Blood Coagulation Testing Smartphone Platform Using Quartz Crystal Microbalance Dissipation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
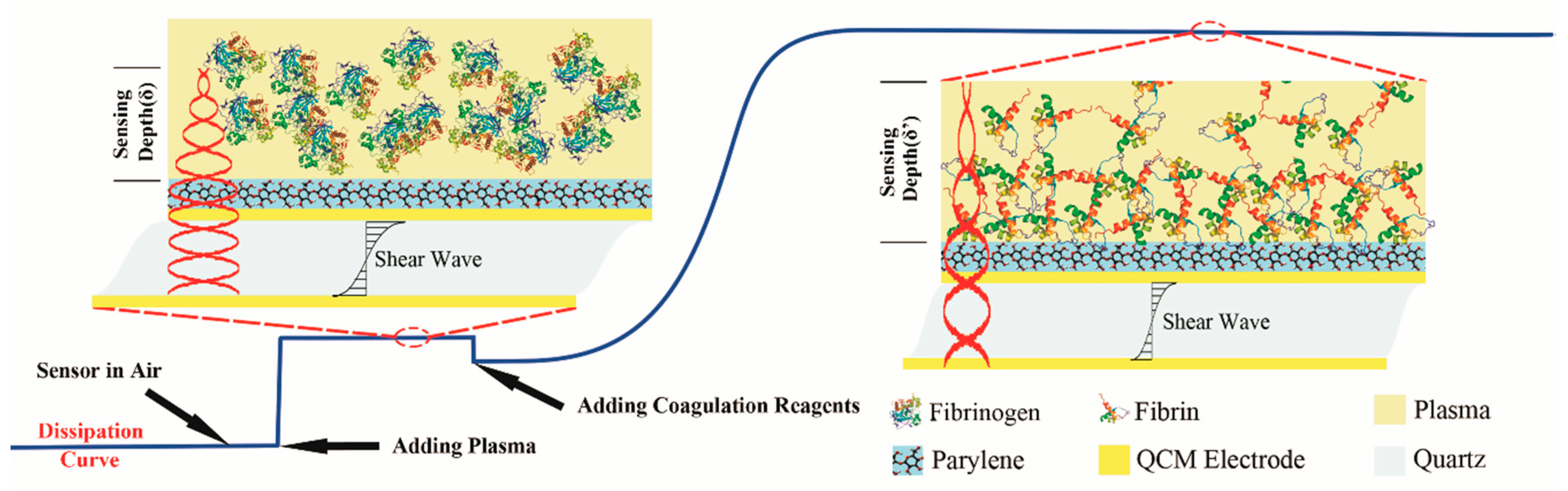
2.2. QCM Dissipation Based Coagulation Measurement Principles
2.3. Platform Electronic Design Principles
- D—The dissipation factor of the QCM sensor,
- BW—3 dB bandwidth of the center frequency, in Hertz,
- fs—The resonant frequency, in Hertz.
- Switches K1 and K1’ were left unconnected to any pathway to start a dark noise calibration;
- DDS chips were used to generate two sine wave signal Se and Sre with the same frequency (fn) and the same phase (0°), the result of the multiplier was processed by low pass filter (LPF) and analog to digital converter (ADC), the voltage was recorded as ;
- Keep the signal Se unchanged and Sre was generated with frequency and phase 90°, the voltage result was recorded as ;
- K1 and K1’ were switched to calibration pathway to start a single frequency zero-point calibration.
- Repeat steps 2–3, voltage results were recorded as and respectively;
- K1 and K1’ were switched to QCM pathway to start a single point frequency response measurement;
- Repeat steps 2–3, voltage results were recorded as and respectively;
2.4. Coagulation Test Chip Design
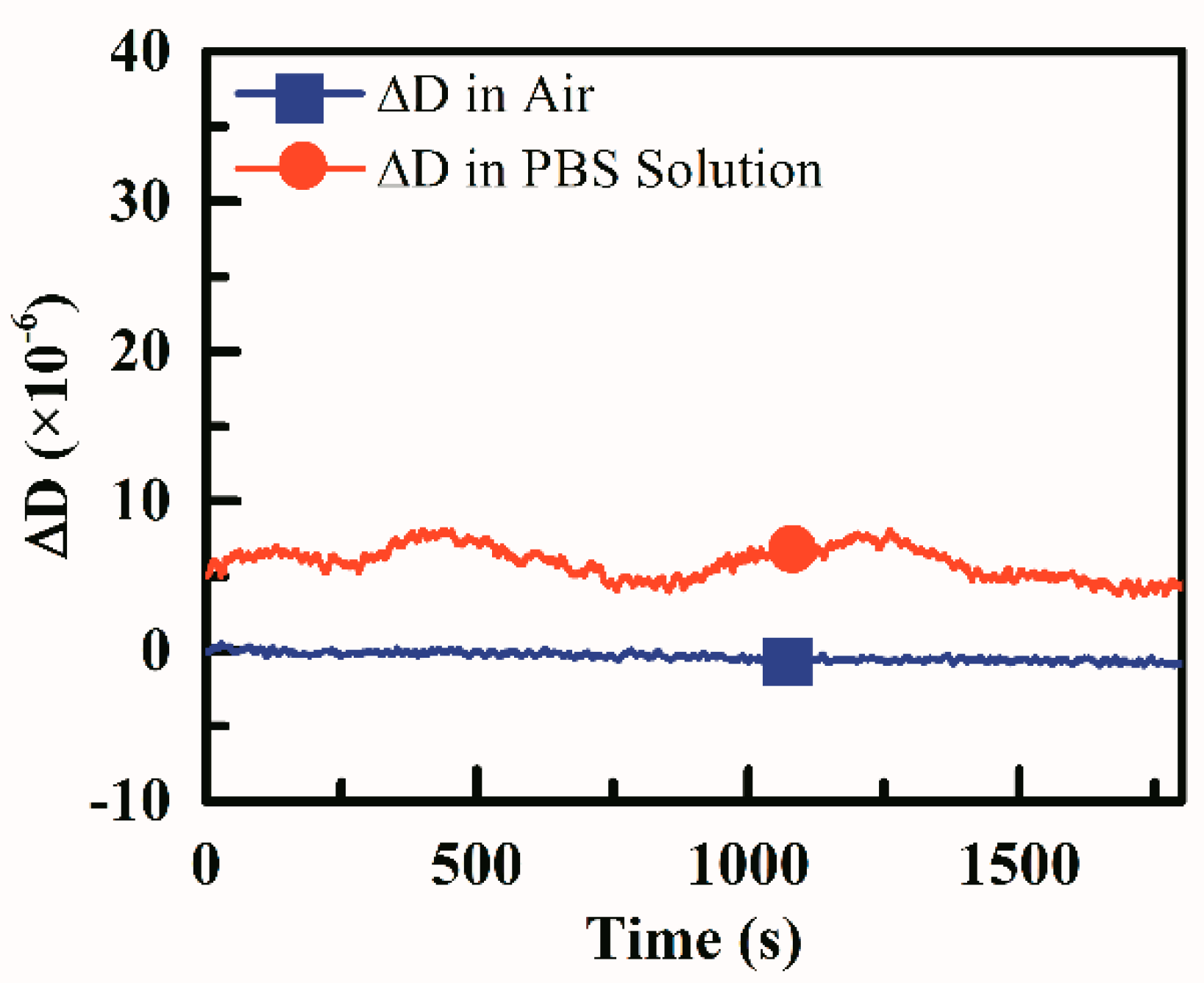
2.5. Dissipation Stability of the Test Device
2.6. APTT and PT Measurements Based on the Platform
3. Results and Discussions
3.1. Dissipation Based Coagulation Measurement
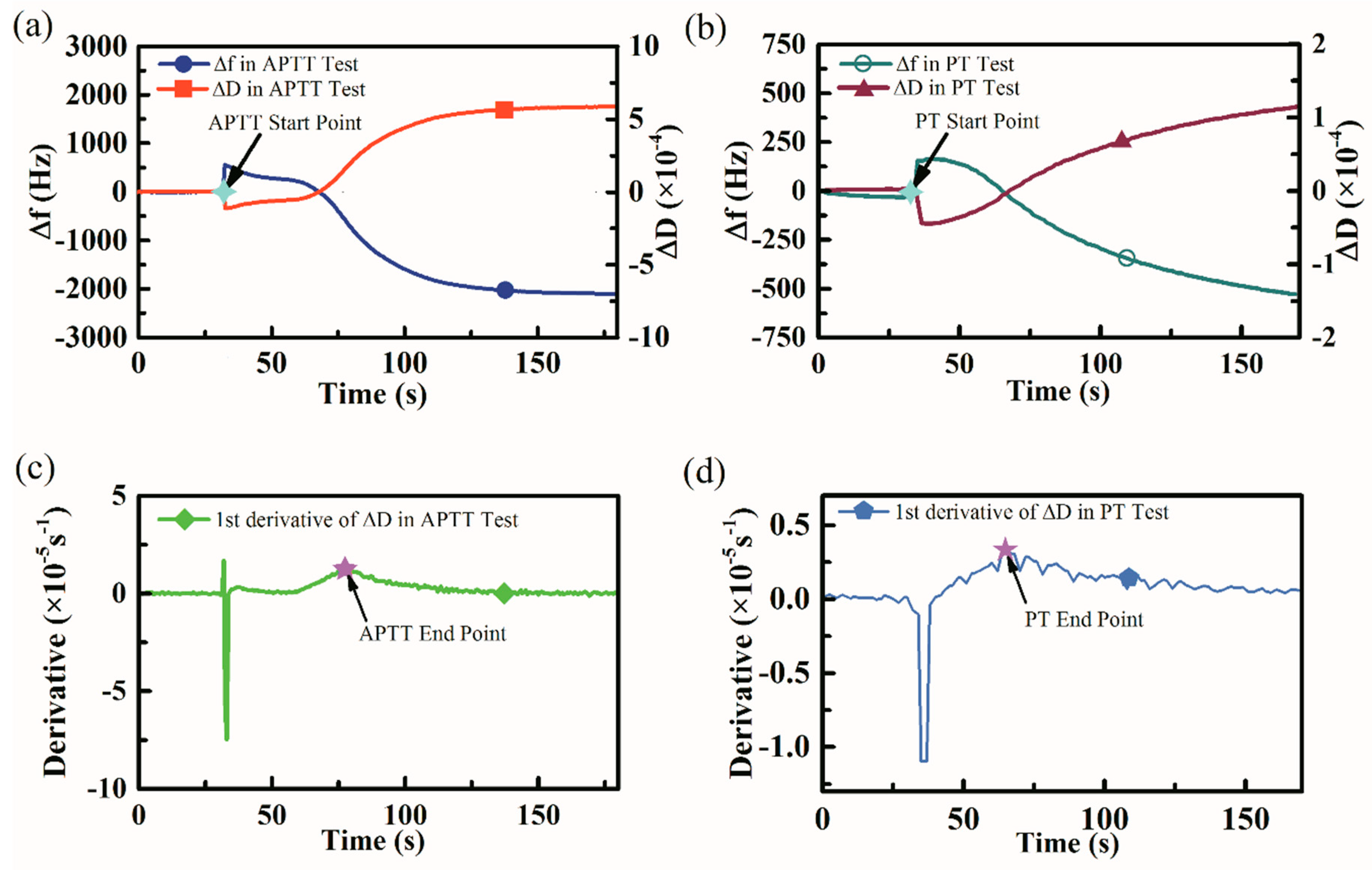
3.2. Coagulation Indexes Results Determination
3.3. APTT and PT Measurement Results
3.4. Reducing the Sample Volume of Coagulation Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bolliger, D.; Tanaka, K.A. Point-of-Care Coagulation Testing in Cardiac Surgery. Semin. Thromb. Hemost. 2017, 43, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Asmis, L.M.; Alberio, L.; Angelillo-Scherrer, A.; Korte, W.; Mendez, A.; Reber, G.; Seifert, B.; Stricker, H.; Tsakiris, D.A.; Wuillemin, W.A. Rivaroxaban: Quantification by anti-FXa assay and influence on coagulation tests: A study in 9 Swiss laboratories. Thromb. Res. 2012, 129, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ebner, M.; Birschmann, I.; Peter, A.; Spencer, C.; Härtig, F.; Kuhn, J.; Blumenstock, G.; Zuern, C.S.; Ziemann, U.; Poli, S. Point-of-care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants. Crit. Care 2017, 21, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M. Coagulation Assays. Circulation 2005, 112, e53–e60. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Mallett, S.V.; Cox, D.J. Thrombelastography. Br. J. Anaesth. 1992, 69, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Padovani, F.; Duffy, J.; Hegner, M. Microrheological Coagulation Assay Exploiting Micromechanical Resonators. Anal. Chem. 2016, 89, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Shih, C.M.; Su, Y.Y.; Su, L.H.J.; Chang, M.S.; Lin, S.J. Quantitative evaluation of thrombosis by electrochemical methodology. Thromb. Res. 2003, 111, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Thuerlemann, C.; Haeberli, A.; Alberio, L. Monitoring thrombin generation by electrochemistry: Development of an amperometric biosensor screening test for plasma and whole blood. Clin. Chem. 2009, 55, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.M.; Vikinge, T.P.; Rånby, M.; Tengvall, P.; Lundström, I.; Johansen, K.; Lindahl, T.L. Surface plasmon resonance (SPR) analysis of coagulation in whole blood with application in prothrombin time assay. Biosens. Bioelectron. 1999, 14, 671–682. [Google Scholar] [CrossRef]
- Iapichino, G.E.; Bianchi, P.; Ranucci, M.; Baryshnikova, E. Point-of-Care Coagulation Tests Monitoring of Direct Oral Anticoagulants and Their Reversal Therapy: State of the Art. Semin. Thromb. Hemost. 2017, 43, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Ganter, M.T.; Hofer, C.K. Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth. Analg. 2008, 106, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Mallett, S.V. Clinical Utility of Viscoelastic Tests of Coagulation (TEG/ROTEM) in Patients with Liver Disease and during Liver Transplantation. Semin. Thromb. Hemost. 2015, 41, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; Dinardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Rodahl, M.; HööK, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. Frequency and dissipation-factor responses to localized liquid deposits on a QCM electrode. Sens. Actuators B Chem. 1996, 37, 111–116. [Google Scholar] [CrossRef]
- Pirinçci, Ş.; Ertekin, Ö.; Laguna, D.; Özen, F.; Öztürk, Z.; Öztürk, S. Label-Free QCM Immunosensor for the Detection of Ochratoxin A. Sensors 2018, 18, 1161. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; García, P.; García, M.; García, J.; Jiménez, Y.; Arnau, A. Design and Validation of a 150 MHz HFFQCM Sensor for Bio-Sensing Applications. Sensors 2017, 17, 2057. [Google Scholar] [CrossRef] [PubMed]
- Efremov, V.; Lakshmanan, R.; Byrne, B.; Cullen, S.M.; Killard, A.J. Modelling of blood component flexibility using quartz crystal microbalance. J. Biorheol. 2014, 28, 45–54. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, M.; Liang, L.; Wang, W.; Xie, J. Airborne particulate matter classification and concentration detection based on 3D printed virtual impactor and quartz crystal microbalance sensor. Sens. Actuators A Phys. 2016, 238, 379–388. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, D.; Liu, X.; Wu, C.; Xie, J. A Miniature System for Separation and Detection of PM Based on 3-D Printed Virtual Impactor and QCM Sensor. IEEE Sens. J. 2018, 18, 6130–6137. [Google Scholar] [CrossRef]
- Bunroddith, K.; Viseshakul, N.; Chansiri, K.; Lieberzeit, P. QCM-based rapid detection of PCR amplification products of Ehrlichia canis. Anal. Chim. Acta 2018, 1001, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.J.; Chang, H.C.; Lin, T.M. A piezoelectric quartz crystal sensor for the determination of coagulation time in plasma and whole blood. Biosens. Bioelectron. 1998, 13, 147–156. [Google Scholar] [CrossRef]
- Ganesan, A.; Rajendran, G.; Ercole, A.; Seshia, A. Multifrequency acoustics as a probe of mesoscopic blood coagulation dynamics. Appl. Phys. Lett. 2016, 109, 413–416. [Google Scholar] [CrossRef]
- Hussain, M.; Northoff, H.; Gehring, F.K. QCM-D providing new horizon in the domain of sensitivity range and information for haemostasis of human plasma. Biosens. Bioelectron. 2015, 66, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Rupp, F.; Wendel, H.P.; Gehring, F.K. Bioapplications of acoustic crystals, a review. TrAC Trends Anal. Chem. 2018, 102, 194–209. [Google Scholar] [CrossRef]
- Dunér, G.; Thormann, E.; Dėdinaitė, A. Quartz Crystal Microbalance with Dissipation (QCM-D) studies of the viscoelastic response from a continuously growing grafted polyelectrolyte layer. J. Colloid Interface Sci. 2013, 408, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, M.; Schreiter, M.; Vörös, J. Comparison of FBAR and QCM-D sensitivity dependence on adlayer thickness and viscosity. Sens. Actuators A Phys. 2011, 165, 415–421. [Google Scholar] [CrossRef]
- Andersson, M.; Andersson, J.; Sellborn, A.; Berglin, M.; Nilsson, B.; Elwing, H. Quartz crystal microbalance-with dissipation monitoring (QCM-D) for real time measurements of blood coagulation density and immune complement activation on artificial surfaces. Biosens. Bioelectron. 2005, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Wendel, H.P.; Schmidt, K.; Langer, E.; Körber, M.K.; Faul, O.; Northoff, H.; von Heymann, C.; Gehring, F.K. QCM-D surpassing clinical standard for the dose administration of new oral anticoagulant in the patient of coagulation disorders. Biosens. Bioelectron. 2018, 104, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Qu, L.; Fu, W. Detection of Fibrinogen and Coagulation Factor VIII in Plasma by a Quartz Crystal Microbalance Biosensor. Sensors 2013, 13, 6946–6956. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Sinn, S.; Drechsel, H.; Ziegler, C.; Wendel, H.-P.; Northoff, H.; Gehring, F. Investigation of Prothrombin Time in Human Whole-Blood Samples with a Quartz Crystal Biosensor. Anal. Chem. 2010, 82, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Ermek, E.; Kilinc, N.; Bulut, S.; Baris, I.; Kavakli, I.H.; Yaralioglu, G.G.; Urey, H. A cartridge based sensor array platform for multiple coagulation measurements from plasma. Lab Chip 2015, 15, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Song, S.; Ma, J.; Zhang, Z.; Wang, P.; Liu, W.; Guo, Q. Micro-electromechanical film bulk acoustic sensor for plasma and whole blood coagulation monitoring. Biosens. Bioelectron. 2017, 91, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Köβlinger, C.; Uttenthaler, E.; Drost, S.; Aberl, F.; Wolf, H.; Brink, G.; Stanglmaier, A.; Sackmann, E. Comparison of the QCM and the SPR method for surface studies and immunological applications. Sens. Actuators B Chem. 1995, 24, 107–112. [Google Scholar] [CrossRef]
- Liu, S.X.; Kim, J.-T. Application of Kevin—Voigt Model in Quantifying Whey Protein Adsorption on Polyethersulfone Using QCM-D. J. Assoc. Lab. Autom. 2009, 14, 213–220. [Google Scholar] [CrossRef]
- Alassi, A.; Benammar, M.; Brett, D. Quartz Crystal Microbalance Electronic Interfacing Systems: A Review. Sensors 2017, 17, 2799. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M.; Zompanti, A.; Grasso, S.; D’Amico, A. Electronic Interface for a Gas Sensor System Based on 32 MHz QCMs: Design and Calibration. IEEE Sens. J. 2018, 18, 1419–1426. [Google Scholar] [CrossRef]
- Rodahl, M. QCM Operation in Liquids: An Explanation of Measured Variations in Frequency and Q Factor with Liquid Conductivity. Anal. Chem. 1996, 68, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, W.; Guo, Z.; Zhang, Z.; Zhu, H.; Yan, R.; Zhou, L. Stability enhanced, repeatability improved Parylene-C passivated on QCM sensor for aPTT measurement. Biosens. Bioelectron. 2017, 98, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Galli Marxer, C.; Collaud Coen, M.; Schlapbach, L. Study of adsorption and viscoelastic properties of proteins with a quartz crystal microbalance by measuring the oscillation amplitude. J. Colloid Interface Sci. 2003, 261, 291–298. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Paul, S.; Paul, D.; Basova, T.; Ray, A.K. Studies of Adsorption and Viscoelastic Properties of Proteins onto Liquid Crystal Phthalocyanine Surface Using Quartz Crystal Microbalance with Dissipation Technique. J. Phys. Chem. C 2008, 112, 11822–11830. [Google Scholar] [CrossRef]
- Robert Valeri, C.; Macgregor, H.; Cassidy, G.; Tinney, R.; Pompei, F. Effects of temperature on the BT and clotting time in normal male and female volunteers. Crit. Care Med. 1995, 23, 698–704. [Google Scholar] [CrossRef]




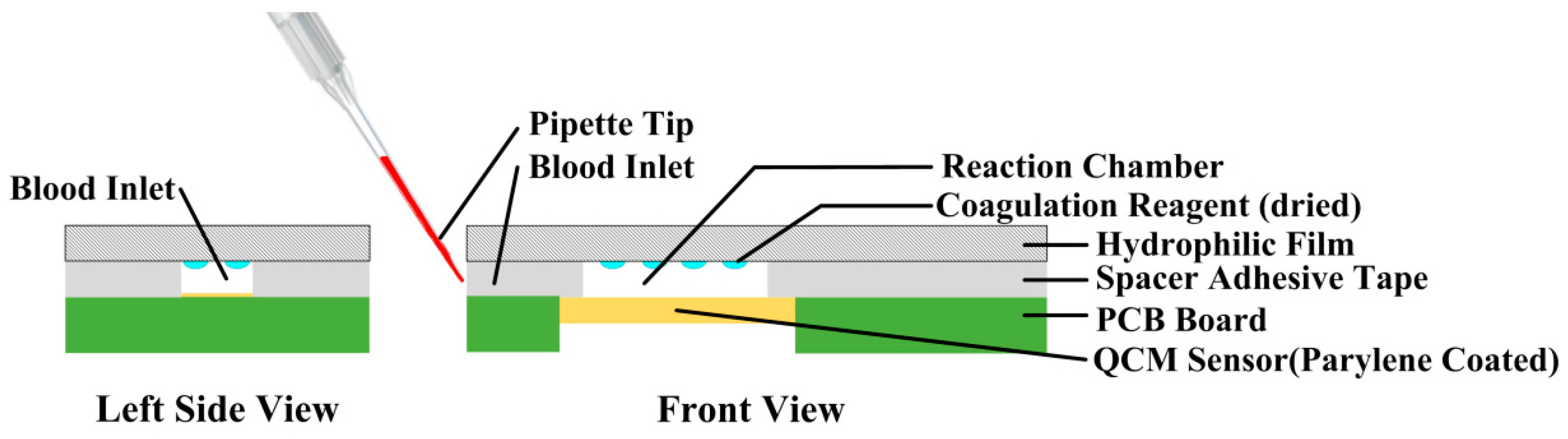
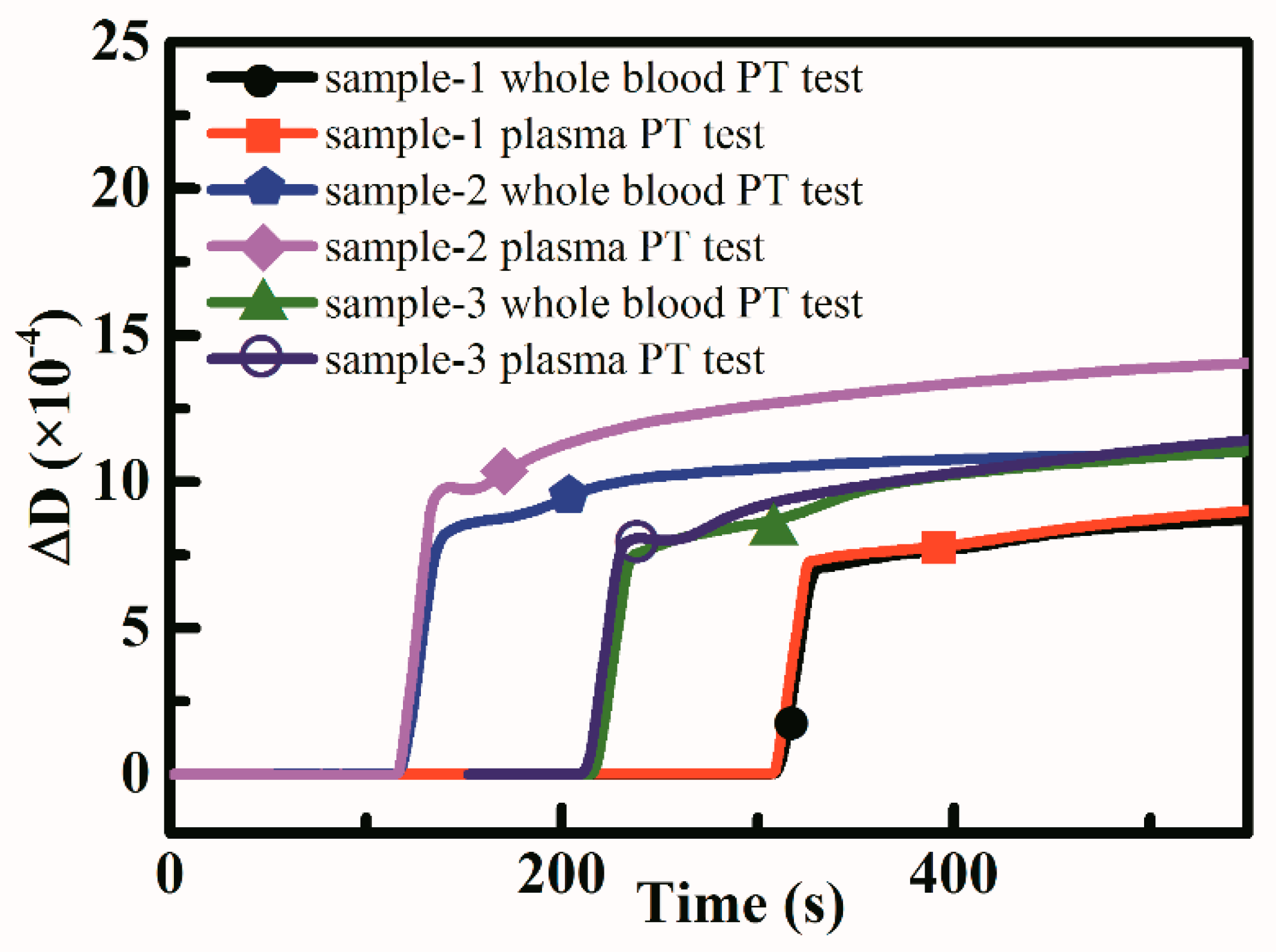
| Samples | Whole Blood Based PT Test Results (s) | Plasma Based PT Test Results (s) | Reference PT Test Results (s) |
|---|---|---|---|
| sample-1 | 72.1 ± 1.5 | 82.2 ± 5.4 | 10.8 |
| sample-2 | 67.0 ± 6.4 | 77.1 ± 3.4 | 10.7 |
| sample-3 | 85.3 ± 8.4 | 88.2 ± 10.8 | 10.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Feng, B.; Zhang, Z.; Li, C.; Zhang, W.; Guo, Z.; Zhao, H.; Zhou, L. Blood Coagulation Testing Smartphone Platform Using Quartz Crystal Microbalance Dissipation Method. Sensors 2018, 18, 3073. https://doi.org/10.3390/s18093073
Yao J, Feng B, Zhang Z, Li C, Zhang W, Guo Z, Zhao H, Zhou L. Blood Coagulation Testing Smartphone Platform Using Quartz Crystal Microbalance Dissipation Method. Sensors. 2018; 18(9):3073. https://doi.org/10.3390/s18093073
Chicago/Turabian StyleYao, Jia, Bin Feng, Zhiqi Zhang, Chuanyu Li, Wei Zhang, Zhen Guo, Heming Zhao, and Lianqun Zhou. 2018. "Blood Coagulation Testing Smartphone Platform Using Quartz Crystal Microbalance Dissipation Method" Sensors 18, no. 9: 3073. https://doi.org/10.3390/s18093073
APA StyleYao, J., Feng, B., Zhang, Z., Li, C., Zhang, W., Guo, Z., Zhao, H., & Zhou, L. (2018). Blood Coagulation Testing Smartphone Platform Using Quartz Crystal Microbalance Dissipation Method. Sensors, 18(9), 3073. https://doi.org/10.3390/s18093073





