimental Setup
5.2. Evaluation of Results
- TP (true positive) represents the number of positives generated when an evaluation result is correct.
- FP (false positive) represents cases where the system identifies as positive an individual who does not have ataxia.
- TN (true negative) represents a case where a normal eye movement is performed, and therefore the system does not identify ataxia.
- FN (false negative) refers to an individual who does have ataxia, but it is not identified by the system.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perlman, S.L. Evaluation and Management of Ataxic Disorders; An Overview for Physicians; National Ataxia Foundation: Minneapolis, MN, USA, 2016. [Google Scholar]
- Velázquez-Pérez, L.; Rodríguez-Labrada, R.; García-Rodríguez, J.C.; Almaguer-Mederos, L.E.; Cruz-Mariño, T.; Laffita-Mesa, J.M. A Comprehensive Review of Spinocerebellar Ataxia Type 2 in Cuba. Cerebellum 2011, 10, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Burk, K.; Fetter, M.; Abele, M.; Laccone, F.; Brice, A.; Dichgans, J.; Klockgether, T. Autosomal dominant cerebellar ataxia type I: Oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J. Neurol. 1999, 246, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Spicker, S.; Petersen, D.; Fetter, M.; Klockgether, T.; Dichgans, J. Fixation instability and oculomotor abnormalities in Friedreich’s ataxia. J. Neurol. 1995, 242, 517–521. [Google Scholar] [CrossRef]
- Zanni, G.; Bertini, E.S. X-linked disorders with cerebellar dysgenesis. Orphanet J. Rare Dis. 2011, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Alston, C.L.; Rocha, M.C.; Lax, N.Z.; Turnbull, D.M.; Taylor, R.W. The genetics and pathology of mitochondrial disease. J. Pathol. 2017, 241, 236–250. [Google Scholar] [CrossRef]
- Yokota, T.; Hayashi, H.; Hirose, K.; Tanabe, H. Unusual blink reflex with four components in a patient with periodic ataxia. J. Neurol. 1990, 237, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Hutton, J.T.; Albrecht, J.W.; Kuskowski, M.; Schut, L.J. Abnormal ocular motor function predicts clinical diagnosis of familial ataxia. J. Neurol. 1987, 37, 698. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.; Jung, T.P.; Cauwenberghs, G. Estimating Direction and Depth of Visual Fixation Using Electrooculography. In Proceedings of the 37th International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 841–844. [Google Scholar]
- Lappe-Osthege, M.; Talamo, S.; Helmchen, C.; Sprenger, A. Overestimation of saccadic peak velocity recorded by electro-oculography compared to video-oculography and scleral search coil. Clin. Neurophysiol. 2010, 121, 1786–1787. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Bulling, A.; Gellersen, H.; Tröster, G. Eye Movement Analysis for Activity Recognition Using Electrooculography. IEEE Trans. Pattern Anal. Mach. Intell. 2011, 33, 741–753. [Google Scholar]
- Belov, D.P.; Eram, S.Y.; Kolodyazhnyi, S.F.; Kanunikov, I.E.; Getmanenko, O.V. Electrooculogram Detection of Eye Movements on Gaze Displacement. Neurosci. Behav. Physiol. 2010, 40, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahayfeh, A.; Faezipour, M. Eye Tracking and Head Movement Detection: A State-of-Art Survey. IEEE J. Transl. Eng. Health Med. 2013, 1, 2100212. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, J. A review on electrooculography. Int. J. Adv. Eng. Tech. 2012, 3, 115–122. [Google Scholar]
- López, A.; Fernandez, M.; Rodríguez, H.; Ferrero, F.; Postolache, O. Development of an EOG-based system to control a serious game. Measurement 2018, 127, 481–488. [Google Scholar] [CrossRef]
- López, A.; Ferrero, F.; Yangüela, D.; Álvarez, C.; Postolache, O. Development of a Computer Writing System Based on EOG. Sensors 2017, 17, 1505. [Google Scholar] [CrossRef] [PubMed]
- Becerra, R. Saccadic Points Classification Using Multilayer Perceptron and Random Forest Classifiers in EOG Recordings of Patients with Ataxia SCA2. In Proceedings of the 12th International Work-Conference on Artificial Neural Networks (IWANN), Puerto de la Cruz, Spain, 12–14 June 2013; pp. 115–123. [Google Scholar]
- Anderson, J.H.; Yavuz, M.C.; Kazar, B.M.; Christova, P.; Gomez, C.M. Eye Position Feedback in a Model of the Vestibulo-Ocular Reflex for Spino-Cerebellar Ataxia 6. In Proceedings of the 23rd Annual EMBS International Conference, Istanbul, Turkey, 25–28 October 2001; pp. 840–843. [Google Scholar]
- Nakanishi, R.; Yamanaga, H.; Okumura, C.; Murayama, N.; Ideta, T. A quantitative analysis of ataxia in the upper limbs. Rinsho Shinkeigaku 1992, 32, 251–258. [Google Scholar] [PubMed]
- Sanguineti, V.; Morasso, P.G.; Baratto, L.; Brichetto, G.; Mancardi, G.L.; Solaro, C. Cerebellar ataxia: Quantitative assessment and cybernetic interpretation. Hum. Mov. Sci. 2003, 22, 189–205. [Google Scholar] [CrossRef]
- Nguyen, K.D. Quantitative Assessment of Cerebellar Ataxia with Kinematic Sensing During Rhythmic Tapping. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 1098–1101. [Google Scholar]
- Marini, F. Quantitative Evaluation Protocol for Upper Limb Motor Coordination Analysis in Patients with Ataxia. In Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6633–6636. [Google Scholar]
- Lee, J.; Kagamihara, Y.; Kakei, S. Quantitative Evaluation of Cerebellar Ataxia Based on EMG Signals. In Proceedings of the 2nd Biennial IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Scottsdale, AZ, USA, 19–22 October 2008; pp. 825–829. [Google Scholar]
- Lee, J.; Kagamihara, Y.; Kakei, S. Development of a quantitative evaluation system for motor control using wrist movements—An analysis of movement disorders in patients with cerebellar diseases. Rinsho byori. Jpn. J. Clin. Pathol. 2007, 55, 912–921. [Google Scholar]
- Lee, J.; Kagamihara, Y.; Kakei, S. Quantitative Evaluation of Cerebellar Ataxia Based on Pathological Patterns of the Muscle Activities. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 902–905. [Google Scholar]
- Tran, H. Automated Finger Chase (Ballistic Tracking) in the Assessment of Cerebellar Ataxia. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3521–3524. [Google Scholar]
- Frost, J.D. Triaxial Vector Accelerometry: A Method for Quantifying Tremor and Ataxia. IEEE Trans. Biomed. Eng. 1978, 25, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Yoshida, K.; Genno, H.; Murata, A.; Matsuzawa, S.; Nakamura, K.; Nakamura, A.; Ikeda, S.I. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias 2015, 2, 607. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, K.H.; Dichgans, J.; Hufschmidt, A. Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain 1979, 102, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Van De Warrenburg, B.P.; Bakker, M.; Kremer, B.P.; Bloem, B.R.; Allum, J.H. Trunk sway in patients with spinocerebellar ataxia. Mov. Disord. 2005, 20, 1006–1013. [Google Scholar] [CrossRef]
- LeMoyne, R.; Heerinckx, F.; Aranca, T.; De Jager, R.; Zesiewicz, T.; Saal, H.J. Wereable Body and Wireless Inertial Sensors for Machine Learning Classification of Gait for People with Fiedreich’s Ataxia. In Proceedings of the 13th International Conference on Weareable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; pp. 147–151. [Google Scholar]
- Cohen, A. Biomedical Signal Processing; CRC Press: Boca Raton, FL, USA, 1986; Volume 1. [Google Scholar]
- Thakor, N.V. Biopotentials and Electrophysiology Measurement. In The Measurement, Instrumentation and Sensors; Webster, J.G., Ed.; CRC Press: Boca Raton, FL, USA, 1999; Volume 74. [Google Scholar]
- Webster, J.G. Medical Instrumentation: Application and Design; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- López, A.; Ferrero, F.J.; Valledor, M.; Campo, J.C.; Postolache, O. A Study on Electrode Placement in EOG Systems for Medical Applications. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 12–14 May 2016; pp. 29–33. [Google Scholar]
- Banikl, P.P.; Azaml, K.; MondaIl, C.; Rahman, A. Single Channel Electrooculography Based Human-Computer Interface for Physically Disabled Persons. In Proceedings of the 2nd International Conference on Electrical Engineering and Information and Communication Technology (ICEEICT), Dhaka, Bangladesh, 21–23 May 2015. [Google Scholar]
- Enderle, J.D. Eye movements. In Encyclopedia of Biomedical Engineering; Cohen, A., Ed.; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Constable, P.A.; Bach, M.; Frishman, L.J.; Jeffrey, B.G.; Robson, A.G. ISCEV standard for clinical electro-oculography. Doc. Ophthalmol. 2017, 131, 1–9. [Google Scholar] [CrossRef]
- Sensory Reception: Human Vision: Structure and function of the Human Eye. In Encyclopedia Britannica; Encyclopædia Britannica: Edinburgh, UK, 1987; Volume 27, p. 179.
- Findlay, J.M.; Walker, R. How are saccades generated? Behav. Brain Sci. 1999, 22, 706–713. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. The Medline Plus Merrian-Webster Medical Dictionary. Available online: http://medlineplus.gov/ (accessed on 11 February 2019).
- Jagla, F. Saccadic eye movements as a marker of mental disorders. Physiol. Res. 2016, 65, 365–371. [Google Scholar]
- BlueGain Cambridge Research Systems. BlueGain EOG Biosignal Amplifier. Available online: http://www.crsltd.com/tools-for-vision-science/eye-tracking/bluegain-eog-biosignal-amplifier/ (accessed on 11 April 2019).
- Wardle, D.A. The time delay in human vision. Phys. Teach. 1998, 36, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Rayner, K.; Smith, T.J.; Malcolm, G.L.; Henderson, J.M. Eye Movements and visual encoding during scene perception. Psych Sci. 2009, 20, 6–10. [Google Scholar] [CrossRef]
- Thompson, P.D.; Colebatch, J.G.; Brown, P.; Rothwell, J.C.; Day, B.L.; Obeso, J.A.; Marsden, C.D. Voluntary stimulus-sensitive jerks and jumps mimicking myoclonus or pathological startle syndromes. Mov. Disord. 1992, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Krauzlis, R.J.; Goffart, L.; Hafed, Z.M. Neuronal control of fixation and fixational eye movements. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160205. [Google Scholar] [CrossRef] [Green Version]
- Amano, K.; Goda, N.; Nishida, S.; Ejima, Y.; Takeda, T.; Ohtani, Y. Estimation of the Timing of Human Visual Perception from Magnetoencephalography. J. Neurosci. 2006, 26, 3981–3991. [Google Scholar] [CrossRef] [Green Version]
- Rucci, M.; Poletti, M. Control and Functions of Fixational Eye Movements. Annu. Rev. Vis. Sci. 2015, 1, 499–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snodderly, D.M. A physiological perspective on fixational eye movements. Vis. Res. 2016, 118, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lim, Y.G.; Kwon, S.J.; Park, K.S. Non-contact blink detection glasses utilizing transparent conductive film for binary communication. Elec. Lett. 2015, 51, 382–384. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chan, Y.C.; Chou, H.C.; Siao, J.W. Eyeglasses Based Electrooculography Human-Wheelchair Interface. In Proceedings of the IEEE International Conference on Systems, Man, and Cybernetics, San Antonio, TX, USA, 11–14 October 2009; pp. 4746–4751. [Google Scholar]
- Barea, R.; Boquete, L.; Rodriguez-Ascariz, J.M.; Ortega, S.; Lopez, E. Sensory system for implementing a human-computer interface based on electrooculography. Sensors 2011, 11, 310–328. [Google Scholar] [CrossRef] [PubMed]



| Ataxia Patients | Healthy | |||||
|---|---|---|---|---|---|---|
| Subject | Age | Gender | Origin | Subject | Age | Gender |
| a | 46 | Male | Hereditary—Unknown | f | 41 | Female |
| b | 54 | Male | Non hereditary—Accident | g | 57 | Male |
| c | 60 | Female | Hereditary—Friedreich | h | 32 | Male |
| d | 64 | Male | Hereditary—Unknown | i | 58 | Female |
| e | 56 | Male | Hereditary—Unknown | j | 46 | Female |
| Subject | ROS | Deviation (°) | Response Delay (ms) | Result |
|---|---|---|---|---|
| a | 0.08 | 21.03 | 156 | Positive |
| b | 0.13 | 5.87 | 615 | Positive |
| c | 0.20 | 2.08 | 149 | Positive |
| d | 0.08 | 1.60 | 1340 | Positive |
| e | 0.13 | 3.32 | 128 | Positive |
| f | 0.08 | 0.45 | 135 | Negative |
| g | 0.11 | 0.71 | 174 | Negative |
| h | 0.11 | 2.26 | 153 | Negative |
| i | 0.08 | 1.82 | 139 | Negative |
| j | 0.08 | 0.94 | 181 | Negative |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, A.; Ferrero, F.; Postolache, O. An Affordable Method for Evaluation of Ataxic Disorders Based on Electrooculography. Sensors 2019, 19, 3756. https://doi.org/10.3390/s19173756
López A, Ferrero F, Postolache O. An Affordable Method for Evaluation of Ataxic Disorders Based on Electrooculography. Sensors. 2019; 19(17):3756. https://doi.org/10.3390/s19173756
Chicago/Turabian StyleLópez, Alberto, Francisco Ferrero, and Octavian Postolache. 2019. "An Affordable Method for Evaluation of Ataxic Disorders Based on Electrooculography" Sensors 19, no. 17: 3756. https://doi.org/10.3390/s19173756