Ruthenium Oxide Nanorods as Potentiometric pH Sensor for Organs-On-Chip Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Fabrication
2.2. Experimental Setup
2.3. Measurement Protocol
3. Results
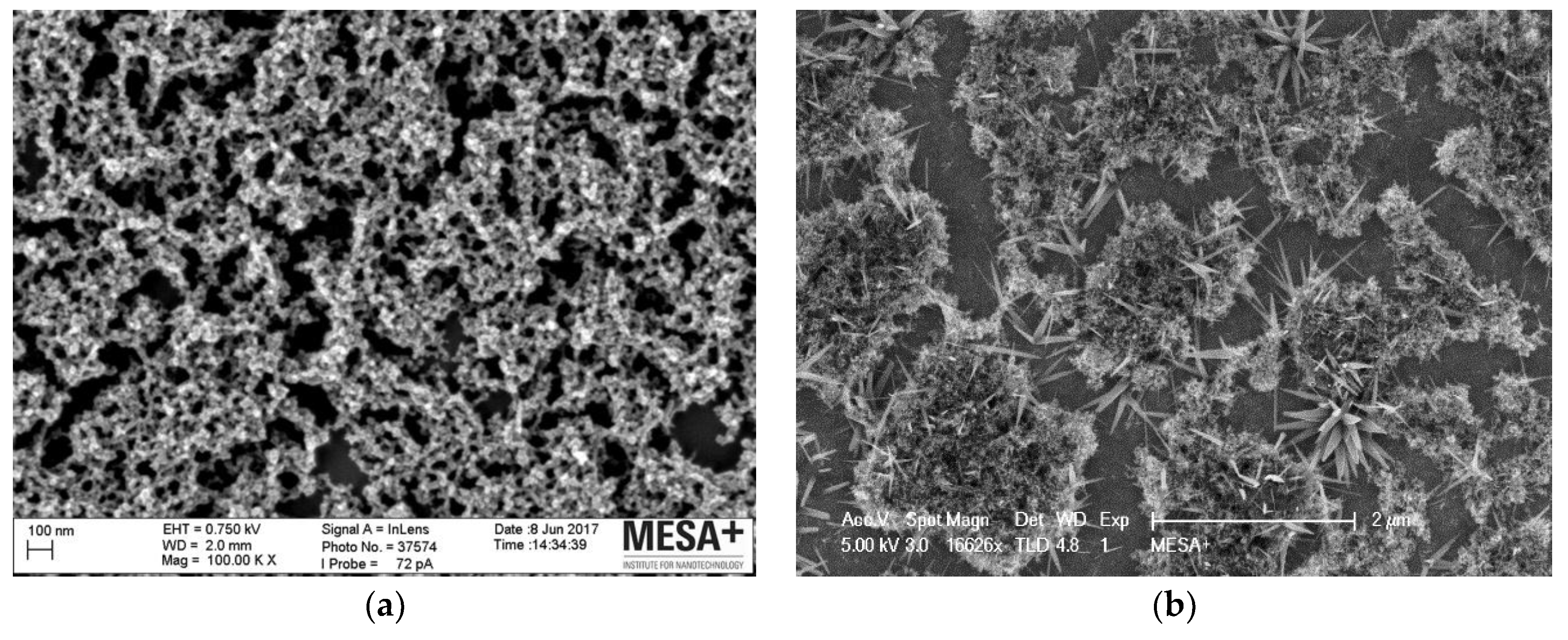
3.1. Fabrication and Characterization
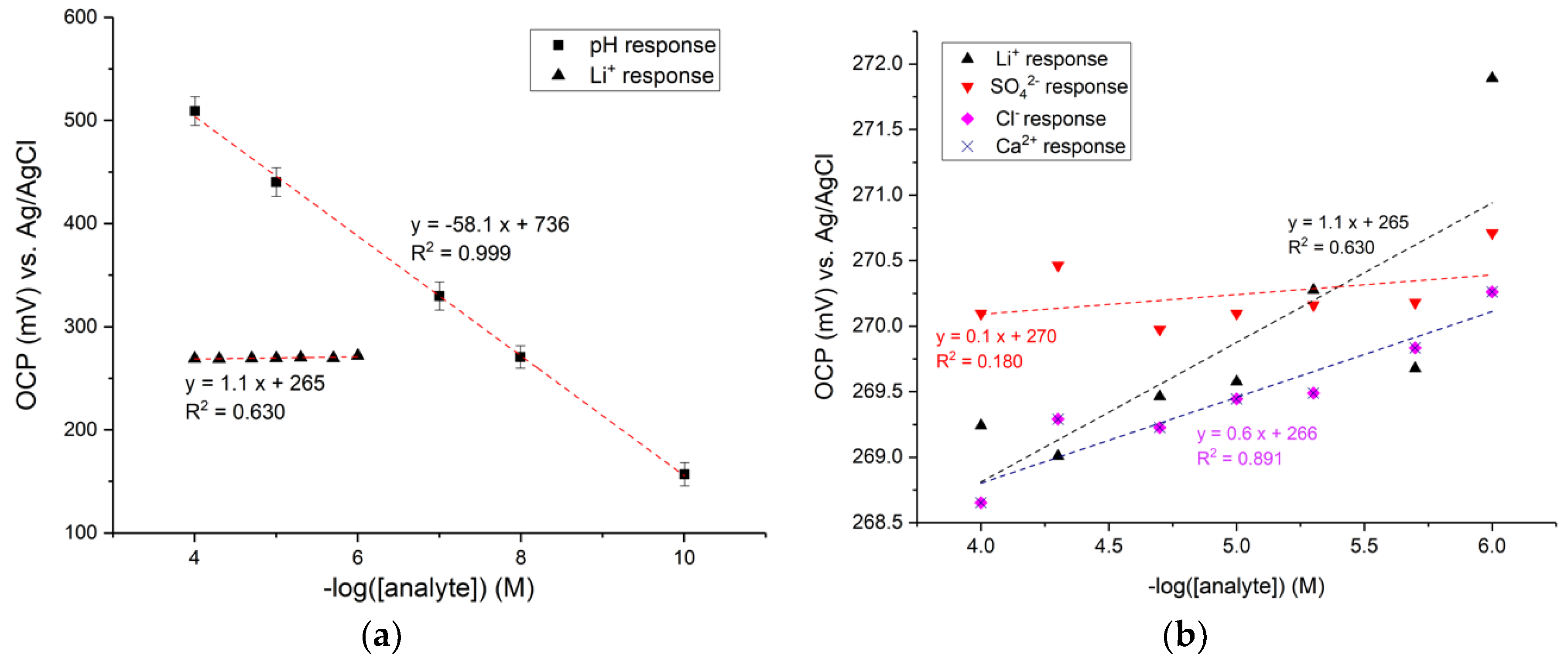
3.2. pH Sensitivity and Selectivity
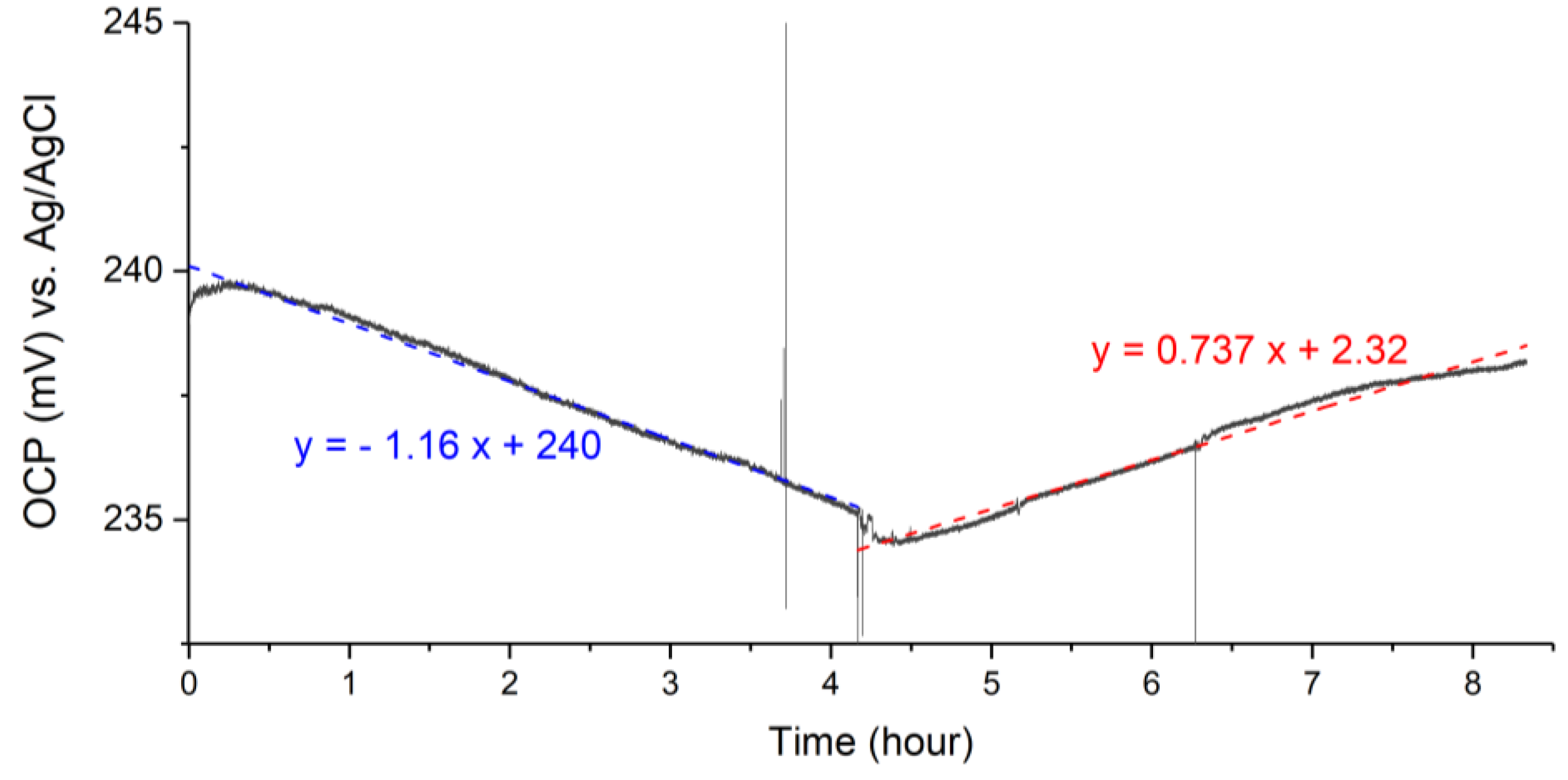
3.3. Drift and Aging
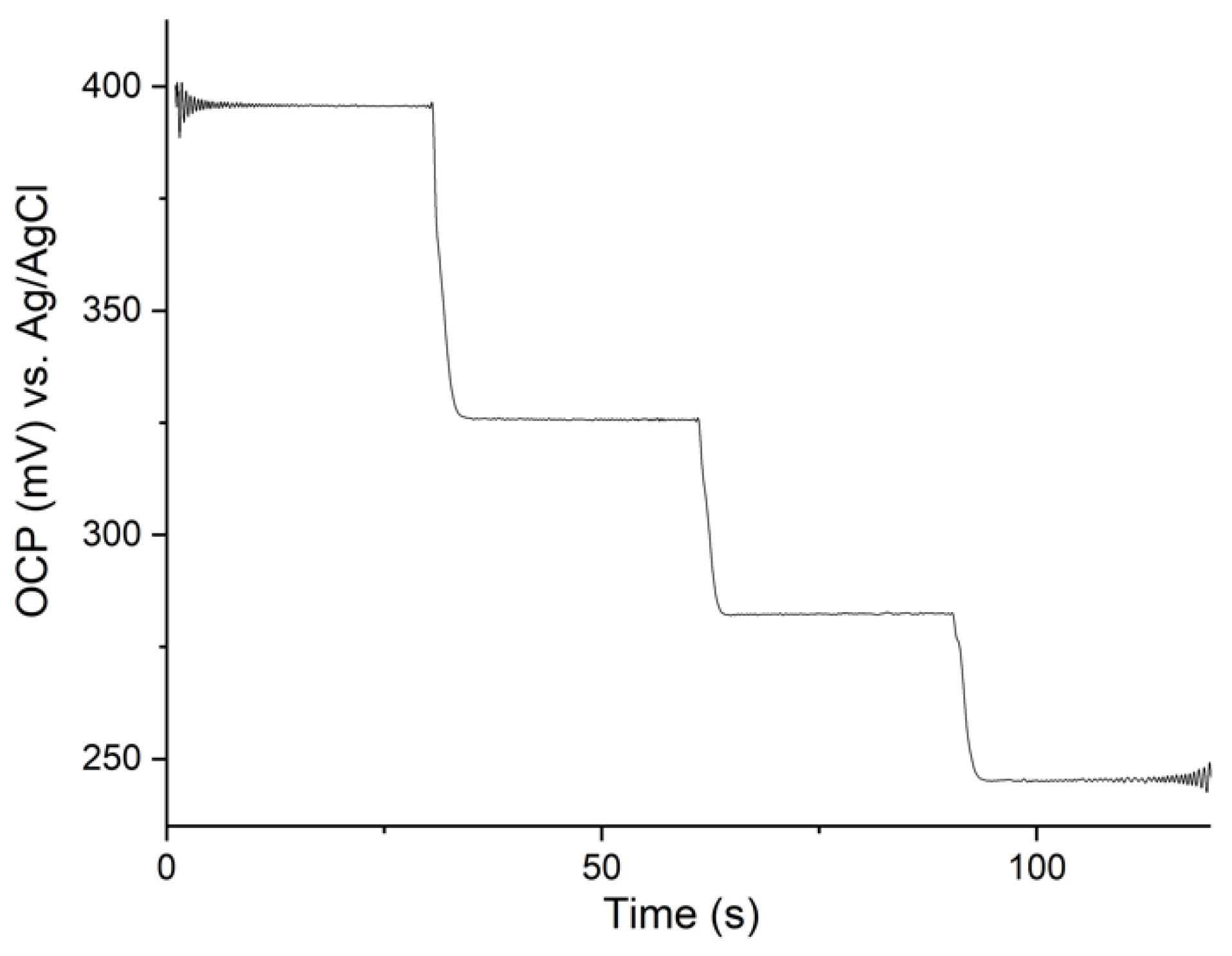
3.4. Response Time
3.5. Oxygen Sensitivity
4. Discussion
4.1. pH Response and Selectivity
4.2. Drift and Aging
4.3. Response Time and Oxygen Sensitivity
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Meer, A.D.; van den Berg, A. Organs-on-chips: Breaking the in vitro impasse. Integr. Biol. 2012, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Głab, S.; Hulanicki, A.; Edwall, G.; Ingman, F. Metal-Metal Oxide and Metal Oxide Electrodes as pH Sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Lauks, I.; Zemel, J.N. pH-sensitive sputtered iridium oxide films. Sens. Actuators 1981, 2, 399–410. [Google Scholar] [CrossRef]
- Olthuis, W.; Robben, M.A.M.; Bergveld, P.; Bos, M.; van der Linden, W.E. pH sensor properties of electrochemically grown iridium oxide. Sens. Actuators B Chem. 1990, 2, 247–256. [Google Scholar] [CrossRef] [Green Version]
- McMurray, H.N.; Douglas, P.; Abbot, D. Novel thick-film pH sensors based on ruthenium dioxide-glass composites. Sens. Actuators B Chem. 1995, 28, 9–15. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955–4985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonsdale, W.; Wajrak, M.; Alameh, K. Manufacture and application of RuO2 solid-state metal-oxide pH sensor to common beverages. Talanta 2018, 180, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, W. De Modification of vertically aligned carbon nanotubes with RuO2 for a solid-state pH sensor. Electrochim. Acta 2010, 55, 2859–2864. [Google Scholar] [CrossRef]
- Lonsdale, W.; Maurya, D.K.; Wajrak, M.; Tay, C.Y.; Marshall, B.J.; Alameh, K. Rapid measurement of urease activity using a potentiometric RuO2 pH sensor for detection of Helicobacter pylori. Sens. Actuators B Chem. 2017, 242, 1305–1308. [Google Scholar] [CrossRef]
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Szwagierczak, D.; Socha, R.P. Fabrication of thick film sensitive RuO2-TiO2 and Ag/AgCl/KCl reference electrodes and their application for pH measurements. Sens. Actuators B Chem. 2014, 204, 57–67. [Google Scholar] [CrossRef]
- Zhuiykov, S. Morphology of Pt-doped nanofabricated RuO2 sensing electrodes and their properties in water quality monitoring sensors. Sens. Actuators B Chem. 2009, 136, 248–256. [Google Scholar] [CrossRef]
- Chen, Z.G.; Pei, F.; Pei, Y.T.; De Hosson, J.T.M. A Versatile Route for the Synthesis of Single Crystalline Oxide Nanorods: Growth Behavior and Field Emission Characteristics. Cryst. Growth Des. 2010, 10, 2585–2590. [Google Scholar] [CrossRef] [Green Version]
- Kreider, K.G.; Tarlov, M.J.; Cline, J.P. Sputtered thin-film pH electrodes of platinum, palladium, ruthenium, and iridium oxides. Sens. Actuators B. Chem. 1995, 28, 167–172. [Google Scholar] [CrossRef]
- Dietz, T.; Kreider, K.G. Review of Materials for pH Sensing for Nuclear Waste Containment; National Bureau of Standards Interagency Report: Gaithersburg, MD, USA, 1985. [Google Scholar]
- Rard, J.A. Chemistry and Thermodynamics of Ruthenium and Some of Its Inorganic Compounds and Aqueous Species. Chem. Rev. 1986, 86, 731. [Google Scholar] [CrossRef]
- Tarlov, M.J.; Semancik, S.; Kreider, K.G. Mechanistic and response studies of iridium oxide pH sensors. Sens. Actuators B Chem. 1990, 1, 293–297. [Google Scholar] [CrossRef]
- Kinoshita, K. Electrochemical Measurements on Pt, Ir, and Ti Oxides as pH Probes. J. Electrochem. Soc. 1984, 131, 1089. [Google Scholar] [CrossRef]
- Mihell, J.A.; Atkinson, J.K. Planar thick-film pH electrodes based on ruthenium dioxide hydrate. Sens. Actuators B Chem. 1998, 48, 505–511. [Google Scholar] [CrossRef]
- Karagounis, V.A.; Liu, C.C.; Neuman, M.R.; Romankiw, L.T.; Leary, P.A.; Cuomo, J.J. A Pd-PdO Film Potentiometric pH Sensor. IEEE Trans. Biomed. Eng. 1986, BME-33, 113–116. [Google Scholar] [CrossRef]


| Material | pH Range | pH Sensitivity (mV/pH) | Response Time (s) | Drift (mV/Hour) | Application | Ref |
|---|---|---|---|---|---|---|
| RuO2-Ta2O5 | - | −55.3 | 5–136 | 7.2 | pH sensing of common beverages | [11] |
| RuO2 | 1–10 | −77.74 | <20 | 93.3 | Detection of Helicobacter pylori | [13] |
| RuO2 | 2–11 | −56 | 60–120 | - | - | [14] |
| Pt-doped RuO2 | 2–13 | −58 | 1–2 | 0.002 | Water quality monitoring | [15] |
| RuO2-MWCNTs | 2–12 | −55 | <40 | - | - | [12] |
| RuO2 nanorods | 2–10 | −58 | <2 | −0.8 | Organs-on-chip | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanumihardja, E.; Olthuis, W.; Van den Berg, A. Ruthenium Oxide Nanorods as Potentiometric pH Sensor for Organs-On-Chip Purposes. Sensors 2018, 18, 2901. https://doi.org/10.3390/s18092901
Tanumihardja E, Olthuis W, Van den Berg A. Ruthenium Oxide Nanorods as Potentiometric pH Sensor for Organs-On-Chip Purposes. Sensors. 2018; 18(9):2901. https://doi.org/10.3390/s18092901
Chicago/Turabian StyleTanumihardja, Esther, Wouter Olthuis, and Albert Van den Berg. 2018. "Ruthenium Oxide Nanorods as Potentiometric pH Sensor for Organs-On-Chip Purposes" Sensors 18, no. 9: 2901. https://doi.org/10.3390/s18092901





