Facile Non-Enzymatic Electrochemical Sensing for Glucose Based on Cu2O–BSA Nanoparticles Modified GCE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Characterization
2.2. Synthesis of the Cu2O–BSA NPs
2.3. Preparation Modified Electrode of Nafion/Cu2O–BSA/GCE
3. Results and Discussion
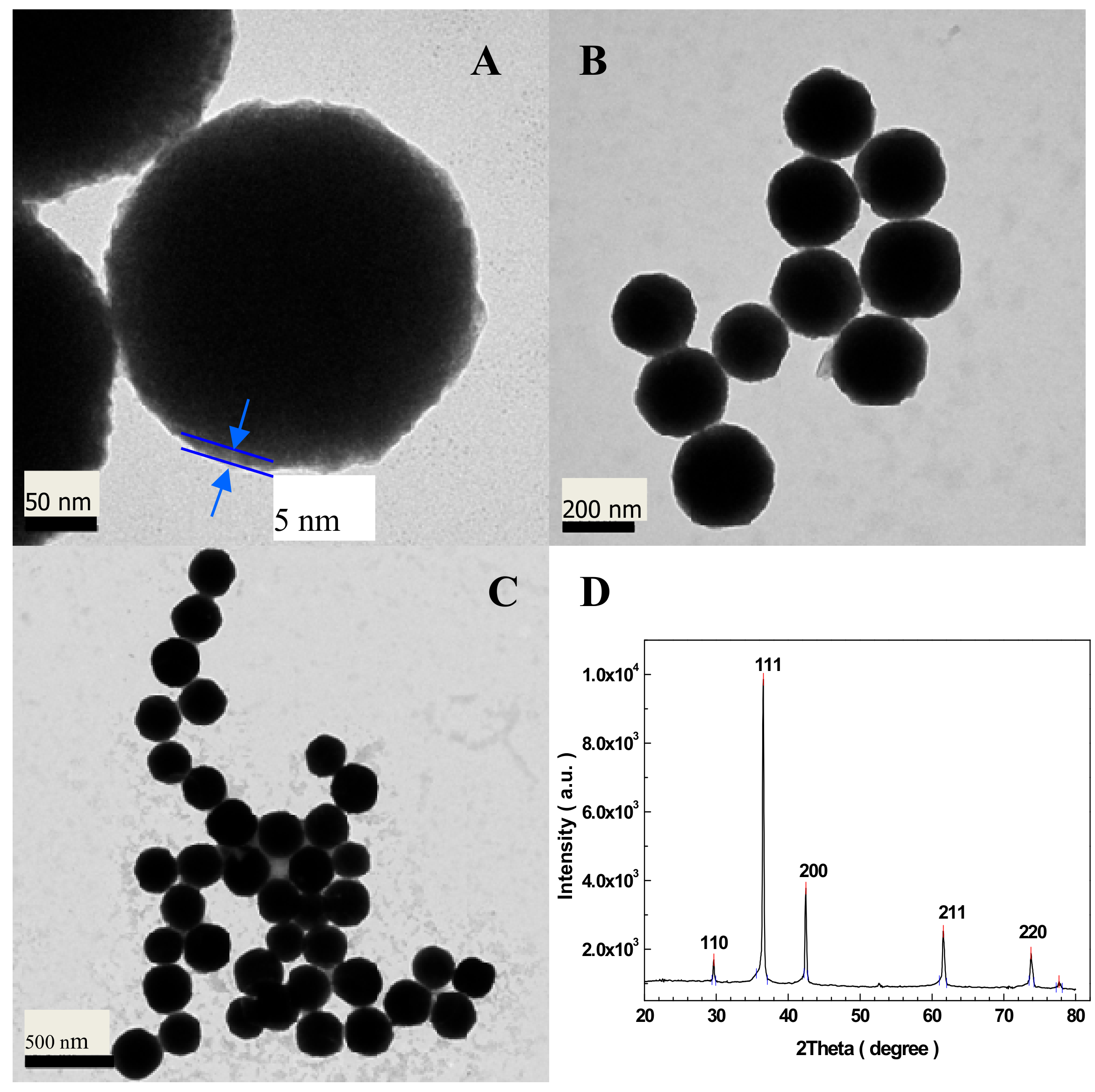
3.1. Structure and Morphology of Cu2O–BSA NPs
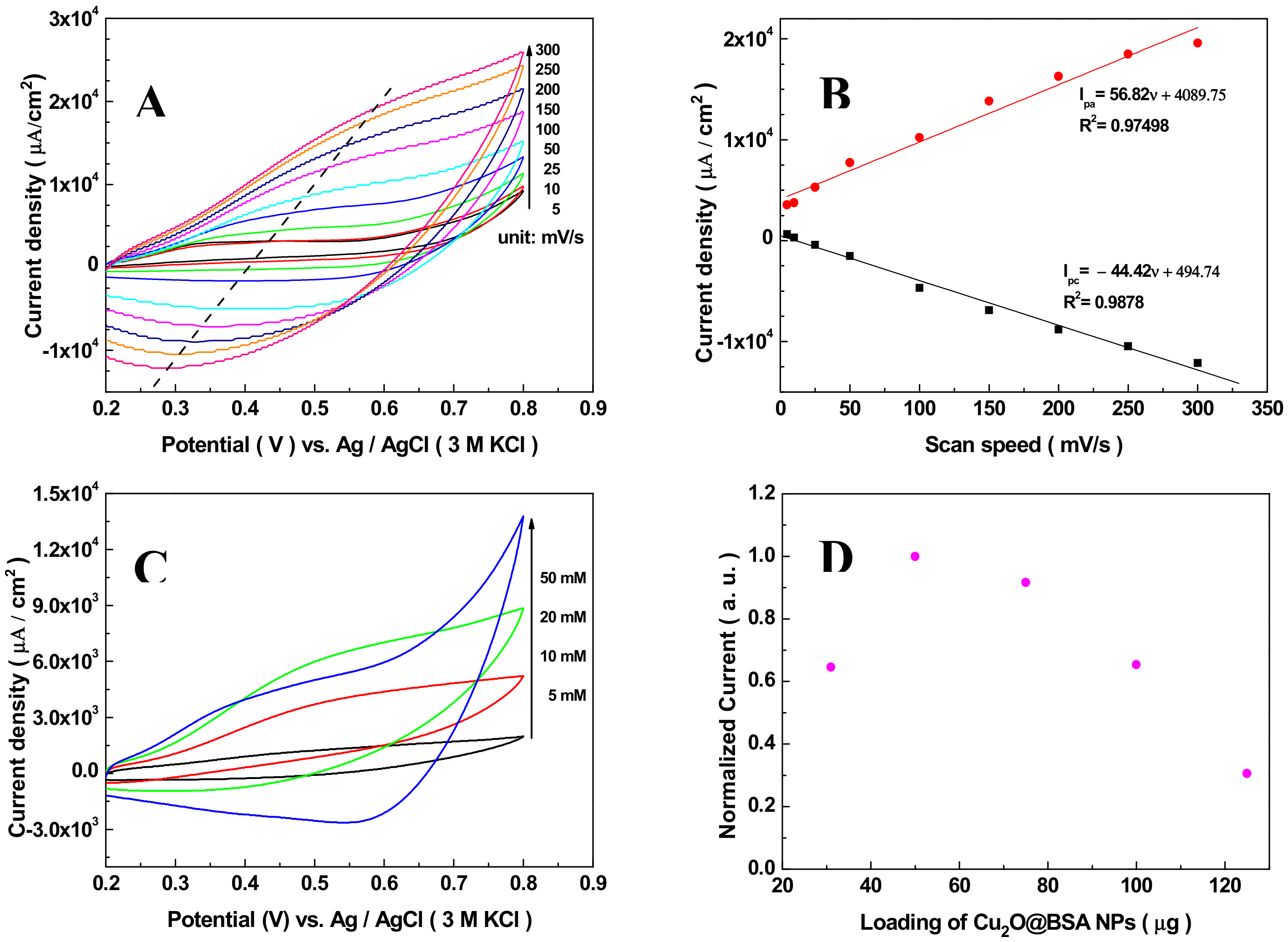
3.2. Electrochemical Characterization of the Sensor
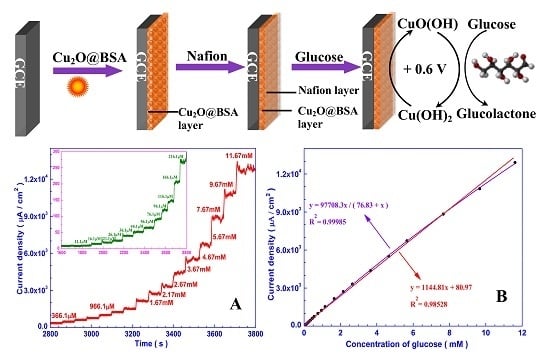
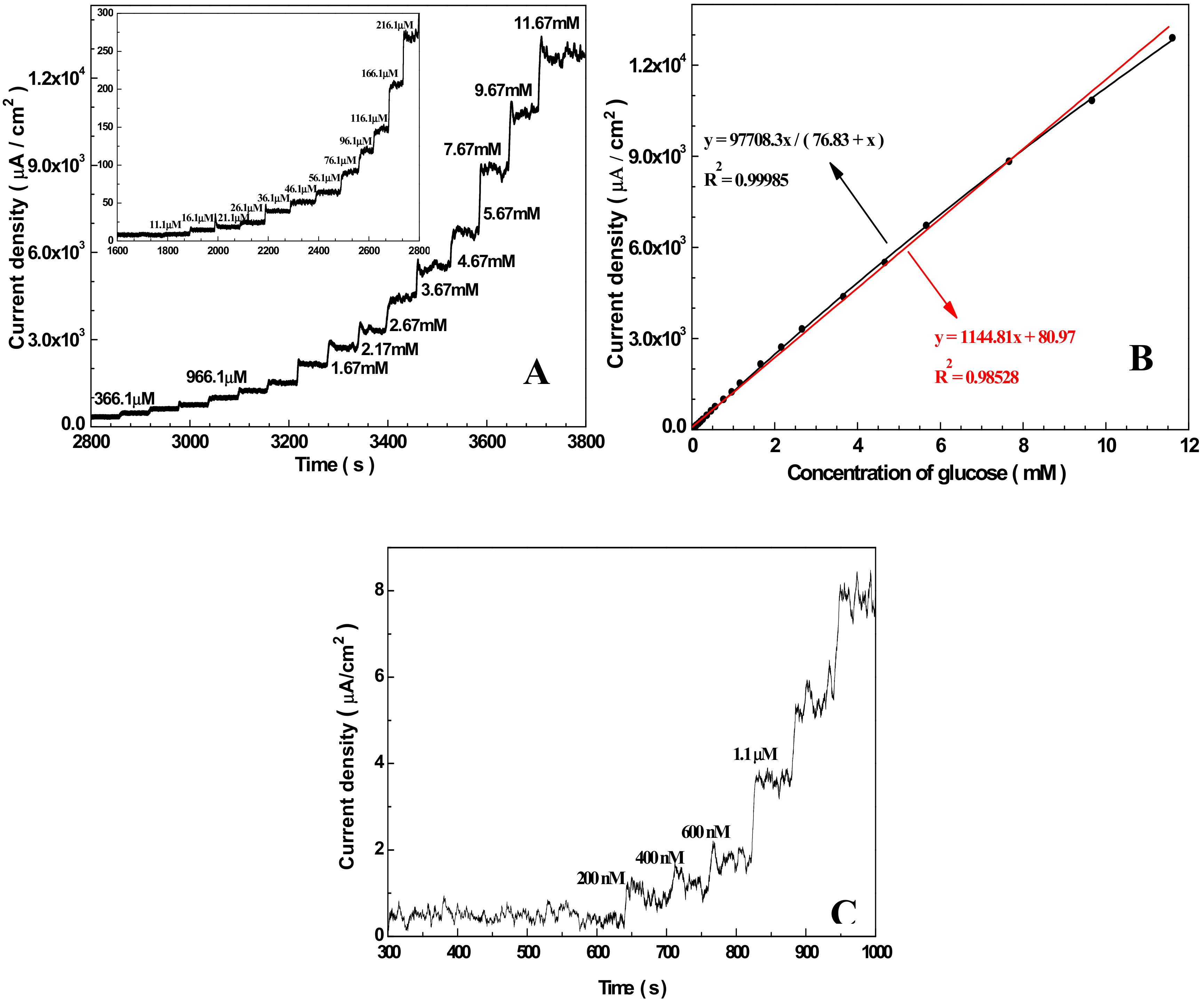
3.3. Amperometric Response of the Sensor Towards Glucose
3.4. Specificity, Reproducibility, and Stability of the Nafion/Cu2O–BSA/GCE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, U.; Luft, G.; Richter, G.J.; von Sturm, F. Development of an implantable electrocatalytic glucose sensor. Bioelectrochem. Bioenerg. 1978, 5, 607–624. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose wensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Wang, G.F.; He, X.P.; Wang, L.L.; Gu, A.X.; Huang, Y.; Fang, B.; Geng, B.Y.; Zhang, X.J. Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Si, P.; Huang, Y.J.; Wang, T.H.; Ma, J.M. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.J.; Yang, D.W.; Xiao, H.L.; Fu, Y.C.; Tan, Y.M.; Yao, S.Z. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal nanostructures for non-enzymatic glucose sensing. Mater. Sci. Eng. C 2017, 70, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.F.; Gu, L.T.; Wang, J.; Li, X.S.; Liang, G.X.; Zhou, J.H.; Zhang, Z. Novel and sensitive chemiluminescence sensors based on 2D-MOF nanosheets for one-step detection of glucose in human urine. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef]
- Li, H.J.; Liu, C.L.; Wang, D.; Zhang, C.S. Chemiluminescence cloth-based glucose test sensors (CCGTSs): A new class of chemiluminescence glucose sensors. Biosens. Bioelectron. 2017, 15, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.J.; Liu, N.; Ma, Z.F. A new luminol chemiluminescence sensor for glucose based on pH-dependent graphene oxide. Analyst 2013, 138, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.B.; Hu, Q.Z.; Kang, Q.; Yu, L. Fabrication of liquid-crystal-based optical sensing platform for detection of hydrogen peroxide and blood glucose. Anal. Chem. 2018, 90, 11607–11613. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Paidi, S.K.; Valdez, T.A.; Zhang, C.; Spegazzini, N.; Dasari, R.R.; Barman, I. Noninvasive monitoring of blood glucose with Raman spectroscopy. Acc. Chem. Res. 2017, 50, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Afroosheh, S.; Lee, J.O.; Cho, H.; Kumar, S.; Siddique, R.H.; Narasimhan, V.; Yoon, Y.Z.; Zayak, A.T.; Choo, H. Glucose sensing using surface-enhanced Raman-mode constraining. Anal. Chem. 2018, 90, 14269–14278. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.K.; Means, A.K.; Dong, P.; Nichols, T.J.; Coté, G.L.; Grunlan, M.A. A Layer-by-Layer Approach to Retain a Fluorescent Glucose Sensing Assay within the Cavity of a Hydrogel Membrane. ACS Appl. Bio Mater. 2018, 1, 1319–1327. [Google Scholar] [CrossRef]
- Wang, D.; Pan, K.; Qu, Y.; Wang, G.F.; Yang, X.F.; Wang, D.S. BaWO4:Ln3+ nanocrystals: Controllable synthesis, theoretical investigation on the substitution site, and bright upconversion luminescence as a sensor for glucose detection. ACS Appl. Nano Mater. 2018, 1, 4762–4770. [Google Scholar] [CrossRef]
- Li, J.M.; Wu, H.L.; Santana, I.; Fahlgren, M.; Giraldo, J.P. Standoff optical glucose sensing in photosynthetic organisms by a quantum dot fluorescent probe. ACS Appl. Mater. Interf. 2018, 10, 28279–28289. [Google Scholar] [CrossRef]
- Pan, Z.B.; Wang, Y.C.; Chakkaradhari, G.; Zhu, J.F.; He, R.Y.; Liu, Y.C.; Hsu, C.H.; Koshevoy, I.O.; Chou, P.T.; Pan, S.W.; et al. A silver metal complex as a luminescent probe for enzymatic sensing of glucose in blood plasma and urine. Dalton Trans. 2018, 47, 8346–8355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.C.; Li, X.; Xu, X.C.; He, Y.F.; Qiu, F.X.; Pan, J.M.; Niu, X.H. Pd nanoparticle-decorated graphitic C3N4 nanosheets with bifunctional peroxidase mimicking and ON–OFF fluorescence enable naked-eye and fluorescent dual-readout sensing of glucose. J. Mater. Chem. B 2019, 7, 233–239. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hong, H.S.; Tang, X.N.; Fang, H.D.; Xu, X.H. Ultrasonic electrodeposition of platinum nanoflowers and their application in nonenzymatic glucose sensors. Electrochim. Acta 2012, 63, 1–8. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, N.; Srivastava, S. Modulating electron transfer properties of gold nanoparticles for efficient biosensing. Biosens. Bioelectron. 2012, 37, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Jin, J.; Yang, G.X.; Lu, T.H.; Zhang, H.; Cai, C.X. Nonenzymatic electrochemical detection of glucose based on palladium-single-walled carbon nanotube hybrid nanostructures. Anal. Chem. 2009, 81, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.F.; Jia, M.Z.; Zhang, M.X.; Hu, J.B. Nonenzymatic glucose sensor based on nickel ionimplanted-modified indium tin oxide electrode. Electrochim. Acta 2013, 96, 285–290. [Google Scholar] [CrossRef]
- Noha, H.B.; Lee, K.S.; Chandra, P.; Won, M.S.; Shim, Y.B. Application of a Cu-Co alloy dendrite on glucose and hydrogen peroxide sensors. Electrochimi. Acta 2012, 61, 36–43. [Google Scholar] [CrossRef]
- Li, X.L.; Yao, J.Y.; Liu, F.L.; He, H.C.; Zhou, M.; Mao, N.; Xiao, P.; Zhang, Y.H. Nickel/copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens. Actuators B Chem. 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Tee, S.Y.; Ye, E.Y.; Pan, P.H.; Lee, C.J.; Hui, H.K.; Zhang, S.Y.; Koh, L.D.; Dong, Z.L.; Han, M.Y. Fabrication of bimetallic Cu/Au nanotubes and their sensitive, selective, reproducible and reusable electrochemical sensing of glucose. Nanoscale 2015, 7, 11190–11198. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.J.; Kumar, T.R.; Yoo, D.J.; Phang, S.M.; Kumar, G.G. Electrodeposited nickel cobalt sulfide flowerlike architectures on disposable cellulose filter paper for enzyme-free glucose sensor applications. ACS Sustain. Chem. Eng. 2018, 6, 16982–16989. [Google Scholar] [CrossRef]
- Sinha, L.; Pakhira, S.; Bhojane, P.; Mali, S.; Hong, C.K.; Shirage, P.M. Hybridization of Co3O4 and α-MnO2 nanostructures for high performance nonenzymatic glucose sensing. ACS Sustain. Chem. Eng. 2018, 6, 13248–13261. [Google Scholar] [CrossRef]
- Huang, M.; Luo, X.; He, D.; Jiang, P. Hierarchical Co(OH)2 nanotube arrays grown on carbon cloth for use in non-enzymatic glucose sensing. Anal. Methods 2017, 9, 5903–5909. [Google Scholar] [CrossRef]
- Tomanin, P.P.; Cherepanov, P.V.; Besford, Q.A.; Christofferson, A.J.; Amodio, A.; McConville, C.F.; Yarovsky, I.; Caruso, F.; Cavalieri, F. Cobalt phosphate nanostructures for non-enzymatic glucose sensing at physiological pH. ACS Appl. Mater. Interf. 2018, 10, 42786–42795. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Zhang, C.M.; Gao, X.H.; Zhuang, Z.H.; Du, C.; Chen, W. 3D network and 2D paper of reduced graphene oxide/Cu2O composite for electrochemical sensing of hydrogen peroxide. Anal. Chem. 2018, 90, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.Q.; Zhong, H.; Kou, T.Y.; Frenzel, J.; Eggeler, G.; Zhang, Z.H. Three-dimensional Cu foam-supported single crystalline mesoporous Cu2O nanothorn arrays for ultra-highly sensitive and efficient nonenzymatic detection of glucose. ACS Appl. Mater. Interf. 2015, 7, 20215–20223. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.R.; Wang, M.J.; Kathiravan, D.; Kurniawan, A.; Zhang, H.H.; Yang, W.L. Interfacial effect of oxygen-doped nanodiamond on CuO and micropyramidal silicon heterostructures for efficient nonenzymatic. ACS Appl. Bio. Mater. 2018, 1, 1579–1586. [Google Scholar] [CrossRef]
- Babu, K.J.; Sheet, S.; Lee, Y.S.; Kumar, G.G. Three-dimensional dendrite Cu−Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection. ACS Sustain. Chem. Eng. 2018, 6, 1909–1918. [Google Scholar] [CrossRef]
- Zhu, W.X.; Wang, J.; Zhang, W.T.; Hu, N.; Wang, J.; Huang, L.J.; Wang, R.; Suo, Y.R.; Wang, J.L. Monolithic copper selenide submicron particulate film/copper foam anode catalyst for ultrasensitive electrochemical glucose sensing in human blood serum. J. Mater. Chem. B 2018, 6, 718–724. [Google Scholar] [CrossRef]
- Chen, X.R.; Liu, D.; Cao, G.J.; Tang, Y.; Wu, C. In Situ Synthesis of a sandwich-like graphene@ZIF-67 heterostructure for highly sensitive nonenzymatic glucose sensing in human serums. ACS Appl. Mater. Interf. 2019, 11, 9374–9384. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Xia, J.F.; Zhang, F.F.; Wang, Z.H. MOF-derived porous Ni2P/graphene composites with enhanced electrochemical properties for sensitive nonenzymatic glucose sensing. ACS Appl. Mater. Interf. 2018, 10, 39151–39160. [Google Scholar] [CrossRef]
- Deepalakshmi, T.; Tran, D.T.; Kim, N.H.; Chong, K.T.; Lee, J.H. Nitrogen-doped graphene-encapsulated nickel cobalt nitride as a highly sensitive and selective electrode for glucose and hydrogen peroxide sensing applications. ACS Appl. Mater. Interf. 2018, 10, 35847–35858. [Google Scholar] [CrossRef]
- Meng, A.; Sheng, L.Y.; Zhao, K.; Li, Z.J. A controllable honeycomb-like amorphous cobalt sulfide architecture directly grown on the reduced graphene oxide–poly(3,4-ethylenedioxythiophene) composite through electrodeposition for non-enzyme glucose sensing. J. Mater. Chem. B 2017, 5, 8934–8943. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Meng, W.; Li, C.; Dai, L.; He, Z.X.; Wang, L.; Li, M.; Zhu, J. Enhanced glucose sensing based on a novel composite Co II -MOF/Acb modified electrode. Dalton Trans. 2018, 47, 3872–3879. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Wang, Y.F.; He, W.H.; Ni, Y.N.; Kokot, S. Nano-composite of Co3O4 and Cu with enhanced stability and catalytic performance for non-enzymatic electrochemical glucose sensors. RSC Adv. 2017, 7, 54460–54467. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.Y.; Zhang, Q.R.; Tong, X.L.; Zhang, Y.; Li, Z.A. Pt catalyzed formation of a Ni@Pt/reduced graphene oxide nanocomposite: preparation and electrochemical sensing application for glucose detection. Anal. Methods 2018, 10, 3845–3850. [Google Scholar] [CrossRef]
- Kano, K.; Takagi, K.; Inoue, K.; Ikeda, T.; Ueda, T. Copper electrodes for stable subpicomole detection of carbohydrates in high-performance liquid chromatography. J. Chromatogr. A 1996, 721, 53–57. [Google Scholar] [CrossRef]
- Kano, K.; Torimura, M.; Esaka, Y.; Goto, M.; Ueda, T. Electrocatalytic oxidation of carbohydrates at copper(ii)-modified electrodes and its application to flow-through detection. J. Electroanal. Chem. 1994, 372, 137–143. [Google Scholar] [CrossRef]
- Gou, X.F.; Sun, S.D.; Yang, Q.; Li, P.J.; Liang, S.H.; Zhang, X.J.; Yang, Z.M. A very facile strategy for the synthesis of ultrathin CuO nanorods towards non-enzymatic glucose sensing. New J. Chem. 2018, 42, 6364–6369. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Das, A.; Samantaray, K.; Singh, S.K.; Mane, R.S.; Shin, H.C.; Yun, J.M.; Kim, K.H. Electrochemical glucose sensing characteristics of two-dimensional faceted and non-faceted CuO Nanoribbons. Cryst. Eng. Comm. 2019, 21, 1607–1616. [Google Scholar] [CrossRef]
- Yang, A.L.; Wang, Y.J.; Li, S.P.; Bao, X.C.; Yang, R.Q. Stepwise synthesis of cuprous oxide nanoparticles with adjustable structures and growth model. Sci. China Technol. Sci. 2014, 57, 2287–2294. [Google Scholar] [CrossRef]
- Cao, H.M.; Yang, A.L.; Li, H.; Wang, L.L.; Li, S.P.; Kong, J.L.; Bao, X.C.; Yang, R.Q. A non-enzymatic glucose sensing based on hollow cuprous oxide nanospheres in a Nafion matrix. Sens. Actuators B 2015, 214, 169–173. [Google Scholar] [CrossRef]
- Akhavan, A.; Kalhor, H.R.; Kassaee, M.Z.; Sheikh, N.; Hassanlou, M. Radiation synthesis and characterization of protein stabilized gold nanoparticles. Chem. Eng. J. 2010, 159, 230–235. [Google Scholar] [CrossRef]
- Tsai, D.H.; DelRio, F.W.; Keene, A.M.; Tyner, K.M.; MacCuspie, R.I.; Cho, T.J.; Zachariah, M.R.; Hackley, V.A. Adsorption and conformation of serum albumin protein on gold nanoparticles investigated using dimensional measurements and in situ spectroscopic methods. Langmuir 2011, 27, 2464–2477. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Kundu, S. Adsorption and conformation variation of BSA protein with the size variation of the metallic nanoparticles in LB film. Coll. Surf. A Physicochem. Eng. Asp. 2015, 468, 56–61. [Google Scholar] [CrossRef]
- Chakraborty, S.; Joshi, P.; Shanker, V.; Ansari, Z.A.; Singh, S.P.; Chakrabarti, P. Contrasting effect of gold nanoparticles and nanorods with different surface modifications on the structure and activity of bovine serum albumin. Langmuir 2011, 27, 7722–7731. [Google Scholar] [CrossRef]
- Shi, X.J.; Li, D.; Xie, J.; Wang, S.; Wu, Z.Q.; Chen, H. Spectroscopic investigation of the interactions between gold nanoparticles and bovine serum albumin. Chin. Sci. Bull. 2012, 57, 1109–1115. [Google Scholar] [CrossRef] [Green Version]
- Mathew, T.V.; Kuriakose, S. Studies on the antimicrobial properties of colloidal silver nanoparticles stabilized by bovine serum albumin. Coll. Surf. B Biointerf. 2013, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, X.X.; Wang, K.M.; Xie, C.; Zhou, B.; Qing, Z.H. Ultrasmall near-infrared gold nanoclusters for tumor fluorescence imaging in vivo. Nanoscale 2010, 2, 2244–2249. [Google Scholar] [CrossRef]
- Hu, C.Y.; Yang, D.P.; Wang, Z.Y.; Yu, L.L.; Zhang, J.L.; Jia, N.Q. Improved EIS performance of an electrochemical cytosensor using three-dimensional architecture Au@BSA as sensing layer. Anal. Chem. 2013, 85, 5200–5206. [Google Scholar] [CrossRef]
- Hu, C.Y.; Yang, D.P.; Wang, Z.H.; Huang, P.; Wang, X.S.; Chen, D.; Cui, D.X.; Yang, M.; Jia, N.Q. Bio-mimetically synthesized Ag@BSA microspheres as a novel electrochemical biosensing interface for sensitive detection of tumor cells. Biosens. Bioelectron. 2013, 41, 656–662. [Google Scholar] [CrossRef]
- Habeeb Muhammed, M.A.; Verma, P.K.; Pal, S.K.; Retnakumari, A.; Koyakutty, M.; Nair., S.; Pradeep, T. Luminescent quantum clusters of gold in bulk by albumin-induced core etching of nanoparticles: Metal ion sensing, metal-enhanced luminescence, and biolabeling. Chemistry 2010, 16, 10103–10112. [Google Scholar] [CrossRef]
- Hu, C.Y.; Yang, D.P.; Zhu, F.J.; Jiang, F.J.; Shen, S.Y.; Zhang, J.L. Enzyme-labeled Pt@BSA nanocomposite as a facile electrochemical biosensing interface for sensitive glucose determination. ACS Appl. Mater. Interf. 2014, 6, 4170–4178. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.R.; Li, Y.W.; Jiang, A.H. Structure, optical property and thermal stability of copper nitride films prepared by reactive radio frequency magnetron sputtering. J. Mater. Sci. Technol. 2011, 27, 403–407. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, G.F.; Zhang, W.; Wei, Y.; Fang, B. Fixture-reduce method for the synthesis of Cu2O/MWCNTs nanocomposites and its application as enzyme-free glucose sensor. Biosens. Bioelectron. 2009, 11, 3395–3398. [Google Scholar] [CrossRef] [PubMed]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187–1197. [Google Scholar] [CrossRef]
- Retnakumari, A.; Setua, S.; Menon, D.; Ravindran, P.; Muhammed, H.; Pradeep, T.; Nair, S.; Koyakutty, M. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 2010, 21, 055103. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Z.L.; Zhou, Y.; Weng, S.F.; Zhang, L.; Wu, J.G. Influence of metal ions on phosphatidylcholine–bovine serum albumin model membrane, an FTIR study. J. Mol. Struct. 2006, 794, 1–11. [Google Scholar] [CrossRef]
- Lu, N.; Shao, C.L.; Li, X.H.; Shen, T.; Zhang, M.Y.; Miao, F.J.; Zhang, P.; Zhang, X.; Wang, K.X.; Zhang, Y.; et al. CuO/Cu2O nanofibers as electrode materials for non-enzymatic glucose sensors with improved sensitivity. RSC Adv. 2014, 4, 31056–31061. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, M.J.; Song, B.; Liu, J.L.; Hua, C.G.; Wei, D.P.; Wong, C.P. Precisely quantified catalyst based on in situ growth of Cu2O nanoparticles on a graphene 3D network for highly sensitive glucose sensor. Sens. Actuators B Chem. 2017, 250, 333–341. [Google Scholar] [CrossRef]
- Yazid, S.N.A.M.; Isa, I.M.; Norhayati, N. Novel alkaline-reduced cuprous oxide/graphene nanocomposites for non-enzymatic amperometric glucose sensor application. Mat. Sci. Eng. C Mater. 2016, 68, 465–473. [Google Scholar] [CrossRef]
- Khedekar, V.V.; Bhanage, B.M. Simple electrochemical synthesis of cuprous oxide nanoparticles and their application as a non-enzymatic glucose sensor. J. Electrochem. Soc. 2016, 163, B248–B251. [Google Scholar] [CrossRef]
- Chen, A.; Ding, Y.; Yang, Z.M.; Yang, S.C. Constructing heterostructure on highly roughened caterpillar-like gold nanotubes with cuprous oxide grains for ultrasensitive and stable nonenzymatic glucose sensor. Biosens. Bioelectron. 2015, 74, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.J.; Han, T.T.; Qu, L.L.; Wu, X.Y. A ultrasensitive nonenzymatic glucose sensor based on Cu2O polyhedrons modified Cu electrode. Chin. Chem. Lett. 2012, 23, 953–956. [Google Scholar] [CrossRef]
- El Khatib, K.M.; Abdel Hameed, R.M. Development of Cu2O/Carbon Vulcan XC-72 as non-enzymatic sensor for glucose determination. Biosens. Bioelectron. 2011, 26, 3542–3548. [Google Scholar] [CrossRef] [PubMed]



| Electrode Material | Sensitivity (μA cm−2 mM−1) | Linear Range (mM) | Detection Limit (μM) | Year | Reference |
|---|---|---|---|---|---|
| Nafion/Cu2O–BSA/GC | 1144.81 | up to 10 | 0.4 | 2019 | This Work |
| Cu2O NPs | 2310 ± 30 | 0.00048–1.813 | 0.14 ± 0.01 | 2017 | [69] |
| Cu2O/graphene/GCE | 1330.05 | 0.01–3.0 | 0.36 | 2016 | [70] |
| Cu2O NPs | 507 | 0.1–2.5 | 26 | 2016 | [71] |
| Cu2O/Nafion/GCE | 2038.2 | 0.00125–0.0375 | 0.41 | 2015 | [50] |
| Cu2O-CLGNs/GCE | 1215.7 | 0.1–5 | 1.83 | 2015 | [72] |
| CuO/Cu2O NFs | 830 | 0.5–10 | Not given | 2014 | [67] |
| Cu2O/Cu | 728.67 | 0.53–7.53 | Not given | 2012 | [73] |
| 3029.33 | 0.01–0.53 | ||||
| Cu2O/Carbon Vulcan XC-72 | 629 | up to 6 | 2.4 | 2011 | [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Yang, A.; Bao, X.; Yang, R. Facile Non-Enzymatic Electrochemical Sensing for Glucose Based on Cu2O–BSA Nanoparticles Modified GCE. Sensors 2019, 19, 2824. https://doi.org/10.3390/s19122824
Dai Z, Yang A, Bao X, Yang R. Facile Non-Enzymatic Electrochemical Sensing for Glucose Based on Cu2O–BSA Nanoparticles Modified GCE. Sensors. 2019; 19(12):2824. https://doi.org/10.3390/s19122824
Chicago/Turabian StyleDai, Zhikuang, Ailing Yang, Xichang Bao, and Renqiang Yang. 2019. "Facile Non-Enzymatic Electrochemical Sensing for Glucose Based on Cu2O–BSA Nanoparticles Modified GCE" Sensors 19, no. 12: 2824. https://doi.org/10.3390/s19122824